Abstract
This paper reports a RP-HPLC method for the determination of Lomustine (CCNU) in mouse tumor tissue. CCNU was separated and determined on a reverse-phase C18 5 µm column with a mobile phase of H2O-acetonitrile (54:46), detected at 254 nm and no internal standard. The calibration curve was linear (r = 0.9999) within the range of 0.98–15.68 µg/ml for CCNU. The recovery ratio of CCNU was 68.64% (n = 5). The relative standard deviation (RSD) was 0.32%. This method is simple and accurate.
Keywords:
INTRODUCTION
Lomustine (CCNU) is an alkylating agent, which could combine with the bases of tumor cells' nucleic acid by covalent bond, and kill the tumor cells. Its adaptive diseases are cerebral tumor, malign lymphoma, lung cancer and malign melanoma. CCNU could be metabolized and eliminated in a short time after oral administration in mice, so ordinary method of determination could hardly get its content in tumor tissue. We found the undermentioned determination method of RP-HPLC, which could determine the trace concentration of CCNU 300 ng/ml.
MATERIALS
Instruments: WATERS 515 HPLC pump, WATERS 996 Photodiode Array Detector; AUTOSCIENCE vacuum pump; Air bath oscillator.
Reagents: CCNU, Shanghai No. 12 Pharmaceutical Corporation; Acetonitrile, methanol, and methylene dichloride, HPLC grade; double distilled water.
Chromatograph conditions: Stainless steel C18 5 µm chromatography column, 250 mm × 4.6 mm; mobile phase: Acetonitrile/H2O (55/45); flow speed: 1.0 ml/min; detection wavelength: 254 nm; injection volume: 20 µl.
METHODS AND RESULTS
1. Animal Experiments
The mice (weight 18 ∼ 20 g, ♂♀ half and half) were separated into 5 groups (12 mice in every group) randomly; they were the control group (N.S.), the CCNU1, CCNU2, CCNU3, and CCNU4 group. The mice were planted S180 sarcoma i.h at oxter, when the sarcoma growed to proper size, one group of the mice were administered with N.S. po, and the other four groups of the mice were administered with CCNU 42 mg/kg po, then we killed the mice and peeled off the tumor, mensurated the weight of tumor, kept them in freezer. The CCNU1, CCNU2, CCNU3, and CCNU4 group was the group of mice that was killed at the No. 1, 2, 3, and 4 day after administration, respectively.
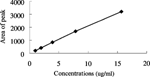
2. Chromatography Behavior of CCNU and Standard Curve
Weighed 5.0 mg CCNU precisely, dissolved it in mobile phase, prepared standard solution whose concentrations are 15.68 µg/ml, 7.84 µg/ml, 3.92 µg/ml, 1.96 µg/ml, and 0.98 µg/ml, then determined their absorption spectrum, we could get the linear relation from peak area (Y) and concentration (X):
is the standard curve of CCNU. is the chromatography behavior of CCNU. CCNU retention time is 6.2–6.3 minutes.
3. Assay of CCNU in Tumor
Ground the tumor tissue with 0.1 mol/L PB solution (Phosphate buffer solution, pH7.4), and got the 20% tissue suspension. Added 10 mL aether and had enough extraction, then the mixed liquid was filtered and kept the filtrate. Extracted this filtrate with separating funnel, the aether layer solution was super-speed centrifugated (15000 rpm, 20 min, 4°C), the aether of the supernatant was vaporized at 40°C, then dissolved the solid with 0.2 mL methanol, filtrated the solution with millipore filter, and injected 20 µl filtrate into the HPLC. According to the peak area of 20 µl solution, we could get the concentration of the CCNU solution. is the result of the CCNU content in the tumor tissue.
Table 1. Assay of CCNU in tumor (n = 12)
4. Recovery Test of the Determination Method
Weighed 2.0 mg CCNU precisely, dissolved it in mobile phase, prepared the solution whose concentration was 2.0 µg/ml. Added this solution 250 µl into 20% tumor tissue suspension (0.1 mol/L PB solution, pH7.4, without CCNU in the suspension) respectively, processed them by the above mentioned method, and the recovery of the method was 68.64%. is the result of the recovery test.
Table 2. Results of recovery and repetition tests
5. Repetition Test of the Determination Method
Injected the 2.0 µg/ml CCNU standard solution repeatedly, the RSD of the repetition test was 0.32%. is the result of the repetition test.
6. Lower Limit of the Determination Method
Injected the CCNU standard solution into HPLC; the lower limit of the determination method was 300 ng/ml.
DISCUSSIONS
CCNU had good stabilization in mobile phase, there was only one little peak before the CCNU peak on the liquid chromatograph after the CCNU solution was deposited for 48 hours. For the sake of lessening the error, we should determine the standard curve as quickly as possible, inject the samples immediately after the extraction of CCNU. We didn't study the metabolism and degradation of CCNU in vivo, and didn't determine its metabolites. On conditions that study the pharmacokinetics of CCNU, we should define and determine the metabolites of CCNU.
Though CCNU could be transformed to some metabolites in the tissue of mice, just analyzing the content of CCNU could indicate the distribution conditions in vivo. Furthermore, for lessening the error of determination, we should peel off and freeze the tumor tissue as quickly as possible.
This work was supported by 2003 Award Foundation of Shandong Province for Excellent Young Scientist.
REFERENCES
- Van Belle, S.J.P. (1992). Determination of vinca alkaloids in mouse tissue by high performance liqiud chromatography. Journal of Chromatography 578: 223. [PUBMED], [INFOTRIEVE], [CSA]
- Xu, S., Deng, A., Cai, J. (1996). Assay of Yipu flavoneby RP-HPLC. Chinese Journal of Pharmaceutical Analysis 16(2): 81. [CSA]
- Center of Drud Examination, National Ministry of Health. (1991). Rudder of human bioavailability test for drug preparations. Chinese Journal of Clinical Pharmacology 8(3): 189. [CSA]
- Stuart, A., Grossman, M.D., Reinhard, C. , et al. (1992). The intracerebral distribution of BCNU delivered by surgically implanted biodegradable polymers. J. Neurosurg. 76: 640–647. [CSA]
- Yong, S., Lisa, X., Qiqing, Z., Haiying, Z. (2003). The controlled release study of Vincristine Sulfate. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology 31(1). [CSA]
- Yong, S., Haiying, Z., Qiqing, Z., Xiaoyu, W. (2000). Improved controlled release study of lomustine. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology 28(2). [CSA]
- Martini, N. (1991). Abstr 7th Nagoya Intern Symposium on Cancer Treat, 39.
- Harris, J.R., Morrow, M., Bonadonna, G. (1993). Cancer of the breast, in Cancer, Principles & Practice of Oncology, DeVita, V.T.Jr, Hellman, S, Rosenberg, S.A. Eds., 4th ed. , JB Lippincott: Philadelphia, pp. 1264–1332.
- Bonadonna, G., Valagussa, P., Moliterni, A. , et al. (1995). Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer, the results of 20 years of follow-up. N. Engl. J. Med. 332: 901. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mauric, L., Durnad, M., Arril, A. (1991). Effects of primary chemotherapy in conservative treatment of breast cancer patients with operable tumors larger than 3 cm, Results of randomized trial in a single centre. Ann. Oncol. 2: 347. [CSA]
- Sun, Y., Yin, W.B., Zhang, R.G. , et al. (1995). Prospective meltimodality treatment of SCLC-experience during the past 18 years. Jpn. J. Cancer ChemoTher. 22(Suppl III): 222. [CSA]
- Sharky, R.M., Juweid, M., Shevtz, J. , et al. (1995). Evaluation of a complementary-determining region-grafted (humanized) anticarcino-embryonic antigen monoclonal antibody in preclinical and clinical studies. Cancer Res. 55: 5935–5945. [CSA]
- Imbert-Marcille, B.M., Berreur, M., Thedrez, P. , et al. (1995). Effects of interferon gamma and tumor necrosis factor alpha association in intraperitoneal radioimmunotherapy: studies on an in vitro micrometastasis model. Anticancer Drug Des. 10: 491–505. [PUBMED], [INFOTRIEVE], [CSA]