1 CHEMICAL AND BIOLOGICAL WARFARE BEFORE 9-11-2001
Wax P
Good Samaritan Regional Medical Center, Phoenix, Arizona, USA
Our ability to respond to the current challenges posed by chemical and biological warfare agents requires an understanding of the past. Despite repeated prior attempts to ban the use of poisons in warfare, the modern age of chembio warfare began during World War I on April 22, 1915 when the Germans released 150 tons of chlorine gas from 6000 cylinders directed at trenches full of Allied troops in Ypres Belgium. As many as 800 died and 2500–3000 were incapacitated from this revolutionary offensive weapon. As one observer noted: “The most stupendous change in warfare since gunpowder was invented had come and come to stay. Let us not forget that.” The Allies employed chlorine gas against the Germans that same year and soon thereafter a chemical arms race would ignite as the combatants worked to weaponize more potent chemicals and improve their delivery systems. By May 1916 the Germans started to use trichloromethyl chloroformate (diphosgene) in combat and by July 1917 added the vesicant agent mustard to their armamentarium. During the war the French used hydrogen cyanide and cyanogen chloride. Approximately one million of the 26 million World War I casualties were attributed to chemical weapons. Biological weapons were also employed during this war but to a far lesser extent. Glanders (pseudomonas) was successfully used as an antianimal biological weapon when a German agent infected 4500 mules in Mesopotamia. Early American endeavors at biochem weaponization during this period included an unsuccessful attempt to produce a ricin dust cloud. During the interwar period a variety of other chemical weapons were produced including white phosphorus, and the stockpiling of mustard (both nitrogen and sulfur mustard) and phosgene continued. In 1936, the first in a series of “nerve” agents were developed when Gerhart Schrader of I.G. Farbon discovered the first organophosphate agent. This chemical would become known as Tabun or GA (German agent A). Two years later Schrader also developed Sarin. Although these nerve agents were not used during World War II, by 1945 12,000 tons of tabun and 1000 pounds of sarin had been produced by the Germans. During the same time the U.S produced 20,000 tons of phosgene, 87,000 tons of mustard agent, 20,000 tons of Lewisite, 12,500 tons of cyanogen chloride and 560 tons of hydrogen cyanide. The chemical warfare agents that were used during this time included mustard bombs by the Italians during their offensive in Ethiopia, and mustard agent and Lewisite by the Japanese in China. The Germans found that hydrogen cyanide (zyclon-B) was a very effective means of mass killing in concentration camps. Japan also experimented with cholera, typhus, and plague in China. Since the end of the Second World War, chemical weapons have reportedly been used in a number of regional conflicts including the Yemen Civil War in the 1960s (mustard agent and possibly nerve agents), Vietnamese War (defoliants and non lethal riot control agents), Soviet–Afghanistan War of the 1980s, and Iraq–Iran War of the 1980s (mustard agent and tabun). Terrorists have also employed chemical and biological weapons. The best-known examples occurred when the Japanese religious cult, Aum Shinrikyo, released sarin in a residential area of Matsumoto Japan in 1994 (killing 7 and injuring 500) and again on the Tokyo subway in 1995 (killing 12, with 5550 seeking medical attention). Two notable bioweapon events in recent years were the outbreak of anthrax in Russia in 1979 and a mass intentional salmonella poisoning in Oregon in 1984. In April 1979, 94 people became ill and 64 died after anthrax spores were accidental released from a suspected Soviet biological weapons facility in Sverdlovsk, Russia (now Ekaterinburg). Seven hundred and fifty people developed food poisoning in Dallas, Oregon in 1984 when followers of the Indian guru Bhagwan Shree Rajneesh spiked salad bars at 10 restaurants with salmonella in an attempt to incapacitate voters in a local election. The surprise 9-11 attack on the world trade center has painfully taught us that, in addition to understanding the past, one must be increasingly vigilant and prepared for the unsuspected.
2 ANTHRAX AS A BIOLOGICAL WEAPON: RECENT EXPERIENCES IN THE UNITED STATES
Cetaruk EW
Division of Clinical Pharmacology and Toxicology, University of Colorado Health Sciences Center Toxicology Associates, Denver, Colorado, USA
Objective: To review and present published data regarding anthrax as a biological weapon, including the clinical presentation, treatment, and epidemiological characteristics of anthrax cases in the United States as a result of the deliberate release of Bacillus anthracis spores in the mail September and October, 2001. Methods: Compilation and review of published information, as well as unpublished government data, regarding anthrax as a biological weapon, focusing on anthrax cases in the United States resulting from terrorist attacks in 2001. Results: In its natural form, anthrax is a zoonotic, or epizoonotic infection, caused by the gram-positive, non-motile, spore-forming rod, Bacillus anthracis. Although it most often infects herbivores, all mammals are susceptible. Humans typically become infected by contact with infected animals or contaminated animals products, such as wool or hides. Clinically, anthrax occurs in three forms. Cutaneous anthrax is acquired by direct contact with anthrax spores or infected animals or animal products through an abrasion or open wound in the skin. The black eschar of cutaneous anthrax gives it its name, anthrakos (Greek: coal, black). Gastrointestinal anthrax, caused by the ingestion of insufficiently cooked contaminated meat, is rare. The inhalational form of the disease results from the inhalation of aerosolized anthrax spores, and is the most likely route to be utilized for a bioweapon. Anthrax was among the primary bioweapons developed by many biological weapons programs, including those of the United States prior to 1969, the Soviet Union, Iraq, Japan, and others. It has become the weapon of terrorists, including the Aum Shinrikyo in Japan, and most recently, by those perpetrating attacks in the United States. Both cutaneous and inhalational anthrax were seen as a result of terrorist attacks via the mail in the United States. Weaponized anthrax spores are most effective when aerosolized in particles 1 to 5 microns in diameter. Particles of this size reach the distal airways where they are phagocytosed by alveolar macrophages, delivered to the mediastinal lymph nodes, and germinate into vegetative bacilli, causing clinical infection. Inhalational anthrax begins as a mediastinitis and progresses to septicemia. Bacillus anthracis has three virulence factors. An anti-phagocytic poly-D-glutamic acid capsule is encoded on a plasmid labeled pXO2. B. anthracis also secretes three exotoxin components: Protective Antigen (PA), Lethal Factor (LF), and Edema Factor (EF), that are encoded on a second plasmid, pXO1. PA is a receptor-binding component, which binds to an unidentified cell surface receptor and is cleaved by a furin, or furin-like protease, into two fragments: PA63, a 63 kDa C-terminal fragment, which remains receptor-bound, and PA20, a 20 kDa N-terminal fragment, which is released into the medium. PA63 forms a heptamer which binds either LF or EF to form lethal toxin and edema toxin, respectively. The resulting hetero-oligomeric complex is internalized by endocytosis and acidification within the vesicle promotes insertion of the PA63 heptamer into the endosomal membrane, producing a channel through which LF or EF translocates to the cytosol, where they induce cytotoxic events. The combination of PA+LF (anthrax lethal toxin) is a zinc metalloprotease that is known to cleave at least two targets, mitogen-activated protein kinase kinase 1 and 2. It has been shown that the host macrophages are integral to the cytotoxic activity of lethal toxin. Lethal toxin causes a hyperinflammatory response, activating the macrophage's oxidative burst pathway, and causing the release of reactive oxygen intermediates and inflammatory cytokines. The combination of PA+EF (edema toxin), a calmodulin-dependent adenylate cyclase, increases intracellular cyclic AMP, causing edema. As septicemia and toxemia progress, shock and death rapidly follow, typically within one to seven days of infection. The initial cases reported in the United States in September 2001, appearing as cutaneous anthrax, went unrecognized until the first case of inhalational anthrax was diagnosed in Florida. The cases in New Jersey and New York subsequently were linked to letters postmarked September 18, 2001. Subsequent cases were linked to letters mailed October 9, 2001, while the source of several cases is still unknown. In all, 22 cases of anthrax, 11 cutaneous and 11 inhalational, were identified, resulting in 5 deaths. The initial 10 cases of inhalational anthrax in the United States were clinically summarized by Jernigan, et al as follows: incubation period: 5–11 days; fever in 100%; severe fatigue/malaise in 100%; sweats in 70%; minimal or nonproductive cough in 90%; chest discomfort/pleuritic pain in 70%; dyspnea in 80%; myalgias 60%; abdominal pain 30%; headache 50%; confusion 40%; sore throat 20%; rhinorrhea 10%; or nausea/vomiting in 90%. Laboratory and radiographic abnormalities for these 10 cases can be summarized as follows: normal to slightly elevated total white blood count with elevated percent of neutrophils or band forms; abnormal chest x-ray or chest CT scan including mediastinal changes in 80–100% (widening, paratracheal/hilar fullness, adenopathy), pleural effusions in 80%, infiltrates in 70% (including multilobar); blood cultures: positive in 70% (in all persons who did not receive prior antibiotics). Ciprofloxacin is the drug of choice for treatment of all forms of anthrax. Epidemiological and criminal studies have identified specific letters, and circumstances of exposure, for many of the cases. Microbiological and genetic studies have found that the anthrax strain used was the same in all letters, and have identified it as the Ames strain. Additional studies and investigations are ongoing to unequivocally determine the source. Conclusion: The threat of biological weapons being used as weapons of mass destruction is no longer merely a threat. A great deal of research has been completed, and more is ongoing, to develop effective medical and emergency responses to bioweapon attacks. The anthrax attacks in the United States hold important lessons that must be learned and applied as we develop and improve our level of preparedness to respond to attacks using weapons of mass destruction.
3 PLAGUE: A POTENTIAL INSTRUMENT OF BIOLOGICAL WARFARE AND BIOTERRORISM
Brent J
Toxicology Associates, University of Colorado Health Sciences Center, Denver, Colorado, USA
The term plague refers to a spectrum of diseases caused by Yersinia pestis, an organism that has been responsible for more human death from infectious causes than any other. Although there are several references to outbreaks of plague before the Common Era the best characterized epidemics are the three most recent plague pandemics. The first two occurred in Europe and killed between one-third and over one-half of the population. The third, occurring in the 19th century, began in China and spread to worldwide. Although there may have been other instances, probably the best characterized use of plague as a biological weapon occurred in World War II when the Japanese used Y. pestis against China. The United States, during its now disbanded biological warfare program, studied plague. There have been a series of allegations of a major Soviet program weaponizing Y. pestis. Although Iraq appears to have been interested in plague, it is not generally listed as one of its major focuses in its biological weapons program. Y. pestis is easy to obtain and mass-produce. Once disseminated it may give rise to both primary and secondary infections, hence perpetuating its effect. The organism: Y. pestis is a gram-negative non-spore forming coccobacillus related to the enteropathogens Y. enterocolitica and Y. pseudotuberculosis. The virulence of Y. pestis is the basis for the World Health Organization model which estimates that an aerosol of 50 kg dropped on a major urban center would cause up to 150,000 cases of plague, of which as many as one-third would die. Clinical manifestations: Plague occurs in bubonic, pneumonic, septicemic, and meningitic forms. In nature, almost 90% of cases are the primary bubonic form and approximately 1% are primary pneumonic plague. Plague meningitis and septicemia may occur secondary to other forms of plague although 10–15% of septicemic plague is primary. Because plague as a bio-weapon would most likely be the primary pneumonic form, spread by the inhalation of organisms, or, less likely, the bubonic form, spread by the release of infected fleas, this presentation will focus on these two entities. Primary pneumonic plague, the most fatal variety of the disease, arises as a result of the direct inhalation of the organism. Patients typically present 1–6 days after inhalation with fever, chills, malaise, headache, productive cough, hemoptysis and bilateral pulmonary infiltrates, rapidly progressing to respiratory failure and death. Bubonic plague results from the direct inoculation of Y. pestis by an infected flea. Thus, in order to intentionally cause bubonic plague it is necessary to disseminate large numbers of infected fleas. Following inoculation the organism spreads to local lymph nodes where it creates a painful dramatic generally non-fluctuant lymphadenitis, known as buboes. Occasionally an ulcerated lesion may be detectable at the site of inoculation. Untreated, the organism may disseminate further causing pneumonic or septicemic plague. The latter has a presentation similar to gram-negative sepsis. Purpura, acral cyanosis and gangrene occur during the septicemic phase giving rise to the label “Black Death.” Following inoculation buboes tend to become evident within approximately one week. Commonly patients will present with severe malaise, often accompanied by a headache, followed shortly thereafter by the appearance of severely painful buboes. Within several days untreated patients develop secondary septicemic plague and/or secondary pneumonic plague. Pathogenesis: Y. pestis derives its pathogenic features from its genetically coded virulence factors that interfere with phagocytosis, prevent inflammation, and facilitate subcutaneous spread via proteolysis. These virulence factors are expressed after the initial inoculation into the host and phagocytosis by macrophages and neutrophils. Once expressed these bacteria proliferate and disseminate. Patients with pneumonic plague expectorate organisms and thus are at risk for being vectors of person-to-person transmission. Diagnosis: The identification of bubonic plague, with its characteristic bubo and severe malaise is suggested by its clinical presentation. Although there are numerous conditions that can present with large painful adenopathy, the major differential for patients that additionally have prominent symptoms of prostration are bubonic plague and tularemia. For naturally occurring disease, being in a plague-endemic area supports the presumptive diagnosis of the latter. Fortunately the antibiotic treatment for both tularemia and plague are similar. Aspiration of buboes allows for culture, microscopic examination, and evaluation by a direct fluorescent antibody technique against Y. pestis capsular antigen. Any body fluid is appropriate for culturing plague. Although there are serologic assays measuring anti-Y. pestis immunoglobulin, these are not clinically useful because the antibody response occurs after disease becomes clinically manifested. There is an enzyme-linked immunosorbent assay (ELISA) for specific antibodies to capsular antigens, however it is not emergently available. Although a polymerase chain reaction (PCR) assay is highly sensitive for Y. pestis, it has not been yet adapted to human clinical use. The diagnosis of plague pneumonia is more subtle. The initial presentation is strongly suggestive of severe pneumonia, which can be caused by a variety of organisms. The identification of a gram-negative source of pneumonia in healthy immuno-competent individuals is markedly unusual and should suggest plague infection. Certainly, any more than one case of pneumonic plague would be highly suspicious for a bio-weapon exposure. Treatment: There is a lack of controlled clinical trials on the treatment of plague so much of the data comes from case series, animal and in vitro studies. The traditional treatment for plague is streptomycin, which appears to reduce mortality. There is also significant animal and in vitro data supporting gentamicin use, although there is less clinical experience with it than with streptomycin. Tetracycline and doxycycline have been studied and appear to be active based on pre-clinical studies, but these are conflicting. There have been several anecdotal case-series supporting the use of these agents. The consensus is that effective but second line. Fluoroquinolones appear to be active based on pre-clinical studies, however there is little human experience with this class of agents in the treatment or prevention of plague. Chloramphenicol appears to be active but is less well studied than streptomycin or gentamicin. It is generally considered to be a first-line agent for the treatment of plague meningitis. Trimethoprim-sulfamethoxazole appears to be active but less so than streptomycin. In the United States a Working Group on Civilian Bio-defense has developed treatment guidelines based on a thorough review of available studies. These call for streptomycin or gentamicin as first-line agents, to be used parenterally where possible. However, if medical resources are overwhelmed and parenteral therapy is not possible, oral treatment with doxycycline, tetracycline, or ciprofloxacin are a second-line strategies. The Working Group have also made a series of recommendations for special populations. The first-line recommendation for plague in pediatric patients is gentamicin or streptomycin. However, they noted that above age 8 tetracycline or doxycycline is an acceptable choice. The Working Group pointed out that children younger than 2 years of age are at risk for Gray Baby syndrome following chloramphenicol treatment. Use of fluoroquinolones in this population is controversial since there have been allegations of drug-induced arthropathy, although the magnitude of this risk is unknown. The Working Group recommends that gentamicin be used as a first-line agent in pregnancy and that streptomycin be avoided because of concern of deafness following fetal exposure. Although tetracyclines are generally considered to have an adverse effect on the fetus, a large case controlled study on doxycycline suggested that this may not pose a similar risk. The Working Group thus recommends that doxycycline be a second-line agent if gentamicin is not available. Vaccination: A formaldehyde-killed whole organism vaccine has been developed which is protective against bubonic, but not pneumonic, plague. This vaccine is not, at present, generally available.
References: 1. Franz, D.R.; Jahrling, P.B.; Friedlander, A.M. et al. Clinical Recognition and Management of Patients Exposed to Biological Warfare Agents. JAMA 1997, 278, 399–411. 2. Vogel, P.; Fritz, D.L.; Kuehl, K. et al. The Agents of Biological Warfare. JAMA 1997, 278, 438–439. 3. Zilinskas, R.A. Iraq's Biological Weapons. JAMA 1997, 278, 418–424. 4. McGovern, T.W.; Friedlander, A.M. Plague. Medical Aspects of Chemical and Biological Warfare. 5. Inglesby, T.V.; Dennis, D.T., Henderson, D.A. et al. Plauge as a Biological Weapon. JAMA 2000, 283.
4 SMALLPOX AND VIRAL HEMORRHAGIC FEVERS: HOW TO CONTROL THESE BIOLOGICAL WARFARE AGENTS
Cetaruk EW
Division of Clinical Pharmacology and Toxicology, University of Colorado Health Sciences Center Toxicology Associates, Denver, Colorado, USA
Objective: To review and present published data regarding smallpox and the viral hemorrhagic fevers (VHFs) as biological weapons, including their clinical presentation, treatment, and epidemiological characteristics. Methods: Compilation and review of published information, as well as unpublished government data, regarding smallpox and VHFs as a biological weapons. Results: The causative agent of smallpox, variola virus, is a member of the genus Orthopoxvirus, subfamily chordopoxvirinae of the family Poxviridae. It is a DNA virus measuring approximately 200 nm in diameter. There is no animal reservoir for variola virus, humans being the only known host. This fact made eradication of smallpox a possibility through worldwide immunization. With the exception of a fatal laboratory-acquired case that occurred in the United Kingdom in 1978, the last endemic case occurred in Somalia in 1977. The global eradication of smallpox was declared by the World Health Organization in 1980. Paradoxically, the successful eradication of endemic smallpox, and subsequent halting of routine vaccination, has created a worldwide population susceptible to smallpox infection. The financial incentive to sell equipment and/or knowledge for the production of biological weapons to terrorist organizations represents a real and significant risk. A resourceful terrorist may attempt to obtain smallpox from the former Soviet Union's Biopreparat biological warfare program, or from one of its former employees. Smallpox is transmitted from person to person by infected aerosols and air droplets spread in face-to-face contact with an infected person after fever has begun. Variola virus is relatively stable in the natural environment. If aerosolized, it probably remains infectious for at least several hours if not exposed to sunlight or ultraviolet light. The disease can also be transmitted by contaminated clothes and bedding, though the risk of infection from this source is much lower. Variola may have been used by Lord Jeffrey Amherst against native Americans by giving them contaminated blankets from the beds of smallpox victims during the French and Indian war. The minimum infectious inoculum is unknown, but is thought to be as small as a few virions. Smallpox infection occurs following implantation of the virus on the oropharyngeal or respiratory mucosa. After the migration of virus to and multiplication in regional lymph nodes, an asymptomatic viremia develops on or about the third day, followed by multiplication of virus in the lymph nodes, bone marrow, and spleen. A secondary viremia begins on about the eighth day and is followed by fever and toxemia. The virus, contained in leukocytes, or free in plasma, then localizes in small blood vessels of the dermis, and beneath mucosa, and subsequently infects adjacent cells causing a rash (exanthem), or mucosal lesions (enanthem), respectively. The rash of smallpox is a synchronous eruption, centrifugal in distribution, in contrast to varicella, which is an asynchronous, centripetal distribution. Smallpox has several variants including Variola minor, flat-type smallpox, hemorrhagic smallpox. As the disease course progresses, patients may develop severe toxemia with a mortality rate of approximately 30%, and 3% if vaccinated. At present, treatment is primarily supportive critical care. Vaccinia vaccine remains the standard for preexposure prophylaxis against smallpox. Vaccinia vaccination, vaccinia immune globulin, and methisazone each possess some efficacy in postexposure prophylaxis. However, the antiviral medication cidofovir may yet be an effective treatment, although human data is still lacking. The prevention of secondary cases is paramount. All medical personnel must were appropriate respiratory personal protective equipment if smallpox is suspected. All exposed individuals must be quarantined a minimum of 17 days. The viral hemorrhagic fevers are a diverse group of human illnesses that are due to RNA viruses from several different viral families: the Filoviridae, which consists of Ebola and Marburg viruses; the Arenaviridae, including Lassa fever, Argentine and Bolivian hemorrhagic fever viruses; the Bunyaviridae, including various members from the Hantavirus genus, Congo-Crimean hemorrhagic fever virus, and Rift Valley fever from the Phlebovirus genus; and Flaviviridae, such as Yellow fever virus, Dengue hemorrhagic fever virus, and others. Although it is known Marburg virus has been weaponized, evidence for weaponization does not exist for many of these viruses. However, many have potential for aerosol dissemination or weaponization, and therefore are mentioned. As most viruses have predilections for a particular cell type, VHFs have a predilection for the vascular bed. This manifests clinically as increased vascular permeability, bleeding, organ necrosis. Symptoms include fever, vomiting, diarrhea, mucus membrane bleeding, shock, ecchymoses. The Biopreparat program had weaponized Marburg virus, therefore it may become available as a WMD. Also, there are naturally occurring outbreaks of Ebola virus in central Africa, which might also be a source of virus for a terrorist organization. Treatment for patients infected with a VHF is primarily supportive. There is no definitive anti-viral drug available, however, ribavarin has been used for some of the VHFs. Great care should be taken to minimize the risk of secondary transmission of the virus by body fluids. VHF viruses can be spread by contact with infected body fluids, or by aerosolization of infected body fluids (e.g. hemoptysis). Strict body surface isolation, respiratory protection, universal precautions, and autoclaving and safe disposal of all contaminated materials are essential to limit secondary cases of infection. Conclusion: The threat of biological weapons being used as weapons of mass destruction is no longer merely a threat. A great deal of research has been completed, and more is ongoing, to develop effective medical and emergency responses to bioweapon attacks. Although smallpox and the VHFs are considered much more difficult to weaponize and disseminate than anthrax, they possess a monumental risk for becoming epidemics due to their communicability, especially smallpox. A swift and aggressive emergency, medical, and public health response will be essential should one of these agents be used by terrorists as a weapon of mass destruction.
5 BOTULISM AS WARFARE AGENT: FEATURES, MANAGEMENT AND TREATMENT
Jaeger A
Service de Réanimation Médicale, Hôpital de Hautepierre, Strasbourg, France
Introduction: Botulinum toxin has already been used as a biological weapon. Aerosols were used on at least 3 occasions between 1990 and 1995 in Japan by the Japanese cult Aum Shinrikyo but these attacks failed. Development of BT as a possible bioweapon began at least 60 years ago, especially during World War II in Japan, Germany and the US. Although the 1972 Biological and Toxin Weapons Convention prohibited offensive research and production of biological weapons, some signatories, such as Iraq and Soviet Union, continued to produce BT toxin for use as weapon. During the last decade, countries suspected to sponsor terrorism are believed to still develop BT toxin as a weapon. The military use of BT as an incapacitating agent is small because of technical constraints to concentrate and stabilize the toxin for aerosol dissemination. However, in a civilian population, deliberate release of BT either by aerosol or contaminated food would be able to cause a large number of casualties. Microbiology and toxin properties: Clostridium botulinum is a spore-forming, strict anaerobic, gram+, bacillus whose habitat is soil and which produces a neurotropic toxin when environmental conditions are favourable (strict anaerobiosis, ambient temperature…). BT is colorless, odorless and tasteless and is inactivated by heat >85°C for 5 minutes. Seven different strains of Clostridium botulinum with distinct antigenic types have been identified (letters A to G). BT is a dichain polypeptide that consists of a heavy chain linked by a single disulfide bond to a light chain. The toxin light chain is a Zn++-containing endopeptidase that blocks the release of acetylcholine from the vesicles. The toxin acts on the cholinergic synapses of the autonomic nervous system and of the neuromuscular junction but does not cross the blood–brain barrier that explains the lack of CNS disturbances. At low concentrations (<10−10 mol) the toxin acts on cholinergic synapses but at higher concentrations(10−7 mol) the toxin inhibits also the neurotransmitter release in dopaminergic and noradrenergic synapses. The exact lethal dose in humans is not known. From animal studies the lethal amount in a 70-kg human would be approximately 0.09–0.15 ug iv or im, 0.70–0.90 ug inhalationally and 70 ug orally. Causes and epidemiology: Naturally occurring human botulism exists in 3 forms: foodborne, wound and intestinal. Inhalational botulism, a man-made form resulting from aerosolized BT, has been demonstrated experimentally in primates, has been attempted by terrorists and has occurred accidentally in humans. When the toxin is absorbed, it reaches the peripheral cholinergic synapses, principally the neuromuscular junction, where it binds irreversibly. Approximately a median of 24 cases are reported annually in the US and 15 to 20 cases per year in France. Most cases are foodborne botulism. Serotype A is most frequent in the US whereas serotype B is prominent in Europe. Serotypes C and D are rare in humans. Clinical manifestations: Botulism is often misdiagnosed, especially in isolated cases. In foodborne botulism, symptoms occur after an incubation period of a few hours to several days (up to 15 days) and include abdominal cramps, vomiting and diarrhea. These gastrointestinal symptoms are thought to be caused by other bacterial contamination and are not observed in other forms of botulism. Typically symptoms include: (1) dry mouth, decreased lacrimal and salivary secretions, (2) cranial and peripheral nerve paralysis: blurred vision, diplopia, ptosis, non reactive mydriasis, dysphonia, dysphagia, muscular weakness, flaccid peripheral muscular paralysis, (3) dysuria with bladder retention, gastrointestinal ileus and hypotension may be present. Typically paralysis involves first the cranial nerves and then reach the upper extremities, the respiratory muscles and finally the lower extremities. Death results from respiratory paralysis which may occur rapidly within one day. Diagnostic testing: BTcan be identified in suspect foods, serum, stool, gastric fluid. The standard laboratory diagnostic test is the mouse bioassay. The toxin is identified by administration of the suspect sample to mice immunized by specific antitoxins (A to G). Results are obtained in 24–48 hours. The limit of detection is about 0.03 ng. Differential diagnosis: Clinical features for differential diagnosis with other paralysis are the lack of fever, of meningitis signs, of CNS disturbances, of sensory nerve damage, the prominence of cranial nerve paralysis, the presence of visual disturbances and the flaccid descending paralysis. Usually other causes of peripheral paralysis can be easily excluded on the clinical particularities, the normality of CSF, the electromyogram which shows normal nerve conduction velocity and a pre-synaptic neuromuscular block. Treatment: Therapy for botulism consists of supportive care and passive immunization. Patients with suspected botulism should be closely monitored and admitted in an ICU. Supportive measures include enteral- or parenteral nutrition, mechanical ventilation for respiratory failure and prevention of complications. Antibiotics have no effect on BT and are only indicated in endogenous contamination and wound botulism. Guanidine which increases the release of acetylcholine in the nerve terminations has been proposed. However, although some improvement has been reported in moderate cases, its efficacy is not established. Passive immunization by neutralizing antitoxin should be administered early within 24 hours following the onset of symptoms. It may prevent and minimize subsequent nerve damage and severity of disease but has no effect on existent paralysis. Two types of equine botulinum antitoxin ABE are available. The antitoxin marketed in the US contains 7500 IU antitoxin A, 5500 IU antitoxin B and 8500 IU antitoxin E per 10 mL vial. The recommended dose is a single vial by slowly intravenous infusion. The antitoxin marketed in Europe contains 187500 IU antitoxin A, 125000 IU antitoxin B and 12500 IU antitoxin E per 250 mL vial. The recommended dose is 2 vials intravenous. Although the doses are quite different, no difference in efficacy has been demonstrated. Botulinum is available from the CDC in the US (tel: (1) 404 6392206) or from Chiron-Behring company in Germany (tel: (49) 1 805251616). Bioterrorism considerations: Several features may suggest a deliberate release of botulinum toxin: an outbreak of a large number of cases, unusual types of toxin (C, D, E, F or G). Contamination of water supply is unlikely because BT is rapidly inactivated by standard potable water treatments and, because of the large capacity of water reservoirs, a comparable large amount of toxin would be needed. The ways which may most probably be used for deliberate release are aerosols or contamination of food. A careful investigation about travel, work and activity history, as well as a careful dietary history is necessary in order to identify a common factor and source of contamination.
References: 1. Arnon, S.S.; Schechter, R.; Ingesby, T.V. et al. for the Working Group on Civilian Biodefense. Botulinum Toxin as a Biological Weapon. JAMA 2001, 285, 1059–1070. 2. Burrows, W.D.; Renner, S.E. Biological Warfare Agents as Threats to Potable Water. Environ. Health Perspect. 1999, 107, 975–984. 3. Holtzer, V.E. Botulim from Inhalation. Med. Klin. 1962, 57, 1735–1738. 4. Knubley, W.; Mc Chesney, T.C.; Mallonee, J. Foodborne Botulism—Oklahoma, 1994. JAMA 1995, 273, 1167. 5. Lacy, D.B.; Tepp, W.; Cohen, A.C. et al. Crystal Structure of Botulinum Neurotoxin Type A and Implications for Toxicity. Nat. Struct. Biol. 1998, 5, 898–902. 6. Shapiro, R.; Hatheway, C.; Becher, J. et al. Botulism Surveillance and Emergency Response: A Public Health Strategy for a Global Challenge. JAMA 1997, 278, 433–435. 7. Shapiro, R.; Hatheway, C.; Swerdlow, D. Botulism in the United States: A Clinical and Epidemiologic Review. Ann. Int. Med. 1998, 129, 221–228. 8. Simpson, L. Botulinum Toxin: A Deadly Poison Sheds Its Negative Image. Ann. Int. Med. 1996, 125, 616–617. 9. Wiener, S.L. Strategies for the Prevention of a Successful Warfare Aerosol Attack. Mil. Med. 1996, 161, 251–256. 10. Woodruff, B.; Griffin, P.; Mc Croskey, L. et al. Clinical and Laboratory Comparison of Botulism from Toxin Types A, B and E in the United States, 1975–1988. J. Infect. Dis. 1992, 166, 1281–1286. 11. Zilinskas, R.A. Iraq's Biological Weapons: the Past as Future? JAMA 1997, 278, 418–424.
6 LUNG DAMAGING AGENTS: RELEVANT FACTORS TO CONSIDER IN MASS EXPOSURE, PRACTICAL GUIDELINES FOR DIFFERENTIAL DIAGNOSIS AND TREATMENT
Meulenbelt J1,2, Spoelstra FM1
1National Poisons Control Centre, National Institute for Public Health and the Environment, Bilthoven, The Netherlands. 2Department of Intensive Care and Clinical Toxicology, University Medical Center, Utrecht, The Netherlands
Introduction: It is important for physicians to be prepared for inhalatory exposure to warfare agents in order to be able to organize adequate medical aid in the case of a terrorist attack. A warfare agent can be dispersed as a gas/vapor or an aerosol (mists, fumes, smokes, or dusts) depending on the agent involved. A number of substances appear also as a fluid, in warm environments the evaporation of fluids is increased, making inhalation of vapors more likely. However, increased humidity increases particle size by hygroscopic effect, and therefore larger particles may precipitate before inhalation is possible. If inhaled, the distribution of particles depends on speed of inhalation, and depth of inhalation (=respiratory minute ventilation). During exercise the respiratory minute ventilation increases considerably and thus the amount of toxic substance that is inhaled. In peripheral airways air motion is relatively slow, occurring primarily by molecular diffusion, consequently precipitation of particles occurs more easily in the peripheral airways. The severity of the symptoms after inhalatory gas exposure depend on the concentration of the intoxicating substance, the duration of exposure, toxic potency of the substance, water solubility, minute ventilation, and the individual susceptibility of the victim. More water soluble toxic gases affect the upper airways and the more central airways. The less soluble gases tend to produce effects in the peripheral airways and alveoli. In order to assess the severity of exposure it is also important to know: (a) color and smell of warfare agent, warfare agent heavier than air, weather conditions (temperature, rain, wind, daylight, fog), landscape (warfare agents heavier than air can accumulate in lower situated areas); (b) victim wearing protective clothing, wearing a gas mask with an adequate filter?; (c) medical history of the victim?; etc. Clinically, three types of responses to acute inhalatory intoxications can be discerned. In the first type (type I) the clinical symptoms appear instantly on exposure and may consist of pain in the upper airways while breathing, nasal discharge and lacrimation. In more severe cases dyspnea due to bronchospasms, bronchial edema, glottis edema and increased mucus production may be present. In the worst cases, the bronchospasms are more intense. Haemoptysis and cyanosis may become manifest. Although pulmonary edema can be observed in type I inhalatory intoxication, it will never be the sole phenomenon. The severity of type I inhalatory intoxication is generally manifest shortly after cessation of the exposure. Generally, compounds causing type I inhalatory intoxication dissolve easily in water and therefore also in the mucus of the upper airways, because mucus predominately consist of water. The process causing symptoms occurs usually at the site where the intoxicating substance encounters mucosal membranes of the airways. After being dissolved, molecules react with elements of the cell walls. The process involved is mostly of an inorganic chemical nature such as oxidation, reduction or pH change. After cessation of the initial exposure the process stops. Bronchoconstriction can be caused by bronchospasms or by inflammation. Via yet unknown mediators the airway epithelial cells may exert an important down-regulatory effect on smooth muscle contraction. When the epithelial cells are damaged this down-regulatory mechanism may be disturbed, which may lead to bronchospasms. Damage to the mucous membranes can also result in release of mediators causing an inflammatory cascade that alters vascular permeability and act as chemotactic factors. The vascular permeability may lead to influx of plasma that can decrease airway caliber, and consequently increasing airway resistance. Furthermore, increased mucus production in combination with plasma influx may cause additional airway obstruction. Patients with preexisting pulmonary diseases such as chronic bronchitis or asthma, are usually more susceptible, particularly concerning the occurrence of bronchospasms and excessive mucus production. Examples of substances causing type I intoxication are chlorine, ammonia, hydrochloric acid vapor, lacrimators (“tear gas,” such as CS {o-chlorobenzylidene malonnitrile}, CN {1-chloroacetophenone}, chloropicrin, DM {diphenylaminearsine}, CR {dibenz(b.f)-1:4-oxazepine} or CA {bromobenzylcyanide}), sulfur trioxide-chlorosulfonic acid (consists of 50% sulfur trioxide and 50% chlorosulfonic acid) or Lewisite vapor. Smoke forming materials such as zinc chloride, titanium tetrachloride and stannic chloride are a group of related metal chlorides producing hydrochloric acid on contact with moisture. In type I intoxications, the clinical effects in combination with blood gas analysis will give the most relevant information about the severity of the exposure. If there is no mucosal irritation of the eyes or nose, it can be concluded that the exposure was not severe. Initially, the chest X-ray is less valuable to access the severity of the intoxication. Mustard “gas” causes primarily effects that can also be observed in agents causing type I injury, but after exposure to higher doses of mustard the lower airways may also be involved, thus causing similar effects that can be observed after exposure to agents causing type II injury (see below). The inflammation reaction after mustard exposure becomes more intense in a period of 4–6 hours. In contrast to type I inhalatory intoxication, in the second type (type II), clinical symptoms are usually absent during the first hours after exposure. Consequently, physical examination of the patient immediately after exposure may not provide information regarding the full extent of the clinical severity of the intoxication. Rarely, minor irritative effects of the upper airways or nausea may be present. Generally, bronchospasm is not a prominent symptom. After several hours, depending on the concentration and the duration of exposure, acute lung injury (ALI) may become clinically manifest. Generally, compounds causing type II inhalatory intoxication dissolve badly in water and therefore penetrate deeper into the lung. Consequently, the process causing symptoms is usually situated much lower in the respiratory tract, i.e. in alveoli and bronchioli terminales. Especially the ciliated cells of the bronchioli and the alveolar type I cells are susceptible to injury. As a result of membrane injuries, the alveolar and terminal bronchiolar cellular layer and basement membranes are interrupted. Following the alveolar damage an influx of plasma and inflammatory cells will occur causing ALI. Acute respiratory distress syndrome (ARDS) represents a severe form of ALI. Both states are characterized by stiff, noncompliant lungs, nonhydrostatic pulmonary edema and hypoxemia. The clinical findings in an ARDS are dyspnea, tachypnea, hypoxemia and decreased lung compliance. The diffuse pulmonary infiltrates on chest radiography represent the consequences of diffuse alveolar damage, which is a nonspecific response of the lung to various forms of lung injury. The full development of ALI/ARDS takes time, because the formation of toxic reactive intermediates continues after cessation of the exposure. Unfortunately, the repair process itself can result in further harm to the lung. Swelling of the alveolar type I cells and the endothelial cells causes a further thickening of the air-blood barrier. Alveolar macrophages are also exposed to and injured by substances reaching the peripheral lung. Macrophage functions that may be affected after oxidant injury are: recognition of particles as foreign material, attachment of particles to the membrane, membrane fluidity, and fagocytosis of particles. Consequently, the clearance of particles from the alveoli is less efficient and increases the exposure of alveolar cells to toxic material and micro-organisms, and therefore increasing the susceptibility to infections. Furthermore, the mucus retention, as a result of more mucus production, damaged ciliary cells and bronchospasms contribute to the sensitivity to infections. Mucus retention may also be caused by obliterating bronchiolitis. Arterial hypoxemia can be induced by ventilation-perfusion mismatch and disorders in the exchange of gasses as a result of interstitial and alveolar edema. Arterial hypoxemia can further be provoked by collapse of the alveoli as a result of reduced surfactant production by the alveolar type II cells and/or denaturation of surfactant by serum proteins. Obliterating bronchiolitis causes arterial hypoxemia by alveolar hypoventilation. The alveolar hypoventilation is provoked by air trapping. Substances responsible for type II intoxication are, for example, nitrogen dioxide, ozone and phosgene. In type II inhalatory intoxication, the chest X-ray in combination with blood gas analysis will give the most relevant information about the severity of the intoxication. Generally, the clinical effects become manifest later and, therefore, initially they are less valuable to assess the severity of the intoxication. In the third type (type III) of response to inhalatory intoxication, substances are absorbed via the lung. Although some compounds involved may cause minor irritation of the upper respiratory tract, they primarily exert their toxic action elsewhere in the body. Because of the great variety of substances that may be responsible for this kind of intoxication, the clinical picture may be diverse. The compounds involved may influence the function of the central nervous system. Severe depression of the central nervous system may cause respiratory depression and, therefore, indirectly inadequate ventilation. Examples of compounds inducing type III inhalatory intoxications are carbon monoxide, cyanide, or organic solvents such as toluene and xylene. For risk analysis, it is essential to be informed about the nature of the substance involved and the type of clinical symptoms it may cause. This is relevant, because if, in the case of type I inhalatory exposure, no symptoms are manifest when the patient consults the physician, it is unlikely that symptoms will appear later. Thus no treatment is needed. In type II inhalatory intoxication, however, judgement is often impossible at the time when the patient visits the physician, because the full extent of the intoxication may only become manifest after several hours. The patient should therefore be kept under observation until more information is obtained regarding the severity of exposure or until clinical effects can no longer reasonably be expected. If, 6 hours after exposure, normal arterial blood gas values have been determined and the chest X-ray is normal, there is little likelihood that a life-threatening lung damage will develop. If in the blood gas analyses CO2 concentration is lowered while oxygen concentration is normal or lowered, lung damage can be expected. In the case hypoxia and elevated CO2 concentrations are observed, the lung damage may even be more severe. When within 6 hours no effects are observed patients can be discharged with instruction. If they experience increasing dyspnea in the hours after discharge then they should be under medical observation again. Therapy: No specific prophylactic or post-exposure therapy for type I and type II lung injury is available. Adequate supportive therapy should be given such as oxygen supply, bronchodilating medicines and mechanical ventilation. Prophylactic administration of antibiotics is not useful. There is no evidence that corticosteroids and radical scavengers diminish the clinical effects after inhalation of toxic agents. With the above guidelines the triage of patients exposed to agents causing type I or type II lung injury can easily be performed in order to prevent unnecessary observation of patients, and therefore creating an optimal use of health care facilities in situations that the need for it is urgent. General guidelines concerning the treatment of type III inhalatory exposure can not be given because of the great variety of substances and the effects involved.
7 MUSTARD GAS: SIGNS, SYMPTOMS AND TREATMENT
Willems JL
Department of Public Health, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium
Case series: Between 1984 and 1986, about 170 mustard gas casualties from the Iran–Iraq war were evacuated to European hospitals. In collaboration with the clinical centres and laboratories involved, and supported by a grant of the US Army Medical Research and Development Command, I performed a critical analysis of the clinical files of 65 of these patients. Observations: The casualties chosen for evacuation had moderate to severe mustard gas injuries, they arrived 4 to 17 days after exposure. For those arriving within 5 days, the most prominent signs and symptoms were mucocutaneous lesions of the eyes, skin, and upper airways; and coughing and sore throat. Skin lesions ranged from dark brown areas of epidermal lysis, beneath which was regenerating epithelium, to deep erosive lesions. Leukopenia was present in almost half the patients, and usually resolved quickly; in contrast, patients with severe leukopenia (200 or less cells/ml) later died. Ventilatory insufficiency requiring artificial ventilation also indicated a poor prognosis. Treatment consisted of antibiotic ointment and homatropine instillation in the eyes, application of povidone–iodine soap, silver sulfadiazine cream or protective creams on skin lesions, closed versus open treatment of skin lesions, inhalation of moist air and N-acetylcysteine, bronchodilators, and systemic antibiotics. Treatment regimens varied because of the number of institutions that treated these patients, and because of the absence of clinical experience with mustard casualties. For instance, systemic drug administration was most extensive for patients that happened to be hospitalised in intensive care units, less for those on burn wards, and least for those on general and dermatology wards. Evaluation of treatment outcomes was precluded by the small sample sizes. Detoxification procedures to remove any remaining mustard were attempted at some centres. However, contamination of newly arrived patients by unmetabolised mustard could not be established by the toxicological assays performed. Therefore, the efficacy of these treatments—among others decontamination with water and chloramines 0.2%—could not be determined, and the need for such treatments is questionable. A high frequency of septicemia was observed for patients receiving hemoperfusion, the most invasive decontamination treatment. Lethal outcome generally occurred within 15 days after exposure. Recovery, allowing return to Iran, occurred after 2 to 10 weeks; the length of hospitalisation in Europe being determined by the healing time for deep skin lesions. Reference: Willems, J. Clinical Management of Mustard Gas Casualties. Ann. Med. Militaris Belgicae 1989, 3 S1: 1–61.
8 S-MUSTARD GAS POISONING—EXPERIENCE WITH 12 VICTIMS
Zilker Th, Felgenhauer N
Toxikologische Abteilung Klinikum r.d. Isar Technische Universität München, Germany
Introduction: The terroristic attack of the 11th of September reminded us that assaults by chemical weapons could also happen. Objective: As we might have to deal again with the management of mustard gas victims we looked back to the year 1984/1985 when we had to treat 12 Iranian soldiers. Results: All wounded soldiers were exposed by a bombing attack on the isle of Madjun near Basra. The smell was more garlickly than like mustard. The soldiers were not protected. All soldiers developed dyspnea, conjunctivitis and burning of the skin especially in the armpits and underneath their belts. Some of them showed vertigo, nausea and fatigue. After 4 to 8 hours all were coughing. Blisters had developed on the contaminated skin. All but one (17 days) arrived in Germany after 4–6 days. Status at admission: 10/12 were somnolent. The skin was red and full of blisters in the face and the trunk, arms, armpits and at the genitals. All of them showed conjunctivitis, photophobia and eyes covered with secretion. 6 of 12 had opaque corneas with erosions. The mucous membranes in the throat showed inflammation, edema, necrosis in parts and fibrin layers. Four showed infiltrations of the lungs on X-ray. All patient had fever and elevated acute phase proteins. The base excess and the pH was high in 8/12 cases. In all cases signs of severe bronchitis with coughing was present. The expectoration contained blood mucous and pus. In 5 patients bronchoscopy shortly after admission exhibited inflammation of the airways down to the segmental bronchi which were highly swollen and showed pseudo-membranes. All patients presented with aphonia, 4 had lost olfaction. All victims were apathic. On EEG an enhanced theta activity was seen. 4 patient were psychotic for a while. One had gastrointestinal bleeding. One patient died on day seven after exposure due to pneumonia and bone marrow suppression. Course of illness: Skin: The blisters would open into ulcers and heal within 4–6 weeks leaving pigmentation. Eyes: None of the patients lost his eyesight. The opaqueness of the cornea resolved. Lungs: 4/12 developed severe pneumonia; one died early; the others got tracheostoma and had several bronchoscopies per day to clean the bronchial system. One of them was discharged with tracheostenosis. Treatment: All patients were treated with antibiotics either parenterally or topically. All but one left hospital by 4–6 weeks in satisfactory condition. Conclusion: Treatment of mustard gas poisoning can be handled with modern intensive care. Nursing the local lesions and removing the pseudo-membranes from the bronchi are the key for success.
9 DELIBERATE RELEASE OF NERVE AGENTS: FACTORS INFLUENCING THE CHOICE OF AGENT, MECHANISMS OF TOXICITY, CLINICAL FEATURES, MANAGEMENT AND RECOMMENDATIONS FOR FURTHER ACTION
Vale JA
National Poisons Information Service (Birmingham Centre) and West Midlands Poisons Unit, City Hospital, Birmingham, United Kingdom
Introduction: The organophosphorus nerve agents are related chemically to organophosphorus insecticides and have a similar mechanism of toxicity, but a much higher mammalian acute toxicity, particularly via the dermal route. For example, in an in vitro study, sarin had 1000 fold more inhibitory effect on acetylcholinesterase (AChE) than parathion. Two classes of nerve agents are recognized, G agents (G allegedly stands for Germany where the early agents were first synthesized) and V agents (V allegedly stands for venomous). Tabun (NATO designation GA), sarin (GB) and soman (GD) were synthesized in Germany in 1936, 1938 and 1944 respectively. GE and GF were synthesized subsequently. The V agents were introduced later and are exemplified by VX (synthesized in the 1950s), though VE, VG and VM have also been produced. Although available, nerve agents were not used in World War II, but were employed by Iraq against that country's own Kurdish population and there have been allegations of use of nerve agents during the Iran–Iraq War. The nerve agent, sarin was also employed in two terrorist attacks in Japan in 1994 and 1995. Factors influencing the choice of nerve agent employed: The following factors are not only potentially relevant to those intending to release a nerve agent deliberately, but an understanding of these factors is also of importance to clinical toxicologists to optimize the clinical and public health responses to a deliberate release. Physicochemical properties: (i) Physical state. Is the nerve agent a volatile or non-volatile liquid? Sarin (22,000 mg/m3 at 25°C) is much more volatile than tabun (610 mg/m3 at 25°C); VX is non-volatile (10.5 mg/m3 25°C); (ii) Vapor pressure. This is a measure of how quickly a nerve agent will evaporate and is increased by a rise in ambient temperature. For example, the vapor pressure for sarin is 0.52 mmHg at 0°C and 2.9 mmHg at 25°C, whereas that of tabun is 0.004 mmHg at 0°C and 0.07 mmHg at 25°C; (iii)Vapor density. Nerve agents with a high vapor density compared to air, such as VX (9.2), stay at ground level and tend to accumulate in low lying areas; (iv) Odor. Tabun is said to have an almond/fruity odor, while the other agents are odorless if pure; (v) Solubility in water; (vi) Stability. This refers to the ability of a nerve agent to survive dissemination and transport to the site of deploy; (vii) Persistence. Non-persistent agents disperse rapidly after release and present an immediate short duration hazard but may be made persistent by a “thickening agent” such as polyethylmethacrylate. In contrast, persistent agents such as VX continue to be a contact hazard and may vaporize over a period to produce an inhalation hazard. Intent: If the intent is to cause permanent injury to a substantial number of those exposed rather than to cause mass panic, a relatively more toxic nerve agent may be chosen by the terrorists. Toxicity of nerve agents: The LCt50 (the exposure (Ct) necessary to cause death in 50% of the population) for VX vapor is 10mg min/m3, whereas the LCt50 for sarin vapor is 100mg min/m3 and tabun vapor is 400mg min/m3 (these data refer to humans at rest). The percutaneous LD50 for sarin is 1700 mg and for VX is 6–10 mg. Ease of synthesis: Many of the precursors to produce nerve agents are available readily and common chemical processes can be adapted easily for their production. It has been calculated that it would cost only some $200,000 to produce 1000 kg of sarin. However, tabun is technically easier to synthesize in bulk than sarin or soman. Intended route of delivery: The major routes of delivery of nerve agents are air (both indoor and outdoor), water (hence the solubility of the nerve agent is important) and food. Meteorological factors: Meteorological factors are important in the case of air delivery as the wind may disperse volatile agents and a higher ambient temperature increases volatility and decreases persistence. Moreover, some agents may freeze on clothing and then vaporize if carried indoors. Rain tends to dilute toxicity and may promote hydrolysis of the nerve agent. Temperature inversions may increase persistency. “Effectiveness”: “Effectiveness” is the capacity of an agent to produce the maximum number of casualties with the least amount of material. The duration of “effectiveness” is dependent on physicochemical characteristics, the amount delivered, the mode of delivery and environmental conditions. Mechanisms of toxicity: Nerve agents phosphorylate a serine hydroxyl group in the active site of the enzyme, acetylcholinesterase, which results in accumulation of acetylcholine and which in turn causes enhancement and prolongation of cholinergic effects and depolarization blockade. Reactivation of acetylcholinesterase occurs by dephosphorylation, and the rates of phosphorylation and dephosphorylation are very variable, which partly accounts for differences in acute toxicity between the nerve agents. With soman in particular, an additional reaction occurs known as “aging.” This consists of monodealkylation of the dialkylphosphonyl enzyme, creating a much more stable monoalkylphosphonyl enzyme, with the result that reactivation of inhibited acetylcholinesterase does not occur to any clinically significant extent. The human in vivo “aging” half-life for soman is 2–6 min, for sarin (human in vitro data) 3 hr and tabun (human in vitro data) 14 hr. In the case of soman, therefore, recovery of function depends on resynthesis of acetylcholinesterase. As a result, it is important that an oxime is administered as soon after soman exposure as possible so that some reactivation of acetylcholinesterase occurs before all the enzyme becomes “aged.” Even though “aging” occurs more slowly and reactivation occurs relatively rapidly in the case of other nerve agents, such as tabun and sarin, early oxime administration is still clinically important. Clinical features: Ocular exposure: Miosis, which may be painful and last for several days, occurs rapidly following exposure to nerve agent vapor and appears to be a very sensitive index of exposure.1 Ciliary muscle spasm may impair accommodation and conjunctival injection and eye pain may occur. Dermal exposure: Contact with liquid nerve agent may produce localized sweating and fasciculation, which may spread to involve whole muscle groups. Systemic features may develop, though the onset is slower than following vapor inhalation. Inhalation: Chest tightness, rhinorrhea and increased salivation may occur within minutes. Systemic features may then develop. Ingestion: Ingestion of contaminated food or water may cause abdominal pain, nausea, vomiting, diarrhea and involuntary defecation. Systemic features may then develop. Systemic features: Abdominal pain, nausea and vomiting, involuntary micturition and defecation, muscle weakness and fasciculation, tremor, restlessness, ataxia and convulsions may follow dermal exposure, inhalation or ingestion of a nerve agent. Bradycardia, tachycardia and hypotension may occur dependent on whether muscarinic or nicotinic effects predominate. If exposure is substantial, death may occur from respiratory failure within minutes. Chronic sequelae: Mild or moderately exposed individuals usually recover completely, though EEG abnormalities have been reported in severely exposed patients.2,3 Management: Adequate self protection should be donned by healthcare workers before decontaminating casualties, as secondary contamination from casualties exposed to sarin vapor has been reported.4,5 If available, pressure demand, self-contained breathing apparatus should be used in contaminated areas. Casualties should be moved to hospital as soon as possible. Ocular exposure: The victim should remove contact lenses if present and they are easily removable. The eyes should be irrigated immediately with lukewarm water or sodium chloride 0.9% solution. Local anesthetic should be applied if ocular pain is present. Dermal exposure: Contaminated clothing should be removed, if possible by the victim, to reduce further nerve agent absorption. Inhalation: The priority is to remove the casualty from further exposure. Establish and maintain a clear airway and give supplemental oxygen as required. In symptomatic patients, intravenous access should be established and blood should be taken for measurement of erythrocyte cholinesterase activity to confirm the diagnosis. If the characteristic features of nerve agent poisoning are present, however, antidotal treatment should not be delayed until the result is available. Atropine: If bronchorrhea develops, atropine (2 mg in an adult; 20 microgram/kg in a child) should be administered intravenously every 5–10 minutes until secretions are minimal and the patient is atropinized (dry skin and sinus tachycardia). In severe cases very large doses of atropine may be required. Oximes: In a clinically relevant time, it is unlikely that it will be known with certainty which nerve agent has been released. Hence, the oxime most readily available should be administered in the appropriate therapeutic dose. Experimental studies in guinea pigs and monkeys have shown that pralidoxime+atropine and obidoxime+atropine were less effective in soman poisoning than HI-6+atropine, though pralidoxime+atropine was more effective than obidoxime+atropine.6 Studies have shown invariably that higher oxime doses, together with atropine, have increased survival further, irrespective of the nerve agent.6 HI-6 was also more effective in GF poisoning than obidoxime+atropine.7 Overall, however, pralidoxime is the oxime of first choice for civilian use in many countries as it is the most widely available, it is cheaper to synthesize than HI-6 and produces fewer adverse effects than obidoxime in equimolar concentrations. If pralidoxime is available (either as the mesylate, chloride or methylsulfate salt) it should be administered to moderately or severely poisoned patients in a dose of 30 mg/kg body weight (2 g in an adult) intravenously over four minutes to reactivate phosphorylated enzyme. Early administration is a priority. In severe cases, pralidoxime mesylate or chloride 30 mg/kg body weight will be required intravenously every four to six hours, depending on the clinical features and erythrocyte cholinesterase activity. Alternatively, an infusion of pralidoxime mesylate or chloride 8–10 mg/kg/hr may be administered. Diazepam: Intravenous diazepam (adult 10–20 mg; child 1–5 mg) is useful in controlling apprehension, agitation, fasciculation and convulsions; the dose may be repeated as required. In some experimental studies, the addition of diazepam to an atropine+oxime regimen increased survival further.6 Deliberate releases of sarin in Japan: Matsumoto: Some 600 people were exposed to sarin released from a truck using a heater and fan in a residential area of the Japanese city of Matsumoto on June 27 1994.8 Fifty-eight residents were admitted to hospital and all recovered: seven casualties died. Eight of 95 rescuers had mild symptoms of OP poisoning. The features experienced by the casualties are summarized in Table 1. Follow up one to two years after exposure of those casualties with the most severe initial features has shown that four had developed epileptiform EEG abnormalities and one had developed a sensory neuropathy,3 though it is not known whether these features were related to sarin exposure. Tokyo: In March 1995 a terrorist attack occurred in the Tokyo subway system during rush hour. Sarin was placed in five subway cars on three separate lines in plastic bags opened so that the agent, which is liquid under temperate conditions, could evaporate. Over 5,000 “casualties” sought medical attention of whom 984 were moderately poisoned and 54 were severely poisoned; 12 died.9,10 However, a substantial number of those presenting (some 4000) had no signs of OP toxicity and 4,973 individuals were seen on day one and sent home. In the following 24 hours many more individuals presented, though none had features of OP poisoning. The features in 111 hospitalized patients are shown in Table 1. Lessons to be learned from these sarin releases and recommendations for further action: Triage: Substantial numbers of casualties presented to a variety of hospitals over a short time period after both incidents which stretched the resources available significantly. Hence, every hospital should now have a major accident plan that covers chemical releases. This plan should be tested at least annually. It should include arrangements to triage substantial numbers of non-poisoned casualties as well as those who are severely poisoned and require admission. Training: It has been claimed11 that if paramedics had been allowed to maintain an airway with an endotracheal tube or to use a laryngeal mask airway without physician oversight more patients might have survived in the Tokyo incident. The implication is that all paramedics/ambulance staff should be trained to an adequate level. Personal protective equipment (PPE): PPE was not donned in the Matsumoto incident and substantial secondary contamination occurred. Similar contamination also occurred in the Tokyo incident where PPE was either not available or not employed. PPE is now readily available and for $200–300 full protection against nerve agents can be afforded. Every hospital should now have suitable PPE available for handling those exposed to nerve agents. Supplies of atropine and oximes: Adequate supplies of atropine and oxime need to be available in every major city or be readily transportable to cities and towns within 1–2 hours.
References: 1. Nozaki, H.; Hori, S.; Shinozawa, Y. et al. Relationship Between Pupil Size and Acetylcholinesterase Activity in Patients Exposed to Sarin Vapor. Intensive Care Med. 1997, 23, 1005–1007. 2. Murata, K.; Araki, S.; Yokoyama, K. et al. Asymptomatic Sequelae to Acute Sarin Poisoning in the Central and Autonomic Nervous System 6 Months After the Tokyo Subway Attack. J. Neurol. 1997, 244, 601–606. 3. Sekijima, Y.; Morita, H.; Yanagisawa, N. Follow-Up of Sarin Poisoning in Matsumoto. Ann. Intern. Med. 1997, 127, 1042–1042. 4. Nozaki, H.; Aikawa, N. Sarin Poisoning in Tokyo Subway. Lancet 1995, 345, 1446–1447. 5. Nozaki, H.; Aikawa, N.; Shinozawa, Y. et al. Sarin Poisoning in Tokyo Subway. Lancet 1995, 345, 980–981. 6. Dawson, R.M. Review of Oximes Available for Treatment of Nerve Agent Poisoning. J. Appl. Toxicol. 1994, 14, 317–331. 7. Clement, J.G. Efficacy of Various Oximes Against GF (Cyclohexyl Methylphosphonofluoridate) Poisoning in Mice. Arch. Toxicol. 1992, 66, 143–144. 8. Okudera, H.; Morita, H.; Iwashita, T. et al. Unexpected Nerve Gas Exposure in the City of Matsumoto: Report of Rescue Activity in the First Sarin Gas Terrorism. Am. J. Emerg. Med. 1997, 15, 527–528. 9. Okumura, T.; Takasu, N.; Ishimatsu, S. et al. Report on 640 Victims of the Tokyo Subway Sarin Attack. Ann. Emerg. Med. 1996, 28, 129–135. 10. Sidell, F.R. Chemical Agent Terrorism. Ann. Emerg. Med. 1996, 28, 223–224. 11. Okumura, T.; Suzuki, K.; Fukuda, A. et al. The Tokyo Subway Sarin Attack: Disaster Management. Part 1: Community Emergency Response. Acad. Emerg. Med. 1998, 5, 613–617. 12. Morita, H.; Yanagisawa, N.; Nakajima, T. et al. Sarin Poisoning in Matsumoto, Japan. Lancet 1995, 346, 290–293. 13. Ohbu, S.; Yamashina, A.; Takasu, N. et al. Sarin Poisoning on Tokyo Subway. South. Med. J. 1997, 90, 587–593.
Table 1. Features in Those Exposed to Sarin in Japan in 1994 and 1995 (Abstract 9)
10 THE POISON CENTER ROLE IN THE RECOGNITION, MITIGATION AND MANAGEMENT OF BIOLOGICAL AND CHEMICAL TERRORISM
Krenzelok EP
Pittsburgh Poison Center, Children's Hospital of Pittsburgh; Schools of Pharmacy and Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
Introduction: The use of unconventional biological and chemical warfare has a 2500-year history. However, the focus of using these agents has turned from warfare to terrorism over the last 30 years. A number of foiled and actual bio-chem terrorist events have enhanced awareness and prompted the need for better preparedness. The sarin gas chemical terrorism incidents in Japan were contemporary tragedies that initiated aggressive legislative action, allocated financial resources and began to encourage general preparedness and vigilance for future bio-chem terrorism events. On October 4, 2001 the sobering and stark reality of biological terrorism became even more apparent as the anthrax era was ushered in. The training simulations in anticipation of biological and chemical terrorism events had not considered this scenario. Poison information centers found themselves immersed in and occasionally overwhelmed by new challenges that included atypical questions that deviated from the norm of expertise and expectations of a poison center. Were poison centers prepared? Are they now prepared? Do poison centers have a role in the recognition, mitigation and management of biological and chemical terrorism? The answer should be apparent, but what is the role? Poison centers must expand their services beyond traditional roles, accept new responsibilities and take advantage of this opportunity to provide additional services. Poison Center Roles: The information and knowledge about biological and chemical agents and the general experience that reside in a poison center, as well as the nature of being a 24/7 resource, establish the poison center as a central component in managing bio-chem terrorism incidents. Furthermore, the public's confidence in and utilization of poison centers whenever any perceived poisoning emergency or toxic hazard occurs, mandates that local and regional government authorities include the poison center in preparedness activities. The poison center role should include planning and preparedness activities, information management and dissemination, clinical management, patient tracking and toxicosurveillance. Planning prior to the occurrence of a bio-chem terrorism event is essential in minimizing the adverse health consequences to the public and the disruption of poison center operations. The center must have a contingency relocation plan so that it can remain viable if communication is compromised or if the terrorist event has incapacitated the center physically. Additionally, the center should have active representation on local government and health care institution preparedness committees that establish incident command centers, communication protocols and media interaction. In the US participation on the Metropolitan Medical Response System (MMRS) committee is essential. The MMRS is a health care coordination system that cares for weapons of mass destruction (WMD) exposed patients from the time of initial exposure through definitive care. The system includes local first response, specialized hazardous materials (HAZMAT) capability, decontamination, the establishment of a regional pharmaceutical cache, mortuary services, medical transport, coordination of the regional hospital system, patient evacuation and other critical health-related services. The poison center plays a critical role in MMRS since it helps to coordinate care by making recommendations about the risks associated with exposure to biological and chemical agents, decontamination, the use of appropriate pharmaceuticals and monitors poisoned patient flow throughout the region. For these reasons poison centers must have significant visibility within the system and poison center staff must be trained to be responsive to these needs. Universal use of the poison center by healthcare providers and residents of a region has the advantage of centralizing data collection. If the poison center utilizes an electronic medical record system, data may be tracked real-time thereby permitting syndromic toxicosurveillance. By monitoring established symptoms and by comparing them to benchmark data, the poison center may be able to identify sentinel events that signal a bio-chem terrorism incident. An early surveillance system may identify illnesses at an early stage and allow interventions that may reduce morbidity and mortality. Poison center data also allow for the detection of “footprints” of exposure by mapping victim location and allowing health officials to anticipate where victims may be found or where prophylactic therapies may be beneficial. Combining poison center data with real-time emergency department discharge diagnosis data, pharmaceutical sales, school illness rates and veterinary clinical diagnoses may help to identify a bio-chem terrorism event in the early stages. Poison centers have an established role in professional education and poison prevention education with the public. Those networks should be utilized to inform healthcare professionals, the public and media about pertinent issues related to bio-chem terrorism. Conclusion: The poison center plays a critical role in the recognition, mitigation and management of biological and chemical terrorism. It is one of several key elements in a successful regional plan to address the issue of biological and chemical terrorism.
References: 1. Krenzelok, E.P.; Allswede, M.P.; Mrvos, R. The Poison Center Role in Biological and Chemical Terrorism. Vet. Hum. Toxicol. 2000, 42, 297–300. 2. Krenzelok, E.P. The Critical Role of the Poison Center in the Recognition, Mitigation and Management of Biological and Chemical Terrorism. Przeglad Lekarski 2001, 58, 177–181. 3. Burda, A.M.; Sigg, T. Pharmacy Preparedness for Incidents Involving Weapons of Mass Destruction. Am. J. Health-Syst. Pharm. 2001, 58, 2274–2284.
11 TOXICOLOGIC CAUSES, MECHANISMS, AND APPROACHES TO TREATMENT OF ANOXIC AND CHEMICALLY-INDUCED BRAIN INJURY
Snodgrass WR
Clinical Pharmacology-Toxicology Unit, Texas State Poison Center—Houston/Galveston, University of Texas Medical Branch, Galveston, Texas, USA
Objective: Anoxic and non-anoxic brain injury usually is devastating and permanent for patients. This review will address causes, molecular mechanisms and approaches to therapy for anoxic and chemically-induced brain injury. Methods: Review of published peer-reviewed medical and scientific studies including clinical trials. Results: The most frequent cause of moderate to severe brain injury is anoxia. One major molecular mechanism of anoxic brain injury is reperfusion injury involving production of free radicals and toxic metabolites of oxygen such as hydroxyl free radical and superoxide anion. Programmed cell death involving the NMDA (N-methyl-D-aspartate) receptor is another pathway of brain injury. A less frequent cause of moderate to severel brain injury is chemically-induced brain injury. Chemical agents that potentially may cause brain injury include carbon monoxide, some organic solvents, heavy metals (e.g., lead (Pb), mercury (Hg)), MPTP, and some metabolic poisons (e.g., cyanide). Chemical agents poisoning may result in cognitive deficits and/or neuro-motor impairment. Molecular mechanisms of chemical agent-induced brain injury include metabolic utilization impairment, neural membrane degradation, mitochondrial injury, membrane transport function destruction, intracellular cytoplasmic accumulation, and cell nucleus/genetic function/control impairment. Rational approaches to prevention, management, and treatment of anoxic or chemically-induced brain injury must derive from an understanding of the molecular mechanisms involved in producing such injury. A few such approaches in the future potentially may include use of antioxidants, NMDA receptor antagonists, lazaroids, nerve growth factors, chemokines, stem cells, and other modalities. Conclusion: Mechanistic research into brain injury now points to several relatively selective and specific potential therapeutic modalities that may offer clinically useful approaches to decreasing or minimizing anoxic and chemically-induced brain injury.
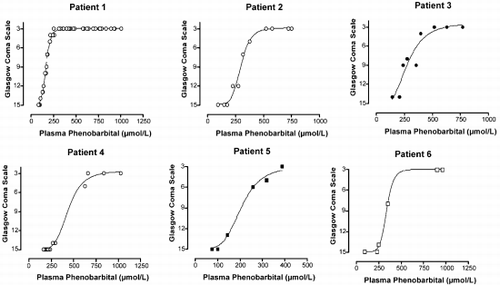
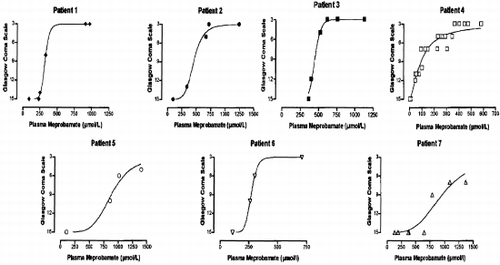
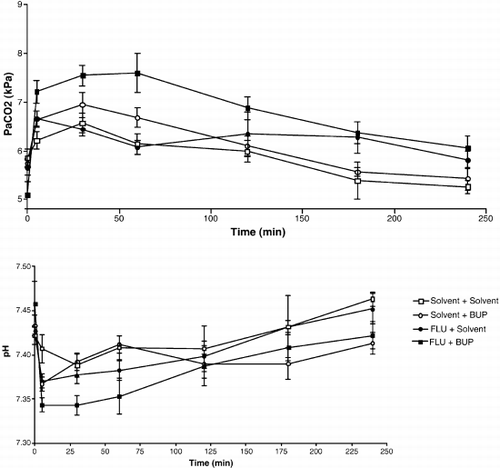
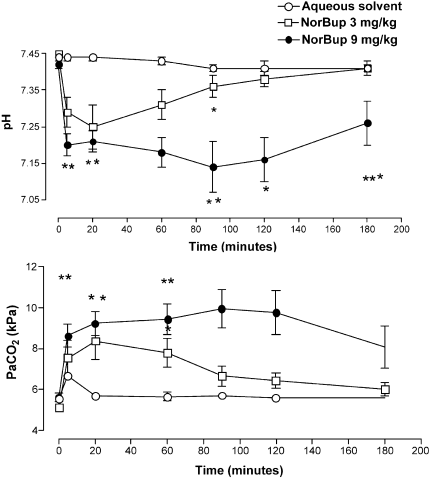
12 POISONING INDUCED COMA: TOXICOKINETIC-TOXICODYNAMIC RELATIONSHIPS
Mégarbane B, Baud F
INSERM U26, Hôpital Fernand Widal and Réanimation Médicale et Toxicologique, Hôpital Lariboisière, Paris, France
Objectives: Pharmacokinetic–Pharmacodynamic (PK–PD) relationships describe in the same individual the quantitative relationships between the drug-induced effect and the timely corresponding drug concentrations. The value of PK–PD relationships in clinical pharmacology is now well recognized. Moreover, for a long time, rough qualitative relationships have been described between neurological presentation and plasma ethanol concentration in acute alcohol ingestion or between coma depth and psychotropic drug concentration in acute poisonings. However, to date, precise quantitative Toxicodynamic–Toxicokinetic (TK–TD) relationships have not been extensively studied in medical toxicology and their potential interest is still poorly investigated. In fact, in toxicology, many difficulties may be encountered in testing such relationships. The date of the ingestion, and the exact ingested dose are generally unknown. To be considered, the observed clinical effect has to be reversible, strictly related to the toxicant and easily measurable in clinical practice. Finally, determination of the toxicant plasma concentrations has to be obtained by routinely performed assays. In this review, Our aim is to present the place of TK–TD interactions in drug involved coma. Methods: We studied TK–TD relationships in meprobamate (MB) and phenobarbital (PB) acute poisonings. Plasma concentrations were measured using an enzymatic (PB) and a colorimetric assay (MB). The depth of coma was assessed using the Glasgow Coma Scale (GCS). Non-linear regression was used for modeling TK–TD relationships. We then discussed the place of such relationships in the diagnosis, the prognosis evaluation, the pathophysiology investigation and the treatment decisions in drug-induced coma. Results: TK–TD relationships were studied in 6 acute PB and 7 acute MB poisoningsl (Figs. 1 and 2). Two patients were previously treated with PB and 3 with MB. Mixed drug poisoning was noted in all MB-poisoned patients and in 2/6 PB poisoned ones. The GCS at the time of hospital admission was 3 in the 6 PB poisoned patients and the mean GCS was 4±1 in the MB-poisoned patients. The mean plasma PB concentration was 710.5±281.9 μmol/l and MB concentration was 1054±318 μmol/l. The TK–TD relationships were well fitted with the sigmoidal Emax model: the mean Hill coefficients were 6.0±1.9 and 6.9±4.0 respectively in PB and MB-poisoning and the mean C50 were 289.7±97.1 μmol/l and 487.9±318.8 μmol/l. A maximal toxic effect (GCS of 3) was associated with a wide range of plasma PB or MB concentrations, indicating clearly the saturation of the drug receptors in these situations of high doses ingestion. During the course of poisoning, the relationships between the depth of coma and the corresponding plasma concentrations were of sigmoidal shape. The high values of the Hill coefficient showed that a small decrease in plasma concentrations near the C50 was associated with a dramatic improvement in the level of consciousness. Two MB-poisoned patients exhibited tolerance to the sedative effects of this drug. In non-tolerant patients, the mean C50 was close to the upper limit of the therapeutic plasma concentration of MB given by our toxicological laboratory (≤200 μmol/l). In the 6 PB-poisoned patients, the mean C50 was approximately equal to 3-fold the upper limit of the therapeutic plasma concentration of PB (≤100 μmol/l). These TK–TD relationships may help diagnosis in the toxic coma, dealing with the comparison of the severity of coma with the plasma concentration currently used as a surrogate of the ingested dose. It seems more pertinent to consider not only a single concentration effect relationship determined on admission, but also a series of relationships, to take in account the distribution phase of the toxicant. Analyzing blood concentrations with respect of the delay elapsed since the ingestion may also help predicting the time of awakening and thus the evaluating the prognosis of coma. In PB and MB poisonings, improvement of the level of consciousness is rapid after a long period of profound coma, depending on the patient tolerance. Quantifying the dynamic tolerance in pretreated patients and understanding the mechanisms contributing to toxicity in overdoses may be afforded by the consideration of TK–TD relationships. Otherwise, ultimate evaluation of antidotes modifying toxicokinetics (such as decreasing the intoxicant bioavailability with activated charcoal or promoting its elimination with hemodialysis) is based on modifying toxicodynamic criteria and thus can be more precisely appreciated with TK–TD relationships. These relationships show clearly that clinical improvement resulting from a reduction of body burden of toxin present in the body is dependent on the amount of toxin, the slope of toxicity and the ratio of the amount of toxin removed to the dose required to produce a toxic effect. Conclusion: TK–TD relationships describe and quantify in the same individual the kinetics of dynamic events. They can be helpful in the assessment of the diagnosis, the prognosis and the treatment of drug-induced coma.,
13 TOXINS AFFECTING THE NEUROMUSCULAR JUNCTION
Nelson LS
New York City Poison Control Center, New York, New York, USA
Objectives: To describe the differential diagnosis of several clinical syndromes that originate with toxin-mediated dysfunction of the neuromuscular junction. Methods: The neuromuscular junction is composed of the terminal of a somatic nerve, the synapse and the post-synaptic motor endplate on the effector muscle. Neural impulses traveling down the somatic nerve initiate a series of events in the axonal terminal that ultimately cause the release of the neurotransmitter acetylcholine. This includes neuronal depolarization, influx of calcium and binding of an acetylcholine-laden vesicle to the terminal membrane and quantal release of its contents. Acetylcholine diffuses across the synapse to stimulate the nicotinic cholinergic receptor on the motor end plate, initiating a subsequent cascade of events in the muscle cell causing muscular contraction. The action of acetylcholine terminates when it is metabolized by the synaptic enzyme cholinesterase. Alteration of neuromuscular function produces a group of recognizable clinical syndromes. Paralysis is the most readily identified syndrome and may be mediated by toxins through one or more of several unique mechanisms. Hypokalemia or tetrodotoxin prevent the normal depolarizing impulse from stimulating neurotransmitter release from the terminal. Specific inhibition of acetylcholine release from the presynaptic terminal by botulinum toxin, hypermagnesemia, or aminoglycoside antibiotics may also occur. Curare prevents acetylcholine from binding to its site on the postsynaptic nicotinic receptor. Cholinesterase inhibitors, particularly the nerve agents, by inhibiting acetylcholine metabolism raise synaptic acetylcholine levels and produce excessive postsynaptic stimulation leading to depolarizing neuromuscular blockade (i.e. paralysis). Excessive muscle contractions are readily recognized as either myoclonus or fasciculations. Uncontrolled quantal release of acetylcholine, as occurs following envenomation by a Latrodectus (black widow) spider or indirectly following strychnine poisoning, produces myoclonus. Fasciculations, or the contraction of individual muscle fibers independently rather than as a unit (which would produce a normal muscle contraction), result most frequently from excessive local stimulation of individual nicotinic receptors following exposure to nicotine or a cholinesterase inhibitor. Conclusion: A complete understanding of the various mechanisms through which toxins may act and the available modalities through which the specific dysfunction may be determined, allows caregivers to provide more focused therapy to poisoned patients.
14 TOXICOLOGICAL EFFECTS ON THERMOREGULATION
Vassallo SU
New York Poison Control Center, New York University/Bellevue Hospital Center, New York, New York, USA
Background: Thermoregulation is a complex process by which hypothalamic temperature is maintained within a narrow range of 37±0.4°C. This hypothalamic “set point” depends on intact function of central and peripheral thermoregulatory controls. Stimulation of temperature-sensitive neurons causes release of neurotransmitters and results in autonomic, somatic and behavioral responses. Neurotransmitters involved in thermoregulation include serotonin, norepinephrine, acetylcholine, dopamine, and intrinsic hypothalamic peptides such as arginine vasopressin and adrenocorticotrophic hormone. Thermosensitive neurons are located in the hypothalamus, spinal cord, skin and abdomen, and may be divided into three types: warm-sensitive, cold-sensitive and temperature-insensitive. Stimulation of warm-sensitive neurons results in vasodilation and sweating; stimulation of cold-sensitive neurons results in piloerection and shivering. Drugs and toxins have pharmacologic effects that interfere with thermoregulation and lead to organ toxicity associated with hypo or hyperthermia. This presentation will discuss the current literature regarding the relationship between certain drugs and toxins and the physiology and biochemistry of thermoregulation. Discussion: Drug-related hyperthermia is mediated through two mechanism; increased production of heat or decreased capacity to dissipate heat produced during exercise or normal metabolic processes. Sympathomimetic agents cause thermogenesis by stimulating hepatic metabolism. Heat produced without muscle contraction is called nonshivering thermogenesis. Catecholamines activate adenylate cyclase, increasing cyclic adenosine monophosphate (cAMP), resulting in mobilization of fat and glucose stores (β-adrenergic receptors). Sympathomimetic agents may also cause hyperthermia by increasing muscle activity secondary to agitation or seizures. Other classes of drugs such as strychnine, Li++ and monoamine oxidase inhibitors increase heat production by causing muscle rigidity. Salicylates and pentachlorophenol increase heat production by uncoupling of oxidative phosphorylation reactions. Increased production of heat may result from drug–drug interactions and altered neurotransmission of serotonin. Anticholinergic substances interfere with heat loss by decreasing sweating. β-adrenergic antagonists and calcium channel antagonists diminish cardiac reserve needed to compensate for heat-induced vasodilation, and diuretics interfere with cardiac reserve through effects on intravascular volume. Thermoregulatory dysfunction may result in hypothermia. β-adrenergic antagonists predispose to hypothermia by interfering with mobilization of substrates for thermogenesis. Cholinergic agents increase sweating and evaporative cooling, and depress utilization of calorigenic substances. Opioids and sedative-hypnotics depress hypothalamic function and behavioral responses to cold stress. Ethanol has a poikilothermic effect, perhaps mediated by serotonergic systems. Ethanol's effects on thermoregulation are due in part to endogenous opioid peptides; naloxone reverses ethanol-induced hypothermia in animals. Phenothiazines have various effects on thermoregulation including a predisposition to hyperthermia secondary to their anticholinergic properties. However, β-adrenergic antagonism by phenothiazines results in central hypothalamic dysfunction and prevents vasoconstriction in response to cold stress, predisposing to hypothermia. Conclusion: Human body temperature is maintained within a narrow range through complex physiologic mechanisms. Pharmacologic agents interfere with normal thermoregulatory responses to heat and cold exposure, predisposing to abnormal elevation or depression in body temperature.
References: 1. Boulant, A. Hypothalamic Neurons. Mechanisms of Sensitivity to Temperature. Ann. NY Acad. Sci. 1998, 856, 108–115. 2. Hensel, H. Neural Processes in Thermoregulation. Physiol. Rev. 1973, 53, 948–1017. 3. Clark, W.G., Lipton, J.M. Brain and Pituitary Peptides in Thermoregulation. Pharmacol. Ther. 1983, 22, 249–297. 4. Vassallo, S.; Delaney, K. Thermoregulatory Principles. In Toxicologic Emergencies, 7th Ed.; Goldfrank, L.R., Eds.; McGraw Hill, in press. 5. Vassallo S.U.; Delaney K.A. Pharmacologic Effects on Thermoregulation; Mechanisms of Drug-Related Heatstroke. Clin. Toxicol. 1989, 27, 199–224. 6. Orts, A.; Alcaraz, C.; Goldfrank, L. et al. Morphine–Ethanol Interaction on Body Temperature. Gen. Pharmacol. 1991, 22, 111–116.
15 INVESTIGATION OF CENTRAL NERVOUS SYSTEM DYSFUNCTION IN ACUTE POISONING
Hantson Ph,1 Duprez T,2 Guérit JM3
1Department of Intensive Care Medicine, 2Department of Neuroradiology, 3Laboratory of Neurophysiology, Cliniques St-Luc, Université catholique de Louvain, Brussels, Belgium
Objective: Central nervous system (CNS) disorders are frequently observed after acute poisoning. Fortunately, these disorders have mostly a functional origin and anatomic lesions are seldom encountered. As the clinical examination has its own limits in poisoned patients staying in the Intensive Care Unit (ICU), complementary investigations have to be performed in order to assess the severity of CNS dysfunction. The objective of this presentation is to illustrate the different types of CNS investigations that can be performed in acutely poisoned patients with CNS dysfunction. Discussion: The electroencephalogram (EEG) is by far the most commonly used among the complementary tests of CNS dysfunction. Experience with EEG investigations in poisoned patients is large and this technique can be easily applied or repeated at the patient's bedside. However, EEG findings are often poorly specific (diffuse slowing, burst suppression,…). The main indication for EEG recording is the early detection of seizure activity. Evoked potentials are a less commonly available technique in the ICU. However, they may offer advantages over EEG recording. A multimodality analysis is to be proposed. It includes the recording of visual (VEPs), somatosensory (SEPs) and auditory (BAEPs) activities after specific stimuli. Multimodality evoked potentials (MEPs) explore cortical and subcortical regions. With CNS acting drugs, aspecific findings are often observed at VEPs or SEPs. However, preservation of BAEPs activities is usually the rule as brainstem is rarely damaged in case of poisoning. Specific findings may be recorded for example in case of methanol poisoning. The visual pathways are a target organ in methanol poisoning and VEPs are helpful for the detection of early retinal dysfunction or optic neuropathy.1 Brain computed tomography (CT) is usually used in the Emergency Department to rule out anatomical lesions in patients admitted with a suspicion of acute poisoning. Investigation of carbon monoxide (CO) and methanol poisoned patients appears useful. Cerebral edema may be present in severely CO poisoned patients. Lesions to the basal ganglia (thalami, putamina, globi pallidi, caudate nuclei) can be demonstrated either in CO or methanol poisoning. The susceptibility of the basal ganglia to toxic agents is not clearly elucidated. Magnetic resonance imaging (MRI) appears as a well suited neuroimaging modality in poisoned patients. In comparison with brain CT, MRI offers a better resolution of cortical lesions and also better explores subcortical (brainstem) structures. MRI is a far more sensitive technique in the detection of subtle water changes within dama ged brain tissue. This is particularly well illustrated by the investigation of methanol poisoned patients. In early observations, brain CT investigations suggested that the putamina were preferentially involved in methanol poisoning. This was ultimately confirmed by MRI in several case reports or series.2 Edematous changes in the putamina are often symmetrical; they may evolve to hemorrhagic lesions or later to pseudocystic lesions. The patients with necrotico-cystic putaminal sequelae often develop parkinsonism. In addition, MRI in methanol poisoning allows detection of lesions in the white matter within the frontal and/or occipital lobes. These lesions correlate with clinical findings (visual impairment). In CO poisoning, MRI offers also a better definition of the lesions than CT imaging. Edematous lesions are also frequently seen in the globi pallidi. They are potentially reversible. In contrast, persistent lesions at delayed imaging corresponds to necrotizing damage. MRI may disclose white matter degeneration, usually in the parieto-occipital areas. This is indicative of a progressive demyelination (Grinke's myelinopathy). In various toxic conditions, abnormal MRI can be noted. The putamina and globi pallidi are also involved in case of cyanide poisoning. Severe hypoglycemia consecutive to insulin overdose seems also to affect the putamina and caudate nuclei, with relative preservation of the globi pallidi. More recently, ecstasy intoxication was also associated to bilateral lesions of the globi pallidi. Finally, toxic leukoencephalopathy due to various substances is now frequently reported since the routine use of MRI for the diagnosis of unexplained neurologic disorders.3 Experience with brain scintigraphy is more limited in acute poisoning. Data from the literature mainly concern CO poisoning. Technetium-99m-hexamethylpropylene amine oxime (HMPAO) brain single photon emission computed tomoscintigraphy (SPECT) seems to be a good tool for early detection of regional cerebral anomalies in acute CO poisoning. Conclusion: The investigation of poisoned patients with acute CNS disturbances has progressed since the introduction of more recent techniques (MEPs, MRI) that allow a better understanding of the nature and topography of the lesions. Among the bedside techniques, MEPs are also better suited than the EEG for the exploration of subcortical dysfunction or to analyze specific injuries. Brain CT remains essential on an emergency basis in order to rule out major lesions to be treated immediately.
References: 1. Hantson, P.; de Tourtchaninoff, M.; Simoëns, G.; Mahieu, P.; Boschi, A.; Beguin, C.; Guérit, J.M. Evoked Potentials Investigation of Visual Dysfunction After Methanol Poisoning. Crit. Care Med. 1999, 27, 2707–2715. 2. Hantson, P.; Duprez, T.; Mahieu, P. Neurotoxicity to the Basal Ganglia Shown by Brain Magnetic Resonance Imaging (MRI) Following Poisoning by Methanol and Other Substances. J. Toxicol. Clin. Toxicol. 1997, 35, 151–161. 3. Filley, C.M.; Kleinschmidt-DeMasters, B.K. Toxic Leukoencephalopathy. N. Engl. J. Med. 2001, 345, 425–432.
16 NEUROBIOLOGICAL AND NEUROPSYCHOLOGICAL CORRELATIVES OF MCS—A PILOT STUDY
Bornschein S,1,2 Hausteiner C,2,1 Drzezga A,3 Theml T,2 Heldmann B,2 Jahn T, 2 Schwaiger M,3 Förstl H,2 Zilker Th1
1Department of Toxicology, II. Medical Clinic, 2Clinic for Psychiatry and Psychotherapy, 3Clinic for Nuclear Medicine, Technical University, Munich, Germany
Objectives: Patients with multiple chemical sensitivity (MCS) experience various symptoms in connection with low levels of environmental chemicals. Many complain about cognitive impairment. It has been hypothesized that this indicates neurotoxic damage. Case studies reported brain changes in functional neuroimaging with PET and SPECT. Our aim was to objectivize possible deficits in brain metabolism and neuropsychological performance in a group of clearly defined MCS patients. Methods: We selected a homogenous sample of 12 patients with symptoms of MCS visiting our department for environmental medicine. They underwent a PET scan of the brain with F-18-FDG and a one- to two-hour neuropsychological examination, both performed in a standardized manner. The PET and neuropsychological findings were compared to those of age and sex matched controls according to an evaluated analytical routine. In addition, a group analysis was conducted. Results: We investigated 7 women and 5 men between 31 and 61 years of age presenting with symptoms typical for MCS. Apart from various other complaints, all of them reported subjectively impaired cognitive performance. 11 patients revealed normal cerebral glucose metabolism. Mild glucose hypometabolism, mainly in the posterior temporal and occipital region on both sides, was found in one patient. In the group analysis, corrected for whole brain volume, no significant functional brain changes compared to a normative sample could be seen. In the uncorrected analysis there was an inhomogenous picture of some small areas with mild hypometabolism in the MCS patients. After correction, however, only one small area in the right gyrus frontalis superior remained. Here, hypometabolism could be demonstrated with approximate significance (p=0.05). In the individual comparisons of patients' PET scans with the normative sample, no significant changes were apparent in this region. 5 patients showed completely normal neuropsychological test results. 6 patients, however, revealed marked deficits in verbal learning and memory, measured with the California Verbal Learning Test. 4 had clear reductions in information processing speed. Conclusion: The subjective cognitive impairments reported by our MCS patients have no objective correlative in functional neuroimaging with PET. Individual brain changes require control examinations. More than half of our patients, however, had measurable deficits in neurocognitive functions. Similar changes are common in psychiatric conditions such as depression, but they can also indicate neurodegenerative diseases. In combination with the PET results, there is no clear evidence that specific brain functioning changes resulting from MCS. For further conclusions, a larger patient sample should be examined.
17 MASS CARBON MONOXIDE POISONING
Butera R, Locatelli C, Fasola D, Agazzi A, Bove A, Petrolini V, Manzo L
Pavia Poison Center, IRCCS Maugeri Foundation and University of Pavia, Italy
Background: Non homogeneous exposure characteristics are a major cause of pitfalls in carbon monoxide (CO) poisoning clinical studies. Objective: To evaluate in a group of patients simultaneously exposed to CO (i) the relationship between carboxyhemoglobin (COHb) levels and symptom severity in the acute phase, and (ii) the incidence of cardiotoxic effects and delayed neurological sequelae. Methods: Forty-three patients exposed to CO 300 ppm for 2 hours while attending a Christmas concert in a church (because of failure in the heating system) were studied. Clinical severity was assessed at the scene and graded according to Italian CO poisoning management guidelines (GIMUPS 2000; 2 suppl. 2: 163–173) using a 4-level score: 1=asymptomatic, 2=mild (nausea, headache, vertigo), 3=moderate (behavioral changes, tachycardia), 4=severe (loss of consciousness, arrhythmias). Normobaric oxygen therapy was started as soon as possible and lasted for 2 to 15 hours; given the exceptional nature of the event it was impossible to perform hyperbaric oxygen therapy. COHb levels were measured in the Emergency Department (5 to 330 minutes after the end of the exposure). Cardiac damage was defined by the presence of electrocardiographic and/or echocardiographic abnormalities and/or myocardial necrosis markers (CK, CK-MB, myoglobin, Troponin T or I) increase. After discharge, follow-up lasted for 5 months. Neurological sequelae assessment was based on subjective complaints, neurologic evaluation and mini-mental status examination; in doubtful cases, detailed neuropsychiatric test battery, MRI and SPECT were performed. Results: Mean COHb levels were 9.5±3.0% (range: 5.5–14.4%) in asymptomatic cases (grade 1, n=13), 9.8±4.1% (range: 4.6–16.5%) in grade 2 (n=10), 13.7±6.3% (range: 6.3–27.7%) in grade 3 (n=13), and 16.1±7.5% (range: 6.5–28.0%) in grade 4 (n=7) (ANOVA test, p>0.05). Regression analysis showed a relationship between COHb levels and time of blood sampling. Mild cardiac damage (i.e. transient ischemic ECG changes with CK-MB and/or Troponin I increase) was observed in 16% of the patients. Delayed neurologic sequelae (cognitive and memory impairment, headache, vertigo, behavioral changes) were observed in 5 patients (11.6%), ensuing 20 to 147 days after poisoning; initial severity score was 2 in 2 patients, 3 in 2 patients, 4 in 1 patient. Two patients showed white matter lesions on MRI and cortex hypoperfusion on SPECT respectively. Conclusion: COHb levels are strictly dependent on the time of blood sampling and do not correlate with CO poisoning severity in the acute phase. Our data suggest that cardiac damage and delayed neurologic symptoms can be observed even in mild or moderate poisoning treated with normobaric oxygen for a few hours.
18 ACUTE ILLICIT DRUG INTOXICATIONS AT EUROPE'S LARGEST INDOOR RAVE PARTY
Sassenbroeck DK Van,1 Verstraete AG,2 Monsieurs KG,3 Calle PA,3 Buylaert WA3
1Heymans Institute for Pharmacology, 2Department of Clinical Biology and 3Department of Emergency Medicine, Ghent University Hospital, Ghent, Belgium.
Objective: I love techno®, Europe's largest indoor rave party was attended by 35,000 people. In order to cope with potential life-threatening intoxications and to prevent overcrowding in the emergency departments, a medical centre was installed at the scene. Case Series: Party attendants found unconscious and transported to the medical centre are included in this study (n=14). They received standard medical management. In three patients, self-reporting the intake of solely ethanol, no further investigations were performed. In 11 patients found with a decreased consciousness at the scene blood samples were taken for toxicological investigations and in 6 patients a Glasgow Coma Score (GCS) was recorded. Patients (pt) presenting in deep coma were transferred to the university hospital where further blood samples were taken and GCS was recorded. The blood plasma was analysed by GC–MS. All patients gave informed consent for the analysis of the repeated samples. Results: Results are presented in the table where GHB is gamma-hydroxybutyrate, EtOH ethanol, AMP amphetamine, MDMA ecstasy, BE benzoylecgonine, THC tetrahydrocannabinol. Opiates and para-methoxyamphetamine (PMA) showed no increased levels. Only the values above the detection limit are shown (Table 1). For GHB, we observed a steep concentration-effect relationship which might however be confounded by co-ingestion of ethanol, ecstasy, amphetamine or cocaine. None of these patients had to be mechanically ventilated and all regained consciousness between 81 and 150 min after arrival in the hospital. Conclusions: Almost half of the patients presenting with a decreased level of consciousness had taken GHB together with ethanol, amphetamines or cocaine. This suggests that GHB must be considered when treating these patients. This is the first attempt to correlate the GCS with GHB plasma concentrations. This relationship proved to be very steep which, in conjunction with the Michaelis Menten elimination kinetics of GHB and the varying doses in the illegal solutions, makes GHB a dangerous party-drug.
19 INTENSIVE SEARCH FOR THE CAUSE OF AN EPIDEMIC OF SEIZURES AFTER DRINKING HERBAL TEA (STAR ANISE TOXICITY)
Wijnands-Kleukers APG,1 Johanns ESD,1 Vries I de,1 Peters PWJ,2 Meulenbelt J1
1National Poisons Control Centre, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; 2Inspectorate for Health Protection and Veterinary Public Health. The Hague, The Netherlands
Background: Accidental intoxication by food products is often difficult to ascertain. An incidentally reported case does not immediately arouse any suspicion. Most often other factors are considered to be responsible for the adverse health effects. Nevertheless the cases reported below clearly illustrate, that it should be taken into account, that foodproducts may also be responsible for possible undesirable health effects. Case Series: In the last week of September 2001 the Inspectorate for Health Protection and Veterinary Public Health (Health Inspectorate) was informed of the occurrence of 8 cases of adverse health effects (among which seizures) after the consumption of a herbal tea. On the 1st of October the National Poisons Control Centre (NPCC) was also approached by a clinician regarding two similar cases after the consumption of a herbal tea. On the same day the NPCC contacted the Health Inspectorate and deliberation took place regarding the possible cause of the health effects. The NPCC suggested that the reported symptoms might be attributed to anisatine (see below), a toxin, in the herbal tea. The suspect herbal tea was subsequently traced and withdrawn from the market by the Health Inspectorate. To alert the public, a warning was placed in the newspapers. After the public had been alerted a total of 51 persons reported symptoms following the consumption of the suspect tea, 19 of which required hospitalisation. The NPCC approached the clinicians in order to ascertain the particular symptoms. The symptoms started 2–4 hours after the consumption of the herbal tea and consisted of general malaise, nausea and vomiting. Sixteen patients also suffered generalised tonic–clonic seizures. In 6 patients these were preceded by auditory hallucinations. Medical history mentioned no previous epileptic events, head injury nor febrile convulsions in childhood. Patients also denied the abuse of alcohol and/or drugs. Physical, neurological and laboratory investigation revealed no underlying pathology. After supportive treatment, patients were discharged from the hospital in good health. Discussion: Incidental intoxications on the consumption of herbal tea have previously been reported. In Mexico new-born babies receiving star anise tea for treatment of abdominal cramps, subsequently suffered tonic–clonic seizures. It was postulated that the star anise tea, contained Japanese star anise (Shikimi fruit, Illicium anisatum L.) instead of Chinese star anise (Illicium verum L.). Shikimi fruit is known to contain the potent neurotoxine anisatine, a non-competitive GABA-antagonist. In laboratory animals anisatine causes hyperactivity of the central nervous system, resulting in gastrointestinal complaints and tonic–clonic seizures. In mice 0.03 mg/kg anisatine had an analgesic and sedative effect, while doses of 0.7–1 mg/kg (LD50) induced fatal seizures. Morphologic and organoleptic investigations indicated that in our case, too, the suspect herbal tea probably contained Shikimi fruit. Because of a bad harvest and consequently problems with the supply of Chinese Star anise, a star anise (probably Shikimi fruit), which was originally intended for decorative purposes, was mixed into the herbal tea. Chromatographic investigations indicated that the suspect herbal tea did indeed contain the neurotoxin anisatin. Conclusions: The consumption of anisatine containing herbal tea was the most likely cause of the adverse health effects reported by the patients. The close co-operation between clinicians, the Health Inspectorate and the National Poisons Control Centre played a vital role in preventing further harm to public health.
20 POISONING-INDUCED NEPHROTOXICITY
Vale JA
National Poisons Information Service (Birmingham Centre) and West Midlands Poisons Unit, City Hospital, Birmingham, United Kingdom
Definition: This review will focus on the development of adverse alterations in renal structure or function occurring in the setting of acute or chronic poisoning. Nephrotoxicity appearing during the course of drug treatment will not be discussed. Relevant anatomy and physiology: The kidneys play an important role in the control and regulation of homeostasis and are also the site of synthesis of hormones, for example renin, aldosterone and erythropoietin, and of vasoactive prostaglandins and kinins. Each kidney contains approximately 1 million nephrons, each of which consists of a glomerulus (through which large amounts of fluid are filtered from the blood) and a long tubule (in which the filtered fluid is converted into urine). The glomeruli and proximal and distal tubules are located in the renal cortex. Fluid flows from the proximal tubule into the loop of Henle situated in the medulla (which has descending and ascending limbs), and then into the distal tubule which is found in the renal cortex. Those nephrons whose glomeruli are close to the cortical surface (cortical nephrons) have loops of Henle that extend only a short distance into the medulla, while those with glomeruli close to the medulla (juxtamedullary nephrons) have longer loops of Henle that extend deep into the medulla, often close to the renal papillae. Although new nephrons cannot be regenerated following renal injury, and are lost as part of normal aging, nephrons are capable of adaptive change to compensate for fewer functioning nephrons. The kidneys comprise only about 0.5% of the body weight, but receive some 22% of the cardiac output (about 1100 mL/min). This substantial blood flow ensures, firstly, that large quantities of oxygen and metabolic substrates are delivered to the kidneys to maintain function and, secondly, that sufficient plasma is available for the high rates of glomerular filtration that are necessary to preserve homeostasis. Vulnerability of the kidneys to toxic damage: The kidneys are particularly susceptible to poisons that decrease blood pressure, as occurs in poison-induced cardiogenic shock, as a reduction in blood flow will produce cellular anoxia. In addition, because of the considerable renal blood supply, especially to the cortex which receives about 80% of the total renal blood flow, circulating poisons are delivered to the kidneys in large amount. As many poisons undergo active transport from the blood into proximal tubular cells and then diffuse into the tubular lumen, proximal tubular cells can be exposed to higher concentrations of a poison than those present in the plasma. Although the renal medulla receives a much smaller blood flow than the cortex, and therefore relatively less toxic material, counter current mechanisms in the loop of Henle generate a high concentration of the poison in the tubular lumen as the poison passes down the nephron into the medulla, producing a concentration of poison many times greater than that in the plasma, leading to intracellular toxicity. Mechanisms of toxicity: Each part of the nephron can be perturbed by a nephrotoxic insult. Pathogenic mechanisms include direct cytotoxicity, where the effect is generally proportional to the concentration of the poison, and immuno-mediated mechanisms such as those that underlie acute interstitial nephritis or glomerulonephritis. A poison may induce cytotoxic injury directly by inhibiting mitochondrial function or key enzymes involved in energy metabolism; the poison may be metabolized in the kidney to an active metabolite by enzymes such as cytochrome(s) P450 or glutathione-S-transferases; the poison or its active metabolite may be converted to a reactive species which then binds covalently to critical sites in proteins or initiates lipid peroxidation leading to cellular damage; or the poison after undergoing metabolism in another organ to a stable metabolite may enter the kidney where further metabolism to generate a reactive species occurs. Alternatively, the poison may initiate a process such as rhabdomyolysis, which leads to renal dysfunction. Although renal dysfunction following rhabdomyolysis is multifactorial (renal vasoconstriction, tubular toxicity and luminal obstruction), myoglobin-induced lipid peroxidation is likely to be one of the main mediators.1 Classification of nephrotoxicity: Poisons may produce changes in renal function that are mild and reversible or, if severe enough, which are permanent and which may cause death. For example, nephrotoxicity may be expressed as dysfunction in tubular reabsorption, with mild transient proteinuria, or as a decreased concentrating ability, for example polyuria or, in its most severe form, the pattern will be that of acute tubular necrosis with the early development of oliguria and cessation of renal excretory function. The causes of acute renal failure can be divided into pre-renal, intrinsic and post-renal. Pre-renal failure in the poisoned patient can be produced by several mechanisms that lead to hypotension and the subsequent decrease in renal perfusion. These mechanisms include poison-induced cardiotoxicity resulting in a lowered cardiac output, poison-induced vasodilatation, poison-induced vasoconstriction and poison-induced loss of effective circulatory volume from diuresis, gastrointestinal fluid loss such as that due to hemorrhage or corrosive damage, and the loss of tissue fluid, for example from a large chemical burn. Illustrative examples: Paracetamol (Acetaminophen): In a review of 2060 unselected patients poisoned with paracetamol and treated over the period 1969–1980, the overall frequency of acute renal failure was 1.6 per cent. Patients who presented more than 10 hr after the overdose were more severely poisoned and 21.2% of these patients developed acute renal failure.2 In other studies, the incidence of acute renal failure was not significantly higher in patients who suffered paracetamol-induced liver failure, as opposed to those who developed liver failure from other causes.3,4 Three distinct types of nephrotoxicity following paracetamol overdose have been identified.5 Firstly, oliguric renal failure may develop, usually within 48 hr of overdose, and is associated invariably with severe hepatotoxicity; it is accompanied by back pain, renal tenderness, proteinuria and microscopic hematuria. Secondly, renal failure has also been reported in patients who do not develop evidence of severe paracetamol-induced hepatotoxicity. In these cases, the severity of the renal dysfunction varies from transient mild oliguria lasting only a few hours to the rapid onset of anuria necessitating treatment with hemodialysis. Thirdly, a substantial number of patients poisoned with paracetamol develop hypophosphatemia due to reduced tubular reabsorption of phosphate.6 Hypophosphatemia is a feature of paracetamol poisoning whether hepatotoxicity is present or not, though the degree of hypophosphatemia correlates well with the severity of paracetamol-induced liver damage.6 Phosphaturia and retinal-binding proteinuria have been proposed as sensitive markers of nephrotoxicity following paracetamol overdose.7 Bipyridyl herbicides: After the ingestion of paraquat, proximal tubular dysfunction occurs which results in proteinuria, microscopic hematuria, glycosuria, aminoaciduria, phosphaturia and excessive leaking of sodium and urate; oliguric or non-oliguric renal failure may supervene and is due usually to acute tubular necrosis. Exceptionally, glomerular and tubular hemorrhages may be found. Although hypovolemia may sometimes contribute to the development of paraquat-induced acute renal failure, more usually nephrotoxicity is produced by the reactive species, superoxide anion radical, initiating lipid peroxidation. Nephrotoxicity has been reported frequently in diquat poisoning and ranges from transient proteinuria to acute renal failure.8 Renal failure develops between 1 hour and 5 days post ingestion and is invariably present in patients who die. The mechanisms of acute renal failure in diquat poisoning are two-fold. Firstly, hypovolemia following sequestration of fluid in the gut reduces renal perfusion. Secondly, diquat has a direct toxic effect on the kidney, probably involving the production of the reactive species, superoxide anion radical and hydroxyl radical, which initiate lipid peroxidation. At post mortem, acute tubular necrosis is the typical lesion observed. Ethylene glycol: The most notable renal histological abnormality that follows ethylene glycol poisoning is intraluminal oxalate crystal accumulation. However, proximal tubular cell injury does not correlate well with the severity of oxalate deposition, which suggests that other ethylene glycol metabolites are pathogenically involved. Recent studies have suggested that ethylene glycol-induced tubular injury is likely to be mediated in large part by two metabolites, glyoxylate and glycoaldehyde.9 These agents probably induce cytotoxicity by generating severe energy (ATP) depletion, potentially stemming from interference with aerobic and possibly anaerobic pathways. In addition, glycoaldehyde can exert toxicity through its ability to denature a number of diverse cellular constituents, including proteins and specific plasma membrane phospholipids. Although oxalate generation may contribute to ethylene glycol associated acute renal failure, present data suggest that this is more likely to result from oxalate-induced crystalluria/cast formation, rather than from a direct cytotoxic effect.9 Cadmium: Nephrotoxicity from acute cadmium exposure is rare, whereas nephrotoxicity from prolonged low-level exposure occupationally and environmentally10 is common. Initially, there is tubular dysfunction with generalized aminoaciduria, glycosuria, phosphaturia, “tubular” proteinuria (“tubular” proteins are of low molecular weight (20,000–40,000) and consist principally of β-microglobulin and retinol-binding proteins), a reduction in concentrating ability and in acid excretion. Albuminuria of glomerular origin may also be present. Even after removal from further exposure, renal damage may not be reversible. There is also an association between chronic cadmium nephropathy and hypercalcuria and hyperphosphaturia, which increases the risk of renal calculi and of osteomalacia (“Itai–Itai” disease). Lead: Acute severe lead poisoning causes proximal tubular dysfunction leading to glycosuria, aminoaciduria and hyperphosphaturia (a Fanconi-like syndrome). This probably reflects damage to brush-border membranes and may be associated with enzyme loss in the urine. Although nitrogen retention occurs, acute renal failure is rare. Acute lead-induced nephrotoxicity is potentially reversible if chelation therapy is given. Chronic heavy exposure to lead leads to a reduced number of functioning nephrons and granular contracted kidneys as a result of tubulo-interstitial disease. Intranuclear eosinophilic inclusion bodies (which consist of a lead-protein complex) are seen in proximal tubular cells and can often be demonstrated in the urine. The lowest levels at which chronic exposure to lead has an adverse effect on the kidney is unknown. A recent study has found that workers with one or more blood lead concentrations >40 μg/dL have some degree of renal impairment.11 It has been found that chelation therapy slows the progression of renal insufficiency in patients with long-term low-level exposure to lead.12 Mercury: Acute mercury poisoning can induce acute renal failure and early manifestations include proteinuria with cellular casts. Mercury inhibits membrane function, particularly of the brush-border membrane of the renal tubules, by interfering with reactive sulfydryl groups. Mercury can also interfere with the function of the mitochondrial membrane and of sulfydryl-containing enzyme systems. Regeneration of tubules may occur if exposure has not been great, but calcification of necrotic proximal tubules occurs more frequently with mercury than in other metal nephropathies. There is increasing evidence that mercury can also produce an immune-complex type of glomerulonephritis, and this is thought to be the mechanism in those occupationally exposed repeatedly to mercury vapor. The histological picture is usually that of membranous glomerulonephritis. The level of proteinuria varies widely and the nephrotic syndrome may develop. Removal from exposure may reduce the degree of proteinuria. In the population exposed to organic mercurials in Minamata Bay in Japan, low molecular weight proteinuria was a typical feature. Solvents: Carbon tetrachloride and other halogenated hydrocarbons can induce a range of tubular damage, including acute tubular necrosis. Tubular disorders, such as Fanconi syndrome and renal tubular acidosis, have been reported following volatile substance abuse, particularly with toluene. An immune-complex type of glomerulonephritis, particularly anti-glomerular basement membrane (anti-GBM) antibody-mediated diseases, such as Goodpasture's syndrome, can follow relatively low-level exposure to a wide variety of hydrocarbons. Studies in patients with glomerulonephritis, and of renal disease in those with chronic occupational exposure to hydrocarbons, have suggested that such hydrocarbon exposure may be causal in the development of their glomerular disease. Clinical presentation is either as a membranous glomerulonephritis or arising as a response to toxic injury of the mesangial cells. In some occupationally exposed individuals, features of coexisting tubular damage, in the form of tubular proteinuria and increased excretion of N-acetylglucosaminidase, may be found in those with features of glomerulonephritis.
References: 1. Holt, S.; Moore, K. Pathogenesis of Renal Failure in Rhabdomyolysis: the Role of Myoglobin. Exp. Nephrol. 2000, 8, 72–76. 2. Prescott, L.F.; Proudfoot, A.T.; Cregeen, R.J. Paracetamol-Induced Acute Renal Failure in the Absence of Fulminant Liver Damage. Br. Med. J. 1982, 284, 421–422. 3. Wilkinson, S.P.; Moodie, H.; Arroyo, V.A.; Williams, R. Frequency of Renal Impairment in Paracetamol Overdose Compared with Other Causes of Acute Liver Damage. J. Clin. Pathol. 1977, 30, 141–143. 4. O'Grady, J.G.; Gimson, A.E.S.; O'Brien, C.J.; Pucknell, A., Hughes, R.D.; Williams, R. Controlled Trials of Charcoal Hemoperfusion and Prognostic Factors in Fulminant Hepatic Failure. Gastroenterology 1988, 94, 1186–1192. 5. Jones, A.F.; Vale, J.A. Paracetamol Poisoning and the Kidney. J. Clin. Pharm. Ther. 1993, 18, 5–8. 6. Jones, A.F.; Harvey, J.M.; Vale, J.A. Hypophosphataemia and Phosphaturia in Paracetamol Poisoning. Lancet 1989, 2, 608–609. 7. Florkowski, C.M.; Jones, A.F.; Guy, J.M.; Husband, D.J.; Stevens, J. Retinol Binding Proteinuria and Phosphaturia: Markers of Paracetamol-Induced Nephrotoxicity. Ann. Clin. Biochem. 1994, 31, 331–334. 8. Jones, G.M.; Vale, J.A. Mechanisms of Toxicity, Clinical Features, and Management of Diquat Poisoning: A Review. J. Toxicol. Clin. Toxicol. 2000, 38, 123–128. 9. Poldelski, V.; Johnson, A.; Wright, S.; Dela Rosa, V.; Zager, R.A. Ethylene Glycol-Mediated Tubular Injury: Identification of Critical Metabolites and Injury Pathways. Am. J. Kidney Dis. 2001, 38, 339–348. 10. Hellström, L.; Elinder, C.-G.; Dahlberg, B. et al. Cadmium Exposure and End-Stage Renal Disease. Am. J. Kidney Dis. 2001, 38, 1001–1008. 11. Lim, Y.C.; Chia, K.S.; Ong, H.Y.; Ng, V.; Chew, Y.L. Renal Dysfunction in Workers Exposed to Inorganic Lead. Ann. Acad. Med. Singapore 2001, 30, 112–117. 12. Lin, J.-L.; Ho, H.-H.; Yu, C.-C. Chelation Therapy for Patients with Elevated Body Lead Burden and Progressive Renal Insufficiency. A Randomized, Controlled Trial. Ann. Intern. Med. 1999, 130, 7–13.
21 NEPHROTOXIC EFFECTS OF X-RAY CONTRAST MEDIA
Andrew E, Berg KJ
National Poisons Information Centre and The National Hospital (Rikshospitalet), University of Oslo, Norway
Introduction: The number of procedures using x-ray contrast media (CM) is increasing. No other drug is administered in such large doses: an average dose of 100 ml CM at a concentration of 300 mg I/ml contains 30 g I. The use of CM world-wide is estimated to 60 million doses per year. CM are the leading cause of drug induced renal failure in hospitals. Chemistry: All currently used CM are based on the tri-iodinated benzene ring. The first generation of high osmolar ionic CM (HOCM) comprised molecules with three iodine atoms which dissociated into two particles in solution. Their high osmolality (1500 mosm/kg) causes osmotoxic effects such as pain and heat sensations because of a general vasodilation and they are nephrotoxic both in animals and in humans. In spite of the introduction of the monomeric non-ionic, low osmolar CM (LOCM in the 1970s, and later the dimeric iso-osmolar CM (IOCM), CM still remain the third leading cause of hospital-acquired acute renal failure, after hypotension and surgery. Pharmacokinetics: CM are small molecules with low protein binding and lipid solubility that are freely filtered through the glomerular basal membrane. More than 99% is excreted in the kidneys by glomerular filtration. CM are concentrated 100 times in urine with a peak concentration of 200–500 mg/ml during the first 4 hours period after administration, thus the osmotic load of CM presented to the distal tubules is very high. Pathogenesis: Three main factors are involved in the pathogenesis of contrast nephropathy (CN): osmotoxic, haemodynamic and tubular effects. The oxygenation of the renal medulla is normally very low. CM increase diuresis, renal metabolic activity and oxygen consumption and aggravate the medullary hypoxia. The osmotic effect of HOCM as well as of LOCM increases intratubular pressure resulting in reduced glomerular filtration rate (GFR). IOCM may still increase intratubular pressure through their high viscosity which causes intratubular obstruction in rats. Vacuolization of the proximal tubular cells (“osmotic nephrosis”) has been described after administration of CM, probably due to giant lysosomes which contain small amounts (<1%) of intracellularly retained CM. Quantitative determination of proximal tubular enzymes in urine has proved extremely sensitive in the evaluation of CN, probably they are markers of the effect of CM on proximal tubular cells. This effect is reversible and enzymuria is not correlated to increase in serum creatinine by CM. CM induce renal vasoconstriction. This is unique for the kidney, as CM induce vasodilatation in all other vascular beds. Renal vasoconstriction results in reduced renal blood flow (RBF) and GFR. CM may also stimulate endothelial factors like endothelin, adenosine, calcium ions and oxygen free radicals. Diagnosis and risk factors: The diagnosis of CN is most often based on changes in serum creatinine levels. Serum creatinine peaks within 1–2 days after CM in patients with normal renal function, and the increase lasts for 1–5 days. In patients with reduced baseline renal function creatinine peaks later and lasts for 7 days or longer, and sometimes is irreversible. In healthy people intravenous CM administration represents a very low risk for CN (>1%). The most important risk factors is reduced renal function and the next is diabetes. The risk for developing CN increased from 2% in low risk patients (non diabetics with serum creatinine <133 μmol/l) to 38% in diabetics with serum creatinine >133 μmol/l in one study. Some exogenous risk factors may be preventable: High CM dose, repeated doses of CM within short interval, dehydration, concurrent use of nephrotoxic drugs (non-steroidal anti-inflammatory drugs, aminoglycosides, in some cases also cyclosporine and ACE inhibitors) and type of CM. LOCM are superior to HOCM as they are less nephrotoxic. This is clearly demonstrated in risk patients. IOCM have a better imaging effect than LOCM, and clinical side effects like pain and heat sensations are less pronounced. The difference between LOCM and IOCM on serum creatinine is, however, marginal. Consequences and treatment: In most cases CN is reversible. But in patients with risk factors like reduced renal function and/or diabetes the risk for further deterioration of renal function is substantial; the risk for renal failure being most pronounced in patients with severe pretreatment renal impairment. In such patients CN sometimes proceeds into nonoliguric or oliguric acute renal failure which has to be treated by dialysis. In hospitalized patients CN increases the average hospital stay with 1–5 days. Even in patients with a moderate increase in serum creatinine (>25%) CM increase the mortality. Patient selection is usually not possible, but in high risk patients like diabetics with impaired renal function, angiography should be postponed until the patient is accepted for dialysis or transplantation. Prophylactic hemodialysis in high risk patients, starting immediate after CM examination, lowers the plasma level of CM, but is without effect on renal function. Concomitant administration of nephrotoxic drugs and CM should be avoided. The contrast dose should also be reduced. The most important prophylactic measure is hydration of the patients. Diuretic treatment with furosemide or mannitol should not be given. Prophylactic treatment with other drugs, like calcium channel blockers, adenosine antagonists like theophylline, endothelin receptor antagonists and antioxidants has been tried. The clinical benefit of such drugs is still a matter of discussion. Conclusion: CM are an important risk factor of iatrogenic renal failure. The most effective strategy for prevention is careful patient selection. Patients at risk should have minimal dose of CM and serum creatinine should be controlled before and after investigation. Volume depletion should be corrected and in high risk patients a hydration protocol should be initiated. LOCM or IOCM should always be used in high risk patients.
22 MECHANISMS OF RENAL TOXICITY IN ETHYLENE GLYCOL INTOXICATIONS
McMartin KE
Department of Pharmacology, Louisiana State University Health Sciences Center, Shreveport, Lousiana, USA
Objectives: This keynote presentation will provide an overview of mechanisms for renal toxicity, especially that associated with ethylene glycol exposure. Discussion: Acute renal failure is a common result of exposure to pharmacologic or environmental chemicals. Toxic renal failure is most often associated with specific cytolytic damage to renal cells, particularly necrosis of proximal tubular (PT) cells. Although low, environmental levels of certain toxicants induce apoptosis of PT cells, in overdose situations, necrosis will predominate. Mechanisms for the necrosis can include oxidative damage to internal metabolic pathways, particularly to the mitochondria, as well as failure of repair pathways to overcome initial damage. To discuss various mechanisms, the example of ethylene glycol (EG) will be presented. Ingestion of EG can induce acute renal failure as indicated by flank pain and tenderness, proteinuria, hematuria, anuria and uremic death if not treated. Pathologically, crystals are often observed in the PT lumen and in PT cells, less so in the papilla and interstitium. Crystals are sometimes seen with normal kidney structure, but most often with tubular necrosis, indicated by PT dilation with a vacuolar, hydropic degeneration of PT cells. The PT lumen can contain desquamated cells and crystal masses. Studies with repeated biopsy during treatment with hemodialysis have shown eventual clearing of renal tubules and PT cell regeneration. Urine and blood chemistry show increased BUN, serum creatinine, urine crystals and urinary protein, particularly increased ⋄1-microglobulin (from PT damage). The mechanism for the nephrotoxicity is linked with the metabolism of EG, since inhibition of the primary step (alcohol dehydrogenase) prevents or reverses the renal failure. EG is metabolized primarily to glycolic acid (GA), glyoxylic acid, oxalic acid (OX) and CO2. OX accumulation is most often associated with the development of acute renal failure. OX is poorly soluble and precipitates in the presence of calcium as calcium oxalate monohydrate or dihydrate crystals. In most experimental studies, renal damage is observed only where crystals are present and only at doses greater than those associated with increases urinary excretion of OX or OX crystals. A traditional explanation of the renal failure has been that oxalate crystals form in the PT lumen, leading to a physical blockage of flow by compression. However, the direct cytotoxicity of crystals or other metabolites such as GA cannot be excluded, leading to the suggestion that tubular necrosis happens independently of crystal formation. For example, recent studies in EG-poisoned humans have correlated accumulation of GA with renal failure. Studies of the relative cytotoxicity of EG metabolites in primary cultures of normal human proximal tubule (HPT) cells have shown that very high concentrations of GA or glyoxylic acid do not produce cell death, while relevant concentrations of OX ion decrease cell viability. Other studies in transformed cell lines derived from the dog and pig kidney have shown similar cytotoxic effects of the OX ion, either alone or in combination with OX crystals. These studies have also suggested that OX may lead to necrosis by oxidatively stressing the PT cell, similar to the mechanism of most nephrotoxic agents. Conclusions: The acute renal failure from EG poisoning results from a tubular cell necrosis similar to that observed with many nephrotoxins. Recent studies have suggested that the mechanism for EG renal necrosis is related to the accumulation of OX ion or OX crystals, which produce PT cell toxicity by an intracellular oxidative mechanism.
23 FOMEPIZOLE TREATMENT PREVENTS RENAL FAILURE IN SEVERE ETHYLENE GLYCOL POISONING: REPORT OF TWO CASES
Moestue S,1 Akervik O,2 Svendsen J,3 Jacobsen D3
1National Poisons Information Centre, Oslo. 2Medical Department, Innherred Hospital, Levanger. 3Division of Medicine, Ulleval University Hospital, Oslo, Norway
Objective: Ethylene glycol (EG) poisoning is a medical emergency characterised by coma, severe metabolic acidosis, cardio-pulmonary failure and acute renal failure. Recently, FDA has approved the new antidote fomepizol (Antizol) as an inhibitor of alcohol dehydrogenase in this poisoning thereby preventing the continuous production of acidic metabolites of EG. Arguments in favour of fomepizole (as compared to ethanol) are less side effects, easier administration and possible prevention of acute renal failure and dialysis treatment if administrated early. We present two cases of EG poisoning where fomepizole was administrated at a relatively late stage of poisoning with pronounced metabolic acidosis. Case Reports: Case 1 (F 32) was admitted few hrs following the ingestion of approximately 500 ml of EG. Upon admission her S-EG was 90 mM (682 mg/dl), pH 7.10, HCO3 5.4 mM, base deficit 23 mM, osmolal gap 137 mOsm/kgH2O, and anion gap 32 mM. S-ethanol was 1 mM (4.8 mg/dl), S-creatinine and electrolytes were normal. Gastric lavage was followed by administration of sodium bicarbonate (420 mmoles). Treatment with fomepizole was started 5–6 h after ingestion and continued for two days. S-EG monitored continuously for 3 days until the level was 4 mM. S-half-life of EG during fomepizole treatment was 16 hrs and acid–base status & S-creatinine was normal during the whole period. Case 2 (M 52) was admitted to local hospital with pronounced metabolic acidosis (pH 6.93, pCO2 1.6 kPa, HCO3 3.6 mM, base deficit 28 mM. Anion gap was 33 mM and osmolal gap 111 mOsm/kgH2O. Methanol, ethanol and isopropanol were not detected. He was given sodium bicarbonate and ethanol IV and transferred to Ulleval Hospital (2 hrs) with a tentative diagnosis of EG poisoning. Upon admission, he was in coma grade IV, still acidotic, S-ethanol was negative and S-EG 45 mM. Anion and osmolal gaps were 38 mM and 47 mOsm/kgH2O, respectively. He was treated with sodium bicarbonate, fomepizole for 3 days and IV fluids which produced a large diuresis (7 L/24 hrs). During fomepizole treatment the EG half-life was about 9 hrs. His metabolic acidosis was rapidly corrected and did not return. He suffered no renal failure and the further course was uneventful except for a minor aspiration pneumonia. Conclusion: The present cases indicate that fomepizole may prevent renal failure and the need for hemodialysis also in patients presenting in later stages of EG poisoning when pronounced metabolic acidosis has developed. As such, the need for referral of patients with EG poisoning from smaller to larger hospitals with dialysis facilities may be reduced.
24 THE ROLE OF THE PORTUGUESE POISONS INFORMATION CENTRES IN PRIMARY AND SECONDARY PREVENTION
Borges A
Centro de Informação Antivenenos, Instituto Nacional de Emergência Médica, Lisboa, Portugal
Introduction: The first and fundamental function of a Poison Information Centre (PIC) is to be operationally capable for 24 hours a day, seven days a week, after rapid identification of a poison, to provide immediate information and advice about diagnosis, toxicity, symptoms, and treatment applicable to any patient who has been exposed to a poison, or is suspected of poisoning or is poisoned. The PIC has other functions, as an important and key role on prevention of poisonings. Poison prevention has three aspects: primary, secondary and tertiary. Primary prevention avoids the toxicological accidents and reduces the risk of appearance of new cases. Secondary prevention avoids or reduces the morbidity and mortality of the poison exposures. Tertiary prevention acts on the poisoning sequelae, decreasing the invalidity and implementing the recuperation and the reintegration of poisoned patients. Prevention is a multidisciplinary task and the collaboration must be close among all partners. Primary Prevention is related with the safety of products, education, information and legislation. The Poison Centre by great experience on clinical toxicology, knowledge of product compositions, statistic data concerning toxic agents, risk factors, circumstances and potential victims can contribute to the primary prevention: influencing the legislation and regulamentations, suggesting appropriate labelling, special packing, adequate use instructions, withdrawal from the market or limitation of the use of some products. Education and information play a very important part in prevention. The PIC has a key role in collaboration or implementation of educational actions, dissemination of information to public authorities, decision-makers, industries, manufacturers, importers, health professionals, civil defence, first responders, teachers, mass media, population (young parents, school children, workers). The PIC has capability of working closely with all partners involved in poison accident response. Secondary prevention involves the rapid identification of poison, the poisoning treatment (the removal of the toxic agent to prevent further local damage or absorption, symptomatic and supportive therapy, use of antidotes, enhancement of poison elimination) and the transport to treatment facilities. The efficacy of secondary prevention depends on the performance of the Poison Center, the first-aid measures done at site of exposure, the availability of patient transport, the good communication links and the availability of antidotes and equipment of the hospitals or other treatment facilities. The PIC has a key role in poison identification, to provide appropriate information and advice about toxicity, symptoms and treatment. Conclusions: The PIC contributes in reduction of the poisoning occurrence and the morbidity and mortality by poisoning. The role of PIC needs to be more publicised, strengthened and the communication channels among the full range of partners including the decision-makers as well as the population must be improved. The PICs are services with good acceptance by the public, with a growing recognition of their work, with experience and knowledge and have unique capabilities as information sources. By all of this, the PICs are fundamental pieces in building of primary and secondary poisoning prevention.
25 THE LIFE, MUSIC AND MYSTERIOUS DEATH OF PJOTR ILYICH TCHAIKOVSKY
Persson H
Swedish Poisons Information Centre, Stockholm, Sweden
Background: In St. Petersburg, October 1893, Pjotr Ilyich Tchaikovsky conducted the first performance of what was to become his last symphony, “Symphonie pathétique”—an overwhelming testimony of passion and suffering. The audience was confused. But when the symphony, a few weeks later, was played for the second time, it brought the house down. On this occasion, however, the composer was not present; he died between the two performances after a short and utterly violent illness. What happened to the so far healthy, 53-year-old composer, who among Russians was next only to the tsar in terms of international fame? Was it a natural death, an accident or a suicide? Was it arsenic, amatoxin or cholera? Objective: To produce an answer. Methods: Excursions through the contemporary documentation (reports from the treating physicians, letters, newspapers), the current scientific literature and in Tchaikovsky's musical universe. A definite answer has been requested, but in vain. Therefore the contradictions, hostile arguing, discrepancies and fanciful theories of the musicologists have been balanced against the original documents, the message of the music and the medical (in particular clinical toxicology) knowledge of today—to form a hypothesis, hopefully bordering on the truth. Results: The life—arduous, astonishing, oscillating between sublime happiness and deep misery. The man—generous and charismatic but also complex and restless, always hunting, always fleeing. Above all a supreme genius. The music—one of the richest and most prosperous artistic achievements of the 19th century: autobiographic (as the symphonies), great entertainment (as the ballets), acts of love (as the concertos and symphonic poems). The health—robust, but constantly and ruthlessly challenged. The death—a mystery to be addressed in the conclusion. Conclusion: Will remain a secret until May 2002, in Lisbon.
26 DOCUMENTATION OF HEALTH HAZARDS IN HUMANS AS A TOOL TO INITIATE PREVENTION OF INTOXICATIONS IN GERMANY—10 YEARS EXPERIENCE OF THE BGVV WORKING GROUP
Hahn A, Heinemeyer G, Gundert-Remy U
Bundesinstitut für gesundheitlichen Verbraucherschutz und Veterinärmedizin (BgVV), Berlin, Germany
Introduction: Unlike pharmaceutical products, chemical substances are not systematically tested in humans. Normally, an extrapolation from animal-studies is performed to characterise possible toxic health risks in humans. Knowledge of substances gained by systematic documentation and analysis of adverse effects of chemicals in humans due to poisonings is therefore a valuable tool for a realistic assessment. To initiate preventive measures by governmental regulations, there is a need for an effective monitoring system for documentation of human health hazards and to characterise the typical scenarios of accidents and exposure related to the respective hazardous products/compounds. This instrument can also serve as a basis for classification and labelling of hazardous products and substances, give arguments for educational advertising and create the basis for co-operation with manufacturers/distributors and ministries. Spontaneous monitoring system § 16e Chemicals Act: Under the regulations of the German Chemicals Act, amended in 1990, physicians who are consulted for treatment or evaluation of sequelae of diseases are requested to report health effects from real or suspected exposures to chemical substances. The BgVV was designated as the central Documentation Centre for Poisonings; Products and Environmental Medicine (DCPPE). Additionally, in different regulations, manufacturers or distributors of products are obliged to provide formulations of chemical products (1990), cosmetics (1998) and biocidal preparations (2002). German Poison Control Centres (PCC) have been put under an obligation to assist the BgVV by submission of data on health hazards resulting from their work. This has led to intensive cooperation in the BgVV Committee: “Evaluation and Treatment of Poisonings” with the German PCCs, Industry, Consumer Councils and Ministries with initiation of two joint research projects: (i) EVA: Data Collection and Data Analysis in Cases of Poisoning in the Poison Information and Treatment Centres in the Federal Republic of Germany and (ii) TDI: The Toxicological Documentation and Information Network. Results: During the years 1990–2001, the following main fields of work have been developed: 1. The development of instruments for documentation and evaluation of cases of poisoning. This includes particularly the assessment of the causal relationship between exposure to a chemical substance or product and manifestation of a disease. Increasingly, the respective cases are documented in a report data base. 2. Evaluation of health impairments by certain compounds with a focus on specific compounds and circumstances. 3. Assessments of health impairments by chemical substances in the environment, especially from industrial accidents 4. As a consequence of documentation and evaluation of health hazards, a “Toxicovigilance” procedure for rapid information to industry, ministries and industrial associations on the health risks of products utilizing the base of immediate and summarised reports has been inaugurated. For severe cases of toxic effects in humans, producers—as well as responsible authorities and ministries—are directly informed and asked for risks reducing measures (PRINS-System). Statistics: The DCPPE received more than 26.500 reports (together with joint PCC research projects). Additionally, the German PCCs were provided with more than 120.000 product records. The distribution of cases in PCCs differs from the BgVV reports: the greatest part (80%) was due to accidents (occupational included), 15% were hazards during normal use and only 2–3% of the cases were related to attempted suicides. 77% of the cases were in adults, 22% in children. Most accidents occurred in private homes. Typical products unintentionally ingested by infants and small children included lamp oils, detergents for dish washing machines and the fluids of heating meters. Cases of poisoning by pesticides were very frequent both in the residential area and at the workplace. Health impairments due to solvents and paints were reported to occur in private homes as well as at the workplace. Exposure to welding fumes (mainly emitted by zinc-coated surfaces) or handling of milking machine cleaners (formation of gaseous chlorine when acid and alkaline cleaners containing hypochlorite are mixed) occurred, as a rule, in the workplace. Health impairment was related to direct exposure (accident, application of products by persons affected, suicide) as well as to indirect exposure from the environment (indoor air, contamination of surfaces, furniture, etc.). Although the case documentation of this spontaneous reporting system does not give a representative overview of health hazards, it serves as an appropriate base to create hypotheses, to initiate single risk reduction activities and to perform additional studies for a better characterisation of the circumstances and to control of the effects of risk reducing measures; some of them have already been reported and discussed at EAPCCT conferences: 1) Direct reports to industry and ministries (PRINS): From 1998–2000 we had 10 immediate reports [(impregnant (1 adult/death), solvent (1 adult/lung edema), lamp oil (1 small child/life-threatening pneumonia), pipe cleaner (1 adult/severe burns), bubble bath (1 elderly person/death), drug (1 toddler/death), industrial cleaner (1 small child/severe burns), depilation creme (1 adult/severe burns), disinfectant (2 elderly persons/deaths)]. The periodically summarised reports were sent to more than 170 manufactures/distributors. These reports led to additional warnings, classification and labelling (e.g. benzalkonium chloride caustic>7.5%) and promoted the development of lamp oil substitutes. 2) BgVV-ESPED Lamp Oil Study: Since lamp oils involve the highest risk of severe health effects in infants and small children due to household chemicals in Germany, the BgVV initiated different measures for risk minimisation: child-resistant closures, warnings, new R 65-Phrase, EC-standardisation of oil-lamps in accordance with the competent ministries, which at least led to sale restrictions from July 1, 2000 onwards in all EU countries. This has consequently led to the introduction of lamp oil substitutes into the German and EU market. To follow the consequences, a BgVV monitory study “Dangerous Lamp Oils” was initiated in conjunction with 450 German Childrens' Hospitals. Preliminary results have shown that the exchange of paraffins by fatty acid esters obviously leads to a marked reduction of risk. 3) BgVV Exposure study: To evaluate the circumstances of exposures during accidents, a study was initiated to collect data describing the circumstances of exposures to a number of selected chemical substances (paints, solvents, glues and pesticides). Cases to be studied are selected from the reports sent to the BgVV, and questionnaires are to be filled out by the exposed persons to collect data that can give a better description of the accident and to allow quantitative exposure evaluations. 4) Workshop: In September 2001, the BgVV hosted a workshop “Children and Pesticides” to reduce the hazards of pesticide exposures. A special focus was laid upon the childhood behaviour as a factor in exposure, toxicokinetics and toxicodynamics as well as on approaches for exposure estimations. Proceedings of this workshop will be published in 2002 by the BgVV. 5) Product labelling: A general BgVV analysis of the product labels especially in the fast-growing and fluctuating international markets of household and cosmetic products has shown that there must be better information on the labels and packages to identify the real tradename in emergencies. To improve the product identification on the basis of the true trade name, the BgVV has initiated investigations to preserve an “Easy-to-Identify Area” on the labels of consumer products in close proximity to the barcode via an EC -standardisation procedure. 6) Risks for the elderly: Some cases of mistaken, but fatal, ingestion of chemical preparations, namely detergents and disinfectants, have shown that elderly persons may represent a population at risk. This may be particularly so for confused persons. With a round table of experts the BgVV discussed appropriate measures to be initiated for reduction of such risks.
27 SUBSTITUTING MACHINE DISH WASHING AGENTS WITH DISILICATES INSTEAD OF METASILICATES CAN EFFECTIVELY PREVENT CHILDHOOD CORROSIVE INJURIES
Brockstedt M, Gregorszewski D, Dilger I
Berlin Poison Center, Berlin, Germany
Objective: In 1994 German industries substituted corrosive machine dish washing agents containing metasilicates by disilicates and carbonate-containing alternatives on a large scale. It was the aim of our study to evaluate, whether these new brands effectively prevent childhood corrosive accidents, if swallowed accidentally, thus allowing the patients to stay at home without further medical treatment. Methods: We performed a 2 year case-control study with a follow-up of all 396 cases of accidental childhood ingestions of non-metasilicate containing brands of machine dish washing agents reported to Berlin Poison center (this excluded sodium hydroxide-containing products, which are not sold in Germany); follow-up was by structured parents interviews or physicians interviews in cases with symptoms within 24 to 96 hours after the initial accident, that was counselled at the poison center immediately. Controls comprised an age-matched group of 288 children ingesting foaming, irritant, but definitely non-corrosive household cleaners, shampoos or hand-washing agents. Results: Out of 396 followed cases 86 (21.7%) initially showed symptoms like crying, drooling, vomiting or unwillingness to drink, all of these were referred to a regional hospital. Out of these 86 cases endoscopy was performed in 17 cases, 13 of which were negative and 4 showed general reddening of the esophageal mucosa (grade I esophageal lesion of Hollinger et al). Those 4 patients also showed reddening of the oral cavity, whereas all other cases had no visible signs of corrosive lesions of the oral cavity. The same held true for all 288 controls after the accidental ingestion of foaming irritant agents, no endoscopies were performed in these controls due to the lack of severe or lasting symptoms. No differences could be observed between the 46 non metasilicate-containing brands in regard to initial symptoms despite a wide range of different ingredients and their concentrations; neither pH or alkali reserve of a product alone allowed for proper classification as being corrosive or not in regard to the observed symptoms; the medical advice to offer something to drink within minutes after the ingestion as stated on all packages of machine dish washing agents in Germany or as advised by the poison center was followed in 260 out of the 396 cases (65.6%) and might have positively contributed to the overall favorable outcomes. In the 4 cases with grade I esophageal lesions one child had not received oral fluids immediately and the 3 others had refused to drink the offered fluids, a clinical sign by itself that would prompt the poison center to let the parents seek medical attention. Finally there were no differences as to observed symptoms between machine dish washing agents in powder form (262) or as so-called tabs (134). Conclusion: From these data we conclude that the newer machine dish washing agents not containing metasilicates (nor sodium hydroxide) prevent serious corrosive lesions to the oral cavity and esophagus in cases of unintentional childhood ingestions and can properly be treated at home, if 1) a glass of water has been given immediately; 2) the child does not display primary or secondary signs of corrosive injury; 3) the product itself is properly identified by a poison center specialist. From the year 2000 calls to Berlin poison center this regimen would apply to 509 out of 533 acute cases of unintentional ingestions of machine dish washing agents, the remaining 24 still containing metasilicates. Since we have changed our regimen due to the study results at least 80% of these patients could stay at home instead of seeking further medical advice, which is in itself a responsible and cost-saving decision that at the same time shows poison centers ability in combining post marketing surveillance with toxicovigilance.
28 THE EPIDEMIOLOGY OF POISONING IN SCOTLAND: THE USES OF EPIDEMIOLOGY IN TOXICOLOGY
Bateman DN
National Poisons Information Service, Edinburgh Centre, Royal Infirmary of Edinburgh, Scotland, United Kingdom
Introduction: In the 1960s hospital admission data in Scotland indicated a large increase in the annual presentation rate of patients with acute poisoning1. This data was used to support the development of the poisons information service in Scotland and specialist treatment facilities within the Royal Infirmary of Edinburgh. Epidemiological data is therefore potentially a useful tool in determining service provision, as well as for investigating the impact of poisoning on public health. Since the 1960s there have been developments in case definition and of data collection systems that now mean it is possible to study epidemiological aspects of poisoning in a variety of ways. These will be illustrated using data sets available within the Scottish Health system. Discussion: A variety of types of data are potentially available, including: a) enquiry data, which may either be patient-specific, as in the case of telephone enquiry data, or be non-patient-specific, as in the case of web site enquiry material as obtained via the TOXBASE system, b) hospital admission details collected locally, which enable trends in hospital activity to be followed over time, and c) national hospital discharge data, available through the Scottish Morbidity Record 01 (SMR01). Call data may enable trends in drug usage to be identified, and if all calls are followed up, to examine the clinical outcome2,3. This is a labour-intensive process, and is obviously dependent upon the proportion of patients about whom calls to a poisons centre are made. Web data is not patient specific, but may still enable useful data to be collected4. As an epidemiological tool for assessing overdose rate enquiry data is, therefore, weaker, than techniques which use hospital data. Hospital admission data may be subject to bias. Thus the change in attitude to the diagnosis by medical staff when self-harm and attempted suicide were decriminalised is one reason for the large increase in patient admissions to hospital in the 1960s. The national dataset includes patient's demographic details, deprivation score, and information on the episode of care, including in particular diagnoses and operations performed. Diagnosis is coded by ICD codes (currently ICD10) and within this coding subset it is possible to identify either individual drugs (eg acetaminophen) or individual drug groups (eg antidepressants). One unique feature of the Scottish dataset is that each patient has an individual anonymised number—the Community Health Index (CHI) number. This number enables patients to be identified and ‘tracked’ through the system so that subsequent hospital discharges, or mortality, can be identified5. Each type of data collected has its strengths and weaknesses. All datasets depend on the consistency of diagnosis and the diagnostic categories used in the analysis. Mortality data may be biased by incorrect diagnosis at post-mortem, best illustrated by the effect of death from opioid/paracetamol combination analgesics on “paracetamol mortality” data. Epidemiological datasets are more useful if linked to fixed populations. Hence admission data from hospitals may be less useful to examine trends if there are changes in referral criteria or catchment area. National data, as collected in Scotland, is a particularly powerful tool because of the link to the CHI number. It can be used to track re-presentations by individual patients in different hospitals, and to study re-admissions involving specific poisons. National statistics also give the possibility of examining rates of presentation in different age or gender groups, and of different social (deprivation) status. In this presentation the use of epidemiological data of various origins will be discussed, and strengths and weaknesses illustrated.
References: 1. Matthew, H. Acute Poisoning. Scot. Med. J. 1966, 11, 1–6. 2. Good, A.M.; Laing, W.J.; Kelly, C.A.; Bateman, D.N. Poisoning in Children Under One Year. Clin. Toxicol. 2001, 39, 306. 3. Laing, W.J.; Gordon, L.D.; Lee, D.S.; Good, A.M.; Bateman, D.N. Have the New Pack Size Regulations Impacted on UK Paracetamol Overdose? Clin. Toxicol. 2001, 39, 30. 4. Bateman, D.N.; Good, A.M.; Kelly, C.A.; Laing, W.J. Web Based Information on Clinical Toxicology for the United Kingdom: Uptake and Utilisation of TOXBASE in 2000. Br. J. Clin. Pharmcol. In press. 5. Bateman, D.N.; Bain, M.; Gorman, D.; Murphy, D. Poisoning in Scotland: Paracetamol, Antidepressants and Opioids, the Problems of the 90s. Br. J. Psychiatr., submitted for publication.
29 A PROSPECTIVE STUDY ON INTOXICATIONS WITH CITALOPRAM
Wijnands-Kleukers APG,1,2 Zoelen G van,1 Vries I de,1,2 Meulenbelt J1,2
1National Poisons Control Centre, National Institute for Public Health and the Environment, Bilthoven, The Netherlands. 2Department of Intensive Care and Clinical Toxicology, University Medical Center, Utrecht, The Netherlands
Introduction: From September 2000 until October 2001 the National Poisons Control Centre conducted a prospective study on acute intoxications with the selective serotonin-reuptake inhibitor (SSRI) citalopram. In literature there has been controversy regarding the safety of citalopram in overdose, especially with regard to cardiac safety. At therapeutic doses citalopram has no effects on conduction and repolarization. In overdose it has been associated with seizures and electocardiographic changes (prolonged QT-intervals, QRS widening, bundle branch block, changes in the ST-T region and ventricular fibrillation) at doses greater than 400 mg. Incidentally bradycardia has been reported. Probably didemethylcitalopram (DDCT), a metabolite of citalopram, is responsible for the cardiotoxic effects. In studies with beagles DDCT, but not citalopram itself, selectively and dose-dependently prolongs the QT interval, with subsequent fatal arrhythmia. DDCT is present at much higher concentration in beagles than in humans (plasmaconcentration generally <6 ng/ml) because of a species-specific metabolism. Sparteine, a markersubstance, is metabolized by cytochrome P450 2D6-isoenzym. DDCT is virtually undetectable in poor metabolizers of sparteine, suggesting that the apparently noninducible cytochrome P450 2D6-isoenzym is important for its formation. The objective of the study was to examine the clinical effects of acute overdose after intoxications with citalopram alone or in combination with other drugs. Is there a potential risk of cardiovascular effects and does this risk increase in combined intoxications. Methods: All cases of possible overdose with citalopram (alone and in combination with alcohol and or other substances) in which the Poisons Control Centre was consulted between September 2000 and October 2001 were taken into consideration. The consulting physicians were asked to complete a standard enquiry form. The following questions were included: patient's age, sex, relevant medical history and drug use, items about the intoxication (estimated dose, time of ingestion, coingested substances, circumstances of the intoxication), clinical effects, additional investigations (laboratory measurements, ECG, X-thorax) and therapies performed. Results: In the study period 192 information requests about citalopram were handled. 146 Enquiry forms were sent, 57 of which were returned. Only the forms that were completed (a total of 42) were studied. The ages of the patients ranged from 3 to 54 years, 11 patients were male and 31 female. In 64.3% of the cases besides citalopram alcohol (14.3%), benzodiazepines (47.6%), antidepressiva (7.1%) and other products (31%) were ingested. The citalopram dose ranged form 100–780 mg (2–13 mg/kg) in mono and 100–2240 mg (1.77–34.5 mg/kg) in combined intoxications. Evaluation of the clinical effects revealed that no serious intoxications were observed in any of the 42 patients. Seven patients were asymptomatic (5 mono and 2 combined intoxications). In all the intoxications drowsiness, tremor, tachycardia and gastrointestinal symptoms were effects most frequently observed. In 2 patients taking citalopram alone cardiac effects occurred (tachycardia in one patient and chest pain in the other). In both, the ECG showed no abnormalities. In one patient an epileptic insult occurred after the ingestion of 780 mg citalopram (13 mg/kg). Unfortunately, detailed medical data were lacking because the inquiry form was not completely filled in. In 8 of the 27 patients taking citalopram in combination with other substances, one or more cardiovascular effects were observed (tachycardia, hypotension, abnormal ECG). Five patients showed sinus tachycardia without electrocardiographic changes. In one patient ECG abnormalities were observed: A 38-year-old female ingested 1500 mg citalopram, 460 mg temazepam, 800 mg pipamperon and 150–250 mg zolpidem. She was somnolent and a hyponatremia (129 mmol/l) was observed. Her ECG showed notched RS complexes and QST elevation of 1 mm in V3, V5 and V6. The ECG normalized within a few hours. Conclusions: In our case-series the general feeling that citalopram-intoxications are usually mild is confirmed. However cardiovascular effects and convulsions can occur, even after the ingestion of small doses. It has not yet been fully clarified whether or not this has to do with genetic predisposition, although the above information suggests this.
30 RELATIVE TOXICITY OF CITALOPRAM AND OTHER SELECTIVE SEROTONIN REUPTAKE INHIBITORS IN OVERDOSE
Isbister GK,1 Dawson AH,1,2 Whyte IM1,2
1Discipline of Clinical Pharmacology, University of Newcastle, Australia. 2Department of Clinical Toxicology, Newcastle Mater Hospital, Australia
Objective: To investigate the cardiac toxicity of the selective serotonin reuptake inhibitors (SSRIs) in overdose. Methods: All SSRI overdoses presenting to Hunter Area Toxicology Service were retrospectively reviewed. Cases were included if a single SSRI had been ingested, or where all co-ingestants had no known effect on QT interval or QRS width. The QRS, QT and R–R intervals were measured and QTc was calculated using Bazett's formula. A control group was taken from overdoses with medications not known to affect the QT interval. A QTc>440 msec was defined as abnormal. Comparisons were made using unpaired t tests and Fischer's exact test. Results: 236 patients were included who ingested citalopram (32), sertraline (82), paroxetine (70), fluoxetine (39) and fluvoxamine (13). The control group contained 318 patients who ingested paracetamol, paracetamol/codeine, temazepam, oxazepam or diazepam. The mean QTc in citalopram overdose patients (451 msec) differed significantly from both the mean of the controls (426 msec; P=0.001) and other SSRIs (431 msec; P=0.006). The number of citalopram overdoses with an abnormal QTc was 66%. Compared with the control group, the odds of developing a QTc>440 msec were 3.73 (95% C.I. 1.73–8.02, p=0.008) for citalopram. Other SSRIs were not significantly different from control. There were no significant differences in QRS width between any SSRI, all SSRIs and controls. Conclusions: Citalopram is relatively more cardiac toxic than other SSRIs, causing a prolonged QTc in overdose. Serial ECG monitoring should be done in citalopram overdose and baseline ECGs taken prior to prescription of citalopram.
31 BUPROPION SR IN OVERDOSE: SUBSIDIZED POISONING
Falkland M,1 McMorrow J,2 McKeown R,1 Cooper GM,1 Buckley NA1
1The Canberra Hospital, Canberra, Australian Capital Territory, Australia, 2Royal Perth Hospital, Perth, Western Australia
Background: Bupropion (Zyban SR™), also known as amfebutamone, was listed on the Pharmaceutical Benefits Sch. (PBS) in Australia in Feb. 2001 as a 150 mg, 120 tablet pack for use within a comprehensive treatment program, as an aid in smoking cessation. Bupropion was not previously marketed in Australia as an antidepressant, as was the case in the U.S. and Europe. Extensive media exposure combined with low cost as a result of the PBS listing resulted in a very high uptake in the community. 239,195 PBS prescriptions for bupropion were dispensed from Feb–May 2001. This implies exposure of approximately 1.2% of Australia's population to bupropion in just four months. Case Series: Many patients with overdoses of bupropion have been admitted to hospitals around the country. We describe a series of patients whose symptoms have included nausea, vomiting, confusion, hallucinations, coma and seizures. In one case, a seizure did not occur until 19 hours after ingestion, most likely due to the slow release nature of the product, and the presence of active metabolites with even longer half-lives. Warnings on the use of this product have been issued by The Australian Adverse Drug Reactions Advisory Committee after receiving large numbers of reports, particularly of neurological and hypersensitivity reactions. To the end of November 2001 the committee had received 1237 reports in connection with the use of Zyban SR. These have included reports of seizures(83) and angioedema(46). Conclusion: Experience in Australia shows that consumer driven prescribing may expose patients to significant risk. Measures to encourage more balanced media reporting of medicines are needed. Appropriate patient selection and follow up counselling is important as the whole seven week course is supplied at the start of therapy. Consideration should be given to supply in smaller quantities. The slow release nature of Zyban SR™ raises questions about management of overdose, and in particular, the length of observation time.
32 ACUTE TOXICITY OF ORAL METHYLPHENIDATE (MP) OVERDOSE IN SWITZERLAND
Koller M, Schnorf-Huber S, Kupferschmidt H, Meier-Abt PJ
Swiss Toxicological Information Centre, Zurich (STIC), and Division of Clinical Pharmacology and Toxicology, Department of Medicine University Hospital Zurich, Switzerland
Objectives: MP is increasingly used for the treatment of attention deficit hyperactivity disorder (ADHD). In Switzerland, prescriptions of MP have doubled during the last 5 years leading to increasing concerns about MP poisoning. The aim of this study was to investigate the symptoms and the outcome of MP poisoning. Methods: All feedback reports from physicians on patients with acute oral MP poisonings between 1968 and 2001 were analyzed retrospectively. Only cases with MP monointoxications containing sufficient information regarding ingested dose and symptoms were included. Results were statistically analyzed by logistic regression with age, dose and decontamination (no or late decontamination vs. early decontamination <1 hr) as independent variables. Intoxication severity was assessed according to the EAPCCT/EC/IPCS Poisoning Severity Score (Persson et al. J. Toxicol. Clin. Toxicol. 1998, 36, 205–213). Results: Among 149 cases with MP monointoxications 39 cases were included into the study. 26 (67%) cases were male, and 13 (32%) cases were female. Age ranged from 2.5 to 68 years (median 13.0). 21 patients received gastrointestinal decontamination within 1 hr post-ingestion. The circumstances of poisoning were unintentional in 12 (31%), intentional in 16 (41%), and others in 11 (28%) cases. 11 (28%) patients remained asymptomatic, whereas 72% of the patients developed either mild (16 cases, 41%) or moderate (12 cases, 31%) symptoms. The most frequent symptoms were neurological (51%), cardiovascular (46%) and/or ocular (13%). Symptoms included tachycardia (33%), agitation (26%), mydriasis (13%), hallucinations/disorientation/delir (8%), tremor (7%), and dizziness (7%). The doses of MP ranged from 0.9 mg/kg to 31.1 mg/kg with a median of 2.55 mg/kg. No significant associations were found between severity and dose (p=0.0535), age and/or decontamination. Conclusion: MP poisoning is relatively benign in most cases. At doses up to 31.1 mg/kg MP, moderate symptoms such as agitation, hallucinations, disorientation and/or marked tachycardia occur in a minority of patients.
33 POISONING FROM NATURAL DRUGS REDISCOVERED FOR RECREATIONAL ABUSE
Hruby K
Poisons Information Centre, Vienna, Austria
Objective: The variety of recreational drugs has been growing during the past years. Drugs from plant origin are cheap and easily available. During the recent years poisonings from overdoses of herbal or fungal drugs have been reported in several European countries. In order to investigate this toxicological facet more closely the databases of the Austrian Poisons Information Centre were analysed as to this subject. Methods: The current literature was screened for case reports and the centre's databases were searched for consultations in reference to drugs taken as psychostimulants or hallucinogens other than conventional designer drugs or illicit narcotics covering the years 1997 through 2000. Furthermore, details regarding the origin of drug, course of poisoning, symptomatology and severity were analysed. Results: Some 20 reports on poisonings from biological drugs taken as recreational intoxicants have been retrieved from the recent European literature. In order to examine this issue more closely telephone enquiries at the Austrian Poisons Information Centre referring to poisonings involving abused drugs of natural origin were assessed. The following species were found to be involved with varying frequency: Psilocybe species, A. muscaria and A. pantherina, Atropa belladonna and datura species, Myristica fragrans, Merremia tuberosa and Argyreia nervosa. Usually, the drugs are taken deliberately as stimulating intoxicants but exceeding the suitable dosage and thus causing toxic symptoms. Due to the occurrence of serious symptoms some patients require specific medical treatment. Those agents causing the most severe troubles appear to be Datura species, and even Myristica fragrans frequently causing an anticholinergic syndrome. Symptoms produced by the others present themselves less threatening mostly being confined to psychotic states. Conclusion: The study reveals a new trend towards natural drugs used by young people for recreational purposes. Difficult controllability and lacking knowledge of the suitable dose when trying to produce a psychedelic state on a more or less experimental basis are the main causes for poisonings with this category of drugs. The occurrence of life-threatening states especially induced by drugs with anticholinergic properties demonstrates the importance of an accurate risk assessment and efficient treatment regimen in such instances.
34 LESSONS LEARNED FROM USE AND ABUSE OF DRUGS AMONG ARTISTS
Ludwig T,1 Borron SW,2 Kite-Powell HL3
1Hamburgisches Krankenhaus Bevensen, 29549 Bad Bevensen, Germany. 2Réanimation Médicinale et Toxicologique, Hôpital Fernand Widal, Paris, France, 3Woods Hole, Massachusetts, USA
Objective: Why do people take drugs?
To alter their mood. Mood or spirit are sometimes thought to be ethereal, divorced from physical being. Yet in practice, people tend to prefer drugs over inspiration as a means to alter their mood or achieve euphoria. Is this different for people with a more advanced spiritual background and capacity, such as artists? Case studies: Composers like Satie and Schumann made a career of lifelong alcohol use and abuse which influenced their creations and impaired their health. Willem van Gogh had a series of risky dates with his green fairy, Absinth. The role of other drugs like cocaine and morphine in the lives of various artists is less evident but still detectable. Preconditions of addiction can be seen in Chopin's and Debussy's lives, in their primary personality, their fate, and their diseases. Addiction, or dependency, evidently influenced both their creativity and way of living? Decisions? as to what drug to take seemingly depend on the type of personality: Debussy was a sanguine egocentric person. During his Bohémian period, he felt uncomfortable with his artistic acceptance, switched from day to night life, and began to take the fashionable drug of that time in Parisien cafés: Cocaine. This resulted in boosted self-confidence and social interaction but also in simultaneously hectic and dawdling behavior, a loss of noble inclination, affairs, deceptions, near-criminal violence, and finally, insomnia and anxiety. He also tried morphine, and tragically had to take huge quantities of it in his last years, suffering from carcinoma. To our surprise and admiration, even then, under awful pain and with his certain death in front of his eyes, he still created wonderful music, including his most marvellous and deepest pieces. Chopin was a noble melancholy man with strong self-confidence in his unique art as both composer and pianist. With Debussy, he shared a lack of tolerance for frustration. When he suffered from tuberculosis, and was given repeated opium medications to mitigate bleeding from the lungs, he soon turned out to be prone to morphine addiction. Opium was apt to compensate for his personal losses in love. Unlike other artists who suffered from tuberculosis but were not opium-dependent, he soon exhibited a deplorable decline in his creative work. Chopin's personal reaction eventually was resignation. Conclusions: We find similar consequences of drug abuse among artists and other people. The connection between chemicals and spiritual changes reinforces our belief that “spirit” exists only as a result of functioning neuronal nets: a strong enough reason to protect them from drugs.
35 NAPOLEON'S DEATH—WAS IT ARSENIC POISONING?
Zilker Th,1 Lin X,2 Henkelmann R2
1Toxicological Department II. Med. Clinic, Technical University Munich. 2Institute for Radiochemistry of the Technical University Munich, Germany
Introduction/Method: The suspicion that Napoleon was poisoned by his friend Earl of Montolon on behalf of the English was fostered for centuries. Since it was possible to measure arsenic in hair, Napoleon's hair always showed elevated levels compared to a general population. We were now able to measure arsenic in a normal population, in 3 hairs of Napoleon, in the hair of Montolon's wife who lived with Napoleon and in a homicidal chronic non fatal poisoning by a neutron activating analysis method. Case report of the homicidal attempt: In 1996 the daughter of the latter victim fell in love with a young man. This man moved into the house of the family. In 1999 the parents of the girl threw him out. The daughter and her friend took a flat on their own. As the daughter still had the key to the house of her parents, the young man took his revenge by coming secretly into their house and by poisoning the food with arsenic. Between October and December 2000 the food was poisoned 6 times. Whenever the father ate some of this food he started to vomit. On 22th of December 2000 he got the last dose. He developed severe polyneuropathy, edema, muscular weakness, loss of sense of vibration and cold feet. After he showed Mees lines in his fingernails arsenic poisoning could be verified by arsenic detection in blood and urine. He came to our department in February. At the end of February hair was taken for arsenic detection. Results: It was possible now to compare the arsenic content of a real chronic homicidal arsenic poisoning with the hair of Napoleon, Mme Montolon and a general population of the presence. Arsenic in hair of the general population was found to be 0.5–0.8 μg/g. Napoleon's hair showed 3 μg/g, Mme Montolon's hair contained 5 μg/g. Our patient's hair was cut into 4 pieces of 1 cm length. From this the following calculation could be made: In November the hair contained 40.4 μg/g, in December 49.7 μg/g. After the discontinuation of the arsenic intake the arsenic concentration in hair was 23.9 μg/g in January and 4.35 μg/g in February. Conclusion: It is unlikely that Napoleon was poisoned by arsenic. At least 3 month prior to his death no arsenic could have been administered to him. As Mme Montolon showed higher levels than Napoleon and did not show signs of arsenic poisoning it is very likely that the arsenic, found in them, stemmed from environmental sources.
36 MECHANISMS OF HEPATOTOXICITY
Tenenbein, M
University of Manitoba, Winnipeg, Manitoba, Canada
Introduction: Injury to the liver is a potential outcome of exposure to many drugs, chemicals and natural toxins. The exposure may be acute or chronic and the mechanism may be dose-related or idiosyncratic. My objective is to discuss the mechanisms of hepatotoxicity due to acute exposures to toxins. The more common type of hepatotoxicity, a consequence of subacute or chronic exposures, will not be discussed. Anatomy and physiology of the liver: The liver is the largest organ in the body and is essential for life. Its average weight is 1200–1500 grams or approximately 2% of total body weight. Knowledge of hepatic histology is essential for insight into various mechanisms of hepatotoxicity. The liver is classically considered to be composed of polyhedral lobules with a central hepatic vein and 4–5 portal triads in the periphery. However, Rappaport's hepatic acinus model is more physiologically sound. In this model, the portal triad is central with hepatic veins in the periphery. The acinus is divided into 3 zones with Zone 1 being closest to the portal triad and Zone 3 being the furthest. The basis for this differentiation is the functional heterogeneity of hepatocytes. There is a non-homogenous distribution of enzymes with those of the mixed function oxidase system being concentrated in Zone 3. Furthermore, there is a non-homogenous distribution of substrate with the highest concentration of drugs and oxygen found in Zone 1. The liver regenerates from Zone 1 cells. These differences have profound implications regarding mechanisms and prognosis of hepatotoxicity due to various agents. The liver is the most metabolically complex organ with important functions including glucose homeostasis, protein synthesis, lipid synthesis, bile synthesis and excretion, reticular endothelial system function and detoxification of exogenous substances. Vulnerability of the liver to toxic damage: There are several reasons why the liver is particularly vulnerable to toxic injury. There is a high concentration of substances in the liver, the liver is a portal to the rest of the body, biliary excretion puts it at risk for cholestatic injury and it is the site of metabolism of foreign compounds. Metabolism of xenobiotics: The metabolism of foreign compounds is the most important etiologic factor for hepatotoxicity because many of the intermediary species are potentially toxic. Indeed, it can be said that most hepatotoxic agents are “pretoxins” that must be converted to “reactive toxins”. Most drugs are non-polar molecules and therefore cannot be efficiently excreted by the body. To prevent accumulation of the agents, they must be converted to polar agents. This is a two-stage process consisting of Stage I, or bioactivation, and Stage II, or detoxification. Stage I reactions, consisting of oxidations, reductions or hydrolyses, create reactive species that undergo conjugation with endogenous substances by Phase II reactions. Glucuronidation, sulfation, and complexation with glutathione are common examples of Phase II reactions. The result is a polar molecule that can be excreted in the urine. Mechanisms of hepatotoxicity: Hepatotoxicity occurs when Phase II processes are insufficient to cope with the output of Phase I activity. The resultant excess of bioactive intermediary metabolites can then produce tissue damage. Examples include electrophilic species covalently bonding to macromolecules and free radicals inducing lipid peroxidation. The cellular targets include the cytoskeleton, mitochondria and cellular ion homeostasis. The outcome is hepatic necrosis. Outcomes other than necrosis include steatosis, cholestasis and inflammation and each have a different pathophysiology. Steatosis is a result of decreased egress of fat from the hepatocyte. This is a consequence of the toxin interfering with the synthesis of the apoprotein required for fat transport. Cholestasis can be a consequence of toxin induced damage to bile synthesis, to bile transport or to the biliary tree. As this is typically an outcome of chronic rather acute drug exposure, it is beyond the scope of this review. Inflammation is the outcome of an autoimmune reaction with the drug, or more often a metabolite of the drug, acting as a hapten. Classification of hepatotoxicity: Hepatotoxicity defies simple classification. Potential schemes such as chemical structure of the toxins, sources of the agent, circumstances of exposure, mechanism of injury or type of liver damage all have shortcomings. A matrix scheme consisting of pathophysiology combined with histopathology is a useful method of classification. The pathophysiologic descriptors are dose-related vs. idiosyncratic and the histopathologic ones are necrosis (zonal or diffuse), steatosis, cholestasis and inflammation. It is important to note that not all instances of idiosyncratic hepatotoxicity are consequences of hypersensitivity. It can be a consequence of a metabolic aberration due to a pharmacogenetic abnormality. Typically, this would be an enzyme deficiency which results in an excess of a toxic metabolite. Illustrative examples: Five hepatotoxins are illustrative of various mechanisms of hepatotoxicity. Because iron concentration is highest in Zone 1, the necrosis due to lipid peroxidation induced by iron generated free radicals, occurs in this zone. Acetaminophen produces Zone 3 necrosis due to insufficient Phase II conjugation of the Phase I generated electrophilic radicals that subsequently covalently bond to hepatic macromolecules. Carbon tetrachloride produces Zone 3 necrosis and generalized steatosis due to Phase I generation of free radicals and to injury of protein synthesis machinery, respectively. Amatoxins produce steatosis and necrosis due to inhibition of protein synthesis. Aromatic anticonvulsants such as phenytoin produce generalized inflammation and necrosis. This is an idiosyncratic reaction due to a pharmacogenetic decrease of Phase II activity. The accumulated bioactive metabolite binds to cellular protein and acts as a hapten. The neoantigen induces an autoimmune inflammatory response. This is known as the Anticonvulsant Hypersensitivity Syndrome. Conclusion: The liver's central role in the biotransformation of xenobiotics places it at risk for drug induced hepatic disease. Hepatotoxicity due to acute exposures to toxins is usually a consequence of insufficient Phase II detoxification, although other mechanisms can occur. An appreciation of these mechanisms is the foundation for rational patient management.
References: 1. Lewis J.H., Ed. Drug Induced Liver Disease. Gastroenterol. Clin. N. Am. 1995, 24 (4). 2. Zimmerman, H.J. Hepatotoxicity. Appleton-Century-Crofts: New York, 1978.
37 THE MOLECULAR BASIS OF TOXICITY OF NUCLEOSIDE ANALOGS AND THEIR ADVERSE CLINICAL EFFECTS
Delaney KA
University of Texas Southwestern Medical School, Dallas, Texas, USA
Objectives: In 1994 an NIH study of the nucleoside analog fialuridine for treatment of chronic hepatitis B was halted due to the development of severe hepatotoxicity in 7/15 study patients. Five patients died. Since that time numerous cases of liver injury associated with treatment of HIV infection with nucleoside analogs that inhibit reverse transcriptase (esp zidovudine, zalcitabine and didanosine) have been recognized. Common clinical features of these cases include severe lactic acidosis with minimal to moderate elevation of hepatocellular enzymes and bilirubin, and failure of hepatic synthetic function. Microscopic examinations of liver specimens show marked microvesicular steatosis with minimal necrosis or structural injury. Clinically these findings suggest a disorder of mitochondrial function, with lactic acidosis as a biochemical manifestation of impaired energy production, and microvesicular steatosis a reflection of related failure of fatty acid metabolism. In addition to liver cell injury, myopathy that is histologically similar to rare myopathies associated with genetic disorders of mitochondrial function occurs in 10% of AIDS patients who take reverse transcriptase inhibitors. HIV-associated lipodystrophy is yet another disorder similar to the well-defined mitochondrial disorder known as multiple symmetric lipomatosis. In addition to clinical similarities to genetic disorders of mitochondrial function, the likelihood of primary impairment of mitochondrial function in these cases is supported by the abnormal electron microscopic appearance of hepatocellular mitochondria in patients with liver injury related to this type of antiretroviral therapy. The object of this presentation is to review histological and clinical manifestations of the cellular toxicity of nucleoside analogs, and then present the results of recent basic science studies that attempt to define the molecular basis of this toxicity. Methods: Review of original case reports and recent basic science investigations of molecular causes of liver injury induced by nucleoside analogs. Results: The hepatic histological manifestation of microvesicular steatosis is similar to that of disorders attributed to mitochondrial toxicity, such as Reye's syndrome, or hepatic toxicity associated with exposure to Bacillus cereus or hypoglycin. AZT has been shown to deplete mitochondrial DNA, probably by “unwitting” impairment of the mitochondrial DNA replicating machinery along with HIV's replication apparatus. More specifically, in vitro studies have demonstrated that inhibitors of HIV reverse transcriptase also inhibit DNA polymerase gamma (the polymerase responsible for replication of mitochondrial DNA), and other mitochondrial enzymes such as adenylate kinase that are involved in the synthesis of ATP and translocation of nucleoside precursors of ATP. The toxicity of reverse transcriptase inhibitors has been shown to correlate with their in vitro capacity to inhibit polymerase gamma. Conclusions: There is mounting evidence that the toxicity of reverse transcriptase inhibitors is due to their unintended capacity to also inhibit mitochondrial DNA polymerase, resulting in mitochondrial dysfunction and manifestations of impaired cellular energy production such as lactic acidosis, impaired B-oxidation of fatty acids, myopathy, and lipodystrophy.
References: 1. Schon, E.A.; Dimauro, S. The Other DNA: Research on Mitochondrial Diseases. http://www.columbia.edu/cu/21stC/issue-1.3/dna-mitoch.html. 2. Chattha, G., Arieff, A.I.; Cummings, C.; Tierney, L.M., Jr. Lactic Acidosis Complicating the Acquired Immunodeficiency Syndrome. Ann. Intern. Med. 1993, 118, 37–39. 3. Feng, J.Y.; Johnson, A.A.; Johnson, K.A.; Anderson, K.S. Insights into the Molecular Mechanism of Mitochondrial Toxicity by AIDS Drugs. J. Biol. Chem. 2001, 276, 23832–23837. 4. Fromenty, B.; Pessayre, D. Inhibition of Mitochondrial beta-Oxidation as a Mechanism of Hepatotoxicity. Pharmacol. Ther. 1995, 67, 101–154. 5. Freiman, J.P.; Helfert, K.E.; Hamrell, M.R.; Stein, D.S. Hepatomegaly with Severe Steatosis in HIV-Seropositive Patients. AIDS 1993, 7, 379–385. 6. Gradon, J.D.; Chapnick, E.K.; Sepkowitz, D.V. Zidovudine-Induced Hepatitis. J. Intern. Med. 1992, 231, 317–318. 7. Kakuda, T.N. Pharmacology of Nucleoside and Nucleotide Reverse Transcriptase Inhibitor-Induced Mitochondrial Toxicity. Clin. Ther. 2000, 22, 685–708. 8. Lai, K.K.; Gang, D.L.; Zawacki, J.K.; Cooley, T.P. Fulminant Hepatic Failure Associated with 2′,3′-Dideoxyinosine (ddI). Ann. Intern. Med. 1991, 115, 283–284. 9. McKenzie, R.; Fried, M.W.; Sallie, R. et al. Hepatic Failure and Lactic Acidosis Due to Fialuridine (FIAU), An Investigational Nucleoside Analogue for Chronic Hepatitis B. N. Engl. J. Med. 1995, 333, 1099–1105. 10. Schafer, F.; Sorrell, M.F. Power Failure, Liver Failure. N. Engl. J. Med. 1997, 336, 1173–1174.
38 MECHANISMS OF HEPATOCELLULAR DAMAGE FROM NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS)
Jones AL
National Poisons Information Service (London), Guy's and St Thomas' NHS Trust, London, United Kingdom
Introduction: Nonsteroidal anti-inflammatory drugs are the centerpiece of pharmacotherapy for most rheumatic disorders and are used in large numbers. The epidemiological risk of clinically apparent liver injury is low i.e. 1 case per 10,000 patient years of NSAID use but when it occurs, it can be serious and can cause diagnostic confusion.1,2,3 Clinical trials that employ routine monitoring of liver function have the highest likelihood of ascertainment of any liver function abnormalities but the usefulness of these studies is often limited by small numbers of patients and limited exposure times as hepatoxicity may be asymptomatic.4 Few trials use uniform criteria for hepatoxicity and the incidence rate of adverse liver events is greatly dependent on this and confounds attempts to compare data between trials on different NSAIDs. Nearly all of the NSAIDs have been implicated in causing liver injury but reports of serious liver toxicity with ibuprofen are rare, though tend to be hepatocellular in nature and the mechanism is thought to be immunological idiosyncrasy.5,6 Sulindac use was associated with a 5–10 fold higher incidence of hepatic injury than other NSAIDS.1 Several NSAIDs have been withdrawn from clinical use because of associated hepatoxicity.7,8,9 The new more selective Cox 2 inhibitors (e.g. celecoxib) are also associated with hepatoxicity10, though are said to have less potential for hepatoxicity11 Hepatoxicity from NSAIDs can occur at any time after drug administration, but most commonly occurs within several weeks of initiation of therapy. In investigation of apparent NSAID induced hepatoxicity it is critical to exclude viral causes (Hepatitis A, B, C, HIV, CMV) and other liver diseases (by measuring serum IgG, IgA, IgM, C3, C4, ANF, antimitochondrial antibody, anti-smooth muscle antibody, rheumatoid factor, caeruloplasmin, ferritin and iron). Fulminant hepatic failure occurs in a tiny proportion of individuals exposed and is often not recognised as a possible adverse event until the post-marketing stage.9,12,13,14 Clinical features of hepatotoxicity due to NSAIDs: Two main clinical patterns of hepatotoxicity due to NSAIDs occur.6 Acute hepatitis with greatly elevated transaminases and jaundice, fever, nausea and sometimes eosinophilia occurs. The alternative pattern is that with serological (ANF +ve) and histological features (periportal inflammation with plasma and lymphocyte infiltration and fibrosis extending into the lobule) of chronic active hepatitis. Mechanism of hepatotoxicity of NSAIDs: Two main mechanisms are responsible for injury; hypersensitivity or metabolic aberration. Risk factors for NSAID-induced idiosyncratic hepatotoxicity include female sex, age>50 years, underlying autoimmune disease. Another risk factor is concomitant exposure to other hepatotoxic drugs.15 Patients who have experienced hepatotoxicity to one NSAID, often have the same reaction if the drug is restarted and the same reaction when a sister drug is given, particularly if the sister drug is structurally similar e.g. diclofenac and tiaprofenic acid. Hypersensitivity reactions often have significant anti-nuclear factor or anti-smooth muscle antibody titres, lymphadenopathy and eosinophilia. Rechallenge with the drug results in a repeat increase in anti-nuclear factor titre. A recent rechallenge from diclofenac generic and non generic prescribing resulted in liver transplantation for a patient.16 Metabolic aberrations can occur as genetic polymorphisms alter susceptibility to a wide range of drugs. It may account for the incidence ratio of 1 per 100,000 prescriptions of NSAIDs. In vitro metabolism of aceclofenac reflects phenotypic variability amongst donor liver cells.17 Recently, it has been found using rat liver mitochondria and freshly isolated rat hepatocytes that diphenylamine, which is common in the structure of NSAIDs, uncouples oxidative phosphorylation and decreases hepatic ATP content and induces hepatocyte injury.18,19 Incubation of mitochondria with diphenylamine, mefenamic acid or diclofenac caused mitochondrial swelling. A spectral shift of the safranine-binding spectra to mitochondria indicated the loss of mitochondrial membrane potentials (one of the characteristics for uncouplers of oxidative phosphorylation). Addition of oligomycin, which blocks ATPase, protected against cell injury.18 In dicofenac induced toxicity in hepatocytes, no significant oxidative stress (decrease in glutathione and lipid peroxidation) or increase in intracellular calcium concentration was seen.19 Diclofenac was more cytotoxic to drug metabolising cells than to non-metabolising cell lines (HepG2, FaO).19 Toxicity thus relates both to impairment of ATP synthesis by mitochondria and related to drug metabolism i.e. it was reduced by the addition of cytochrome P450 inhibitors (prothiaden and ketoconazole to culture medium).19 The in vitro cytotoxicity correlated well with the formation of 5-hydroxydiclofenac and particularly the n,5-dihydroxydiclofenac metabolite—the latter in particular can inhibit ATP synthesis.19 Conclusions: It is important to be vigilant to the hepatotoxic potential of any NSAID, as increased awareness, surveillance and reporting of these events can lead to better understanding of the risk factors and pathophysiology associated with NSAID toxicity. At risk groups for idiosyncratic hepatotoxicity have been identified. Idiosyncratic reactions due to hypersensitivity or metabolic aberration are responsible for toxicity in the vast majority of cases. Although hepatotoxicity is listed as a class warning for NSAIDS, diclofenac and sulindac are most commonly associated with the problem.20 Successful orthotopic liver transplantation may be undertaken in patients with fulminant hepatic failure due to NSAIDS.
References: 1. Walker, A.M. Quantitative Studies of the Risk of Serious Hepatic Injury in Persons Using Nonsteroidal Anti-Inflammatory Drugs. Arthritis Rheum. 1997, 40, 201–208. 2. Manoukian, A.V.; Carson, J.L. Nonsteroidal Anti-Inflammatory Drug-Induced Hepatic Disorders. Incidence and Prevention. Drug Safety 1996, 15, 4–71. 3. Garcia-Rodriguez, L.A.; Williams, R.; Derby, L.E.; Dean, A.D.; Jick, H. Acute Liver Injury Associated with Nonsteriodal Anti-Inflammatory Drugs and the Role of Risk Factors. Arch. Int. Med. 1994, 154, 311–316. 4. Dierkes-Globisch, A.; Schafer, R.; Mohr, H.H. Asymptomatic Diclofenac-Induced Acute Hepatitis. Dtsch. Med. Wochenschr. 2000, 125, 797–800. 5. Zimmerman, H.J. Update on Hepatotoxicity Due to Classes of Drugs in Common Clinical Use: Non-Steroidal Drugs, Anti-Inflammatory Drugs, Antibiotics, Antihypertensives and Cardiac and Psychotropic Agents. Seminars in Liver Disease. 1990, 10, 322–338. 6. Rabinovitz, M.; Van Thiel, D.H. Hepatotoxicity of Non-Steroidal Anti-inflammatory Drugs. Am. J. Gastroenterol. 1992, 87, 1696–1704. 7. Koff, R.S. Liver Disease Induced by Nonsteroidal Anti-inflammatory Drugs. In: A Profile of Adverse Effects, Borda, I.T.; Koff, R.S.; Eds.; Hanley and Belfus: Philadelphia, 1992, 133–145. 8. Boelsterli, U.A.; Zimmerman, H.J.; Kretz-Rommel, A. Idiosyncratic Liver Toxicity of Nonsteroidal Anti-inflammatory Drugs: Molecular Mechanism and Pathology. Crit. Rev. Toxicol. 1995, 25, 207–235. 9. Rabkin, J.M.; Smith, M.J.; Orloff, S.L.; Corless, C.L.; Stenzel, P.; Olyaei, A.J. Fatal Fulminant Hepatitis Associated with Bromfenac Use. Ann. Pharmacother. 1999, 33, 945–94. 10. Nachimuthu, S.; Volfinzon, L.; Gopal, L. Acute Hepatocellular and Cholestatic Injury in a Patient Taking Celecoxib. Postgrad. Med. J. 2001, 77, 548–550. 11. Maddrey, W.C.; Maurath, C.J.; Verburg, K.M.; Geis, G.S. The Hepatic Safety and Tolerability of the Novel Cyclo-Oxygenase-2 Inhibitor Celecoxib. Am. J. Ther. 2000, 7, 153–158. 12. Purdum, P.P.; Shelden, S.L.; Boyd, J.W.; Shiffman, M.L. Oxaprozin-Induced Fulminant Hepatitis. Ann. Pharmacother. 1994, 28, 1159–1161. 13. Jones, A.L.; Latham T.; Shallcross, T.J.; Simpson, K.J. Fulminant Hepatic Failure Due to Diclofenac Treated Successfully by Orthotopic Liver Transplantation. Transplantation Proc. 1998, 30, 192–194. 14. Merlani, G.; Fox M.; Oehen, H.P.; Cathomas, G.; Renner, E.L.; Fattinger, K.; Schneeman, M.; Kullak-Ublick, G.A. Fatal Hepatotoxicity Secondary to Nimesulide. Eur. J. Clin. Pharmacol. 2001, 57, 321–326. 15. Bareille, M.P.; Montastruc, J.L.; Lapeyre-Mestre, M. Liver Damage and Nonsteroidal Anti-Inflammatory Drugs: Case Non-case Study in the French Pharmacovigilance Database. Therapie 2001, 56, 51–55. 16. Greaves, R.R.; Agarwal, A.; Patch, D.; Davies, S.E.; Sherman, D.; Reynolds, N.; Rolles, K.; Davidson, B.R.; Burroughs, A.K. Inadvertent Diclofenac Rechallenge from Generic and Non-generic Prescribing, Leading to Liver Transplantation for Fulminant Liver Failure. Eur. J. Gastroenterol. Hepatol. 2001, 13, 71–73. 17. Ponsoda, X.; Pareja, E.; Gomez-Lechon, M.J.; Fabra, R.; Carrasco, E.; Trullenque, R.; Castell, J.V. Drug Biotransformation by Human Hepatocytes. In Vitro/ In Vivo Metabolism by Cells from the Same Donor. J. Hepatol. 2001, 34, 19–24. 18. Matsubuchi, Y.; Yamada, S.; Horie, T. Possible Mechanisms of Hepatocyte Injury Induced by Diphenylamine and Its Structurally Related, N. SAIDS. J. Pharmacol. Exp. Ther. 2000, 292, 982–987. 19. Bort, R.; Ponsoda, X.; Jover, R.; Gomez-Lechon, M.J.; Castell, J.V. Diclofenac Toxicity to Hepatocytes: A Role for Drug Metabolism in Cell Toxicity. J. Pharmacol. Exp. Ther. 1999, 288, 65–72. 20. Bjorkman, D. Nonsteroidal Anti-inflammatory Drug Associated Toxicity of the Liver, Lower Gastrointestinal Tract and the Esophagus. Am. J. Med. 1998, 105, 17S–21S.
39 VULNERABILITY TO ACETAMINOPHEN (PARACETAMOL) LIVER INJURY DURING THERAPEUTIC DOSING: DOES IT EXIST?
Dart RC
Rocky Mountain Poison and Drug Center, Denver Health, Denver, Colorado, USA
Objectives: The potential toxicity of acetaminophen (paracetamol) in the setting of therapeutic use or “low dose” overdose ingestion has generated intense controversy. If rare cases of fulminant hepatic failure occur during therapeutic use, regulatory authorities have suggested that it may need to be withdrawn from the over-the-counter analgesic market. Based on its metabolism, several patients groups with enhanced vulnerability to acetaminophen toxicity have been proposed: the alcoholic patient, the patient treated with anticonvulsants, the starved or malnourished patient, and the HIV-infected patient. Each of these conditions either increases the production of N-acetylparabenzoquinoneamine (NAPQI) or reduces the cellular supply of glutathione or both. Of these groups, the alcoholic patient is perhaps the most vulnerable because both increased NAPQI production and decreased hepatocellular stores of glutathione. Methods: Using the alcoholic patient as a prototypical vulnerable population, a formal systematic review was performed to assess the evidence available documenting the vulnerability of alcoholic patients to acetaminophen toxicity following ingestion of therapeutic doses (cumulative dose of 4 grams/day or less). The question examined was “Is the use of repeated therapeutic doses of acetaminophen by the alcoholic patient associated with any degree of liver injury?” Data sources included Medline, EMBASE as well as data submitted to the US Food and Drug Administration. Over 7000 articles and reports were individually retrieved, categorized and abstracted using a standardized procedure. Articles involving any mention of alcohol ingestion and ingestion of one or more doses of acetaminophen were identified and analyzed. Results: A clear-cut dichotomy became apparent: prospectively designed studies found no effect in the alcoholic patient following repeated ingestion of therapeutic doses of acetaminophen, however, there are few studies available. In contrast, most retrospective papers reported an association of fulminant hepatic injury with the therapeutic use of acetaminophen in the alcoholic patient. Most retrospective data were individual case reports or small case series. Randomized prospective trials and the best retrospective data will be presented. Evaluation of individual reports revealed several observations that could reduce toxicity from acetaminophen. Use in children and adults present different challenges. Further analysis showed similar results for patients treated with anticonvulsants, starved or malnourished patients, and patients with HIV disease. Prospective data were few, however, they also failed to document liver injury following repeated ingestion of therapeutic doses. In contrast, all cases associating injury from therapeutic use of acetaminophen were retrospective and relied solely on the patient history to determine the dose. Many methodological issues were identified: confounding (22% of American adults use acetaminophen each week), historical inaccuracy (many reports included evidence, such as markedly elevated acetaminophen levels, that conflicted with the history), the improving veracity of data based entirely on the history of ingestion and several other factors. It appears likely that the fundamental issue is the difference between therapeutic intent and a true therapeutic dose. Acetaminophen may be used for therapeutic intent but in doses that greatly exceed the therapeutic dose. Another issue is confusion about the threshold concept of acetaminophen toxicity. For toxicity to occur, severe glutathione depletion beyond the threshold of injury must occur. Simple increased production of NAPQI is not sufficient. Conclusion: The data suggest that susceptibility to acetaminophen toxicity may exist for selected groups, however, convincing data of acceptable rigor indicating a risk from therapeutic doses of acetaminophen in any of these patient groups have not been reported. Further research needs to use prospective research design or methods to identify the ingested dose more accurately.
40 TREATMENT STRATEGIES FOR TOXIN-INDUCED LIVER INJURY
Brent J
Toxicology Associates, University of Colorado Health Sciences Center, Denver, Colorado, USA
Toxin-induced liver injury affects not only the liver but significantly the central nervous system (CNS). These CNS manifestations are often the most prominent clinical feature of hepatic injury and the most common cause of death. The CNS effects fall under the general rubric of hepatic encephalopathy (HE). The physiological disturbances associated with toxin-induced hepatic injury involve a loss of the synthetic and detoxifying abilities of the liver, leading to elevation in serum ammonia, endogenous opioids and benzodiazepines (BZPs), mercaptans, phenols, and short and medium chain fatty acids; alteration in plasma amino acid composition, and decreased coagulation factors. Among the consequences of these disturbances is the loss of integrity of the blood brain barrier (BBB). Most of the evidence implicates elevation of the serum ammonia concentration as being responsible for this effect. The consequences of the defective BBB leading directly to the HE, include an increase in manganese and the various circulating products enumerated above, into the brain, ultimately causing astrocytopathy and cerebral edema. Astrocytes have a major role in the regulation of brain volume and it is the metabolic abnormalities in them that directly gives rise to the cerebral edema. Since the astrocytes regulate the concentration of glutamate, the astrocytopathy leads to increased synaptic glutamate concentrations and stimulation of glutamate receptors. There is a direct correlation between increased ammonia concentrations and the likelihood of brain stem herniation.1 Decreasing serum ammonia levels is protective against cerebral edema.2 The treatment of toxin-induced hepatic injury encompasses standard supportive measures, the use of various experimental therapies, artificial hepatic support devices, and transplantation. The supportive treatment generally consists of early intubation, monitoring, and control of intracranial pressure, and serum osmolarity, and the avoidance of seizures. Attempts to reduce serum ammonia concentration generally consist of the administration of antibiotics to reduce ammonia production by gut flora and lactulose to enhance gastrointestinal ammonia excretion. There have been multiple experimental therapies proposed for the treatment of hepatic injury. N-acetylcysteine diffuses into hepatocytes and acts as a precursor for glutathione, is an antioxidant, a TNF-alpha antagonist, and increases hepatic oxygen delivery and extraction. It has not, however, been shown to enhance survival in hepatic injury except for hepatotoxins such as acetaminophen, where its use has been validated. Because patients with hepatic injury have decreased branch chain amino acids (BCAA) there is concern that the predominant amino acid species might be the aromatic potentially false neurotransmitter group. Thus changing the amino acid profile to increase the BCAA component might be beneficial. However, although they may improve encephalopathy they have no effect on survival. The BCAA may be used to maintain nitrogen balance during protein restriction, which is common in patients with hepatic injury. This seems to be their primary beneficial effect. Although there are increased levels of circulating endogenous BZDs there is little to support the use of flumazenil in this condition. Although there have been numerous studies that have shown it to be better than placebo, all have methodological flaws. Other studies have not been able to verify this efficacy. Because flumazenil's half-life is short there would be no long-lasting effect anticipated. Similarly, although there are increased levels of circulating endogenous opioids in hepatic injury the use of opioid receptor antagonists has not been evaluated adequately in clinical trials. L-ornithine-L-aspartate (LOLA) is a substrate for the urea cycle thus decreasing serum ammonia concentrations and cerebral edema in experimental models of acute hepatic injury.2 Sodium benzoate has been used for treating hyper-ammoniemia in patients with congenital defects of ammonia metabolism. Benzoate reacts with ammonia to form hippurate, which is renally excreted. There are few adverse effects associated with this therapy except for the increased sodium load. The degree to which this strategy is helpful in the treatment of toxin-induced hepatic injury is unknown. L-carnitine has been attempted as a treatment aimed at lowering serum ammonia concentrations. However, results of clinical trials with this substance have been conflicting. Voglibose (AO-128) is a disaccharidase inhibitor that thus causes otherwise digestible disaccharides to act like exogenous lactulose. Its effect in toxin-induced hepatic injury is unknown. Two of the five enzymes in the urea cycle are zinc-dependent. However, there is no evidence that zinc concentrations are limiting for enzyme activity. Thus, zinc supplementation should be done only in cases of documented deficiency. Because the major source of serum ammonia is production by urea producing organisms in the gut there have been attempts to alter the bacterial flora of the GI tract with non-urea producing organisms. However, results of this strategy have been inconsistent. Hypothermia protects against ammonia-induced cerebral edema.3 A report in humans with acute liver injury suggest that mild hypothermia, which has the effect of decreasing ammonia transport across the BBB, may protect against cerebral edema.4 However, there is insufficient evidence to support the routine use of this technique. The H-1 antagonist pyrilamine has been demonstrated to improve hepatic encephalopathy in liver failure models in animals.5 It is unknown whether such an effect would occur, or be of clinical utility in humans. Cerebral function and perfusion is improved in animal models of hepatic injury by the inhibition of glutamine synthesis. Glutamine is the primary osmol in astrocytes and thus is directly responsible for the cerebral edema. It is unknown whether inhibition of glutamine synthesis in humans with toxin-induced hepatic injury would be beneficial. Extra-corporeal hepatic support systems include both artificial detoxification systems and the bioartificial liver. Artificial detoxification systems may replace some of the hepatic detoxification functions but do not provide any synthetic activity. None have been shown to have a significant impact on survival. The bioartificial liver systems are intended to both detoxify and supply synthetic functions and have been shown to have efficacy in animal models of acute hepatic injury. There are three systems currently in clinical trials, two use porcine hepatocytes and one uses an immortalized human hepatoblastoma cell culture. There are two clinical trials that have been completed on the bio artificial liver. The Cedars–Sinai trial in Los Angeles is the largest published experience, comprising 31 patients using the BioAssist device, which uses a primary culture of porcine hepatocytes. This study showed improvements in hepatic encephalopathy, intracranial pressure, and several biochemical parameters of liver injury. However cerebral blood flow was not improved nor were factor 5 concentrations or pro-thrombin time. Of the 18 patients in this series with acute liver failure they reported a 94% survival rate. However, this was an uncontrolled trial and the selection criteria were unclear (Watanabe 1997). The Kings College trial was a randomized clinical study using the Hepatix system, which utilizes an immortalized human hepatoblastoma cell culture. This trial randomized 24 patients to receive standard care versus standard care plus artificial liver support and detected no difference in survival between the two groups.
References: 1. Clemensen, J.O.; Larsen, F.S.; Kondrup, J.; et al. Cerebral Herniation in Patients with Acute Liver Failure Is Correlated with Arterial Ammonia Concentration. Hepatology 1999, 29, 648–653. 2. Rose, C.; Michalak, A.; Pannunzio, M. et al. Mild Hypothermia Delays the Onset of Coma and Prevents Brain Edema and Extracellular Brain Glutamate Accumulation in Rats with Acute Liver Failure. Hepatology 2000, 31, 872–877. 3. Cordoba, J.; Crespin, J.; Gottstein, J.; Blei, A.T. Mild Hypothermia Modifies Ammonia-Induced Brain Edema in Rats After Portacaval Anastomosis. Gastroenterology 1999, 116, 686–693. 4. Jalan, R.; Olde Damink, S.W.M.; Lee, A.; Hayes, P.C. Moderate Hypothermia for Uncontrolled Intracranial Hypertension in Acute Liver Failure. Lancet 1999, 354, 1164–1168. 5. Lozeva, V.; Valjakka, A.; Lecklin, A. et al. Effects of the Histamine H1 Receptor Blocker, Pyrilamine, on Spontaneous Locomotor Activity on Rats with Long-Term Portacaval Anastomosis. Hepatology 2000, 31, 336–344. 6. Master, S.; Gottstein, J.; Blei, A.T. Cerebral Blood Flow and the Development of Ammonia-Induced Brain Edema in Rats After Portacaval Anastomosis. Hepatology 1999, 30, 876–880. 7. Watanabe, F.D.; Mullon, C.J.; Hewitt, W.R. et al. Clinical Experience with a Bioartificial Liver in the Treatment of Severe Liver Failure. A Phase I Clinical Trial. Ann. Surg. 1997, 225, 484–491. 8. Sussman, N.L.; Chong, M.G.; Koussayer, T. et al. Reversal of Fulminant Hepatic Failure Using an Extracorporeal Liver Assist Device. Hepatology 1992, 16, 60–65.
41 EXTRACORPOREAL ALBUMIN DIALYSIS: AN OPTION IN TREATING FULMINANT HEPATIC FAILURE DUE TO INTOXICATION
Eyer F,1 Felgenhauer N,1 Pfab R,l Kreymann B,2 Zilker Th1
1Toxikologische Abteilung; 2Nephrologische Abteilung der II. Medizinischen Klinik, Technische Universität, München, Germany
Objective: Several intoxications can lead to reversible or irreversible liver failure. Examples are severe paracetamol overdose, ingestion of amatoxin containing mushrooms, MDMA abuse and rare poisons like potassium dichromate and carbon tetrachloride. In these cases extracorporeal hepatic support devices (ECHS) could be used to save time for spontaneous organ regeneration or as a bridging technique until a suitable transplant is available. In our department, three patients with severe irreversible liver failure due to intoxication (Galerina marginata, MDMA and potassium dichromate) were treated with albumin dialysis prior to liver transplant. Case Series: Patient one presented with fatigue and jaundice after MDMA-abuse. Highly elevated transaminases, bilirubinaemia and seriously decreased hepatic function showed progressive liver damage. Other reasons for liver failure were excluded. Despite substitution with fresh frozen plasma and clotting factors, clinical condition worsened and the onset of hepatic encephalopathy made liver transplant necessary. Albumin dialysis was started resulting in stable coagulation, sufficient blood pressure and decreasing hepatic encephalopathy. Liver transplant was successfully achieved ten days after MDMA abuse. Patient two had ingested 17 g potassium dichromate. Beside corrosive damage of the esophagus, stomach and duodenum; the patient developed metabolic acidosis, renal and hepatic failure. Blood exchange and hemo-filtration with DMPS were started, followed by CVVHD. As liver enzymes showed high elevation and encephalopathy ensued, albumin dialysis was started. 19 mg of potassium dichromate-equivalents were removed, ICP showed a decrease from 23 mmHg to 19 mmHg during albumin-dialysis and relapsed to nearly 40 mmHg after dialysis was discontinued. Liver transplant could be done seven days after potassium dichromate ingestion. Patient three consumed a mushroom meal with amatoxin-containing Galerina marginata that he had mistaken for Kuehneromyces mutabilis. A typical gastrointestinal syndrome occurred 10 hours after ingestion followed by signs of progressive liver failure. When visual disorder, asterixis and a slow-wave EEG were registered, albumin dialysis was initiated while the clinical signs remained stable and liver transplant was done seven days after ingestion. Conclusion: In three cases of toxic liver failure, albumin dialysis could be used as a successful bridging technique until liver transplant could be carried out, resulting in stable blood pressure, sufficient coagulation (under substitution) and improving consciousness (HE III° to I°). Successful use of this technique in other cases of acute liver failure is well documented in the literature. Clinical toxicologists should keep in mind this ECHS-technique as a therapeutic option in cases of toxin-related liver damage until spontaneous recovery ensues or liver transplant can be performed.
42 NON-PHARMACOLOGICAL CARDIOACTIVE STEROIDS
Hoffman RS
New York City Poison Center, New York, New York, USA
Objective: Describe the pharmacology, clinical presentation, assessment and treatment of patients exposed to non-pharmacologic cardioactive steroids. Methods: A review of basic research, clinical material, and original research performed by the author were used to identify the following points. Cardiac glycosides comprise a largely homogeneous class of molecules that are formed of a steroid nucleus with an unsaturated lactone ring at the C17 position and at least one sugar molecule attached at the C3 position. Digoxin and digitoxin, the two most commonly prescribed cardiac glycosides, belong to the cardenolide subclass, as defined by having a 5-membered lactone ring attached to their steroid nucleus. While this basic structure is similar to most plant-derived cardiac glycosides, animal-derived molecules such as those found in toads and humans are bufodienolides, denoting a 6-membered lactone ring. Furthermore, since some agents lack the sugar residues, they cannot be called glycosides and are known as aglycones or cardiac genins. As such, the term cardioactive steroid is preferred to cardiac glycoside in that it encompasses all of the compounds under consideration. Over 400 cardioactive steroids are described; most plant-derived agents come from the Figwort (Scrophulariaceae), Lily (Lilliaceae), Milkweed (Asclepiaceae) and Dogbane (Apocyanaceae) families. Additional agents come from common and yellow oleander as well as toads from the Bufo species. All known cardioactive steroids block Na+–K+-ATPase by binding to a specific site on the extracytoplasmic face alpha subunit known as the “oubain binding site”. Binding requires the C17 lactone ring, a hydroxyl group at C14, and a cis fusion of the A–B and C–D rings. These structural requirements distinguish cardioactive steroids from cholesterol and steroid hormones. Although the sugar residues are not required for binding to Na+–K+-ATPase, they alter the pharmacokinetic properties of these toxins. Inhibition of Na+–K+-ATPase results in an increase in intracellular sodium content, which in turn blocks the normal sodium–calcium exchange mechanisms to produce intracellular hypercalcemia. Resting membrane potential becomes less negative manifested electrophysiologically by early after-depolarizations. If early after-depolarizations reach threshold, triggered ventricular dysrhythmias result. Also, the higher than normal resting intracellular calcium content increases the magnitude of calcium dependent calcium release from the sarcoplasmic reticulum. This later effect is responsible for the positive inotropic effects of these agents. Additional mechanisms that may be responsible for toxicity include variable sodium channel blockade, augmented sympathetic discharge, increased vascular tone, and vagotonic effects. Clinical effects of cardioactive steroid toxicity share some similarities regardless of the agents involved. Although digoxin and digitoxin toxicity are well characterized, there is also sufficient information available to make generalizations with regard to oleander and toad toxins. Nausea and vomiting are common, followed by altered mental status and cardiac dysrhythmias. Dysrhythmias are generally characterized as varied degrees of heart block and bradycardia, followed by ventricular ectopy. Hyperkalemia is typically present, but may vary with agent, degree of toxicity and host factors such as age, renal function and muscle mass. Most polyclonal immunoassays designed for therapeutic drug monitoring of digoxin or digitoxin will exhibit sufficient cross reactivity to allow for some detection of the non-pharmacologic cardioactive steroids. One way to confirm the diagnosis is to obtain serum levels using two different assays. While similar results should be obtained for digoxin or digitoxin, widely disparate values resulting from varied cross reactivity will help identify the presence of a non-pharmacologic agent. Monoclonal assays are less reliable. It should be noted, that regardless of the assay used, the numerical value obtained has no proven correlation with clinical toxicity or prognosis. Digoxin-specific antibody fragments have demonstrated utility against digoxin and digitoxin. Although less well studied, both animal experiments and human case reports support the use of polyclonal antibodies or fragments in clinical cases of toxicity from oleander and toad venom, and by analogy other cardioactive steroids. Indications for therapy would include life-threatening dysrhythmias, hyperkalemia, and renal failure. Initial dosing must be empirical (10 vials of digoxin specific Fab), as pharmacokinetic calculations are not possible. Subsequent doses should be administered based on clinical response. Conclusions: Diagnosis and treatment strategies of patients exposed to non-pharmacologic cardioactive steroids can be based on principles learned from digoxin and digitoxin. Levels can help confirm the diagnosis, but cannot be used to provide prognostic information or guide therapy.
43 MYOCARDIAL STUNNING OR NECROSIS: A NEW SCINTIGRAPHIC TOOLS FOR EVALUATING CARBON MONOXIDE CARDIOTOXICITY
Hubalewska-Hoła A, Pach D, 1Pach J, Szybiński Z
1Department of Clinical Toxicology and Nuclear Medicine Unit of Endocrinology Department, Jagiellonian University Medical College, Kraków, Poland
Objectives: Several techniques have been used to detect early manifestations of carbon monoxide cardiotoxicity but an extent of the heart injury is still unclear. The application of myocardial scintigraphy allows the evaluation of heart injury with better sensitivity. It is important both for treatment and late sequalae prognosis. The cardiotoxic effects of CO result from myocardial hypoxia and ischemia, and from direct toxic effect of CO on the myocardium. Acute CO poisoning causes ischemia- and hypoxia-related myocardial damage to the heart with subsequent lactic acidosis. The metabolic consequence is diminishing the amount of ATP production. Directly binding to cytochrome c oxidase, significant increase in releasing of free radicals and nitric oxide from platelets, releasing of neurotransmitters and physiological cytotoxic mediators may inactivate mitochondrial respiratory chain and produce oxidative stress resultant in myocardial stunning, and/or interference with normal physiological functions. Clinically, the CO toxicity is manifested by myocardial depression, myocardial ischemia, arrhythmias and ECG alterations. A microscopic evaluation revealed pathological changes in myocardium (hemorrhage, myocardial degeneration and mononuclear infiltration). In earlier studies we assessed the cardiac injury using 99mTc-MIBI myocardial perfusion scintigraphy. The blood clearance of 99mTc-MIBI is rapid and biexponential, with an initial fast phase followed by a slow phase. The mechanism of the tracer trans-cellular transport is not clear, but it may be passive, depending on concentration and electrical gradient. The retention of MIBI depends on cellular and mitochondrial electrical potential. The tracer activity is associated with the mitochondria in an energy-dependent manner as a free cationic complex. The fundamental myocellular uptake mechanism involves passive distribution across plasma and mitochondria membranes, and at equilibrium, sestamibi is sequestered within the mitochondria by the larger negative mitochondria trans-membrane potential. Reverse distribution into blood stream is stopped by the high membrane potential of the cardiac cells and depends on myocardial metabolic rate. If the metabolic rate is lower than normal, the decrease in myocardial tracer accumulation is observed. An experiments using irreversible myocardial damage by cytochrome c oxidase inhibitor (sodium cyanide) resulted in a decrease in 99mTc-MIBI activity. Scintigraphic lesions following the metabolic abnormalities in carbon monoxide intoxicated patients can indicate on necrosis or transitory ischemia only. The mode of cardiac injury is different than that due to coronary occlusion, and usually a longer time (duration) is needed to process the heart damage. It seems be important to detect an extension of early (not reversible) necrosis of the myocardium. Amiscan™ kit for preparation of Tc99m-glucarate is radiopharmaceutical diagnostic agent being developed just for the imaging and diagnosing of acute myocardial necrosis. It contains monopotassium D-glucarate. Glucarate is an minor metabolite of glucose pathway endogenously produced in mammals. So far two infarct-avid imaging agents are known: 99mTc-pyrophosphate and 111In-anti-myosin antibody, the latter being not commercially available, however both have limitations. In the absence of reperfusion, 99mTc-pyrophosphate scintigrams should be delayed 24–48 hours. Pyrophosphate accumulates in bones, and this uptake into skeletal structures can obscure the definitive diagnosis of myocardial infarction, especially that in CO poisoned patients the small foci of necrosis are mostly observed. The shorter half life of 99mTc (6 hours) compared to 111In permits higher doses to be injected, which results in higher photon flux, shorter imaging time and improved image quality. Amiscan, in contrary to Tc-phrophosphate and 111In-Myoscint limitations, accumulates during the very early phases of myocardial necrosis. Results of an evaluation of subjects with Amiscan suggest that 99mTc-glucarate can identify zones of acute myocardial necrosis in the short time after the event. The tracer concentrates in areas of acute, severely injured myocardial tissues with earliest myocardial infarction visualization possible within a few minutes of cardiac insult, and optimal visualisation about 3 hours, depending on the model. Normal tissue concentrates minimal 99mTc-glucarate amounts. Cellular and sub-cellular uptake studies have shown that 99mTc-glucarate localizes in necrotic tissues binding primarily to the nucleoprotein sub-fractions and to a lesser extent to the DNA fractions. Glucarate is taken up rapidly by necrotic myocardium and cleared relatively rapidly from the circulation resulting in early, high target-to-background ratios. In studies of Amiscan in patients with acute myocardial infarction, sensitivity was found to be 100% when Amiscan is administered within 9 hours of the onset of chest pain. Patients with unstable angina all had negative scans for a specificity of 100%. Khaw BA compared the distribution of Tc99m glucarate with 111In-Myoscint in subjects after reperfused myocardial infarction, and showed a direct correlation in glucarate and antimyosin uptake (p<0.0001). He concluded, like other authors, that this tracer should be favourable used as a specific early marker of myocyte necrosis. In our earlier study the 99mTc-MIBI SPECT changes were noted in all acutely CO poisoned patients. The changes were dependent on poisoning severity and could be seen because of necrosis, and/or stunned myocardium (99mTc-MIBI kinetics is dependent on membrane integrity and to a lesser extent on aerobic metabolism). The aim of the study was to distinguish the extension of early necrosis, if any, from reversible changes caused by CO cardiotoxicity. Material and methods: 20 acutely carbon monoxide poisoned patients treated at the Kraków Department of Clinical Toxicology (8 male, 12 female aged from 16–39) with any cardiac history or cardiac risk factors, were subjected to Tc99m glucarate and 99mTc-MIBI scintigraphy on day 1 to 5 post admission. COHb level, blood lactate concentration, duration of exposure, ECG and echocardiography examinations were carried out on admission. The enzymes activity (ALT, AST, CPK) was evaluated after 24 hours. The scintigraphy scans were performed in each patient with Siemens two-head gamma camera equipped with parallel high resolution collimators. The Tc99m-Glucarate (700MBq) was injected intravenously and immediately afterward planar sequential 1-minute images were acquired for the first 60 minutes and then the static image was performed. The delay static images were carried out 3 and 24 hours after tracer injection. 99mTc-MIBI scan was performed 1 day later. The changes in 99mTc-MIBI scan were graded: I0—diminished and non-homogenic tracer uptake, II0—diminished and small foci of tracer absence, III0—visible diminished uptake of tracer+one bigger “cold spot”, IV0—large and numerous “cold spots”. Results: No elevation in enzyme activity was noted. The echocardiography did not reveal substantial changes in any patient. The electrocardiography examination revealed tachycardia in 14 of the 20 examined patients. Myocardial necrosis was excluded in 11 (55%) of the examined patients because of negative 99mTc-Glucarate scans: 5 of them had I0 but 6 had II0 changes seen in 99mTc-MIBI scans. The unfocal, disperse accumulation of 99mTc-Glucarate was found in 5 persons (1patient—I0; 2 pts—II0; 2 pts—III0 in MIBI uptake scale). The typical focal accumulation of the tracer indicating necrosis was seen in 4 of the examined patients. All of them had III0 in 99mTc-MIBI uptake scale, extreme values of HbCO (23.2–42.9%) and the lactate concentration ranged from 2.97–13,29 mmol/L. Conclusions: 1. The changes in 99mTc-MIBI scintigraphy scans of myocardium confirmed the cardiac hypoxia and resultant metabolite disturbances in the CO poisoned patients but differentiation of the myocardial necrosis from transitory ischemia was not possible using 99mTc-MIBI tracer; 2. The myocardial necrosis was confirmed in 4 patients (20%) by 99mTc-Glucarate scintigraphic examinations; the unfocal, disperse accumulation of 99mTc-Glucarate without typical focal necrosis was found in 5 persons (25%); myocardial necrosis was excluded in 11 patients (55%), because of negative the 99mTc-Glucarate scans; 3. Myocardial scintigraphy using both 99mTc-MIBI and 99mTc-Glucarate tracers seems be most helpful for evaluation of carbon monoxide cardiotoxicity
44 METABOLIC ASPECTS OF ORGAN TOXICITY: BLOOD AND BLOOD-FORMING ORGANS
Eyer P
Walther-Straub-Institut für Pharmakologie und Toxikologie der Ludwig-Maximilians-Universität München, München, Germany
Objective: Blood is one of the largest organs and constitutes approximately 8% of human body weight. Since blood plays a central role in the exchange of substances between other organs it is exposed to many foreign compounds and their reactive metabolites that escape the site where they are generated. On the other hand blood is equipped with cells of high pro-oxidative capacity. Thus erythrocytes have received ammunition with extraordinary high amounts of oxygen that is activated by its binding to heme iron, making oxyhemoglobin capable of various oxidation and oxygenation reactions. Granulocytes and monocytes are equipped with antimicrobial defense systems that can generate copious amounts of reactive oxygen species and compounds of extremely high oxidation potential such as hypochlorite. Hence all these cells run considerable risk because reactive intermediates may injure their generating cells and cause methemoglobinemia, hemolysis, bone marrow depression, and various blood dyscrasias. In addition, cells of peripheral blood and blood-forming organs may become antigenic due to covalent binding of reactive compounds to the cell membrane, thus initiating immunological reactions with the possibility of innocent bystander cells being attacked too. Unforeseeable differences in the individual susceptibility to foreign compounds, mostly concerning only a small portion of the population, are observed. We are becoming aware that polymorphically expressed metabolic reactions may be involved in idiosyncrasias that have not been understood in the past. Epigenetic effects, such as in HIV infection, are another source of large differences in susceptibility. This overview will discuss principal metabolic aspects and provide some examples of abnormal metabolic events that are involved in toxic actions on blood and blood-forming organs. Metabolism and toxic mechanisms: There is a large amount of evidence that cytotoxic reactions of xenobiotics are frequently brought about by electrophilic metabolites rather than by the parent compounds themselves. The liver is in most cases the central organ to produce more hydrophilic derivatives of the foreign compounds we incorporate daily, but other organs like the GI tract may share this task. To enhance hydrophilicity and thus renal excretion, oxidation reactions with ultimate formation of hydroxylated compounds is the most usual approach, followed by phase II reactions with formation of glucuronide and sulfate conjugates. The oxidation reactions are brought about by enzyme systems of high plasticity and of low substrate specificity that can be induced on a demand basis, e.g. the cytochrom P450 superfamily and flavine-containing oxygenases. Hence a high genetic and epigenetic variability of these enzyme activities is not surprising. Examples of this variability are the widely varying rates of toxification of parathion yielding paraoxon or formation of morphine from codeine. Most cytotoxic metabolites stem from nitrogen-containing aromatic compounds. Metabolic oxidative activation of sufficiently stable compounds include ring-hydroxylated derivatives, e.g. aminophenols and N-hydroxylated metabolites, i.e. phenylhydroxylamines and hydroxamic acids. From these proximate reactive compounds ultimate reactive intermediates are mostly formed in the target cells, including quinone imines, nitrosoaromatics and oxygen- or nitrogen-centered radicals. These electrophiles may bind to sulfhydryl (mainly cysteine) and amino groups (mainly lysine and histidine) in proteins, may induce hemoglobin oxidation or sustain redox cycling with formation of reactive oxygen species. Cytotoxic reactions in blood cells are aggravated in people with inborn defective antioxidative capacity, such as in G6PDH anomalies and methemoglobin reductase deficiency, or in acquired diseases, such as HIV and deficiencies in the antioxidative vitamins, mainly α-tocopherol and ascorbic acid. Examples: Methemoglobinemia and hemolysis due to aminophenols (4-dimethylaminophenol, diclofenac and partly primaquine) are brought about by the cooxidation of oxyhemoglobin and the aminophenol, resulting in a phenoxyl radical that oxidizes ferrohemoglobin to ferrihemoglobin. Disproportionation of the phenoxyl radicals yields quinone imines that bind covalently to sulfhydryl groups in proteins or low-molecular weight sulfhydryls. Phenylhydroxylamines from aromatic amines (dapsone, sulfamethoxazole, and primaquine) or from nitroarenes such as chloramphenicol also are cooxidized with oxyhemoglobin under formation of ferrihemoglobin and a nitrosoarene. The latter has also high sulfhydryl affinity and can lead to several sulfur-containing products. Aplastic anemia associated with chloramphenicol was related to such a reaction. Myeloic leukemia elicited by benzene is probably due to quinoid reaction products with genotoxic properties. Granulocytopenia and thrombopenia due to carbamazepine appears to be an immunological disorder elicited by haptenization from a reactive intermediate. In all cases electrophilic metabolites are the ultimal causative agents that react with biomolecules. Therapeutic measures: Avoidance of critical drugs and chemicals in persons at increased risk due to inborn errors or acquired deficiencies is mandatory. Met hemoglobinemia in persons with normal G6PDH can be meliorated by certain phenothiazine dyes (methylene or toluidine blue) and ascorbic acid. Extreme methemoglobinemia is treated with hyperbaric oxygen, resulting in sufficient amounts of oxygen physically dissolved in the plasma to meet the oxygen demand of the tissue. In those cases massive hemolysis is usually observed which requires a fractional exchange transfusion to remove the nephrotoxic amounts of free hemoglobin and to make available intact red blood cells. It has to be demonstrated whether intoxications with compounds forming reactive metabolites towards thiols may profit from N-acetylcysteine. Finally, other organs than blood and blood-forming organs may be affected by the xenobiotic and its reactive metabolites and require specific treatment. Conclusions: Blood and skin are frequently affected by idiosyncratic reactions towards xenobiotics, resulting in significant morbidity and mortality. Despite progress in the identification of reactive metabolites and some understanding of the toxic reactions, the basic mechanisms often remain elusive. Research is needed to explore the intricate pathophysiological reactions in more detail and to develop diagnostic tests to identify susceptible subgroups in order to prevent adverse reactions.
45 TOPICAL TREATMENTS FOR HYDROFLUORIC ACID BURNS—A BLIND CONTROLLED EXPERIMENTAL STUDY
Höjer J, Personne M, Hultén P, Ludwigs U1
Swedish Poisons Information Centre and 1Dept of Emergency Medicine, Karolinska Hospital, Stockholm, Sweden
Objective: Calcium gluconate gel 2.5%, after initial water rinsing, has a proven effect on hydrofluoric acid (HF) burns and constitutes a widely accepted standard therapy. Hexafluorine (Prevor, France) is a novel compound developed for decontamination of HF eye and skin exposures. Scientific documentation is insufficient however, therefore this study was undertaken. Methods: Sprague Dawley rats (300–325 g, n=35) were anaesthetised and shaved on the back. Four filter papers (Ø 10 mm) were soaked into 50% HF and applied separately on the back of each rat for three minutes. Thirty seconds later, the animals were randomly rinsed with either 500 ml Hexafluorine (original rinsing equipment) during three minutes (group H), 500 ml water (using the same rinsing equipment) during three minutes (group W), 500 ml water during three minutes followed by a single application of calcium gluconate gel (group Ca) or received no treatment at all (group 0, controls). The animals were closely observed for five days and received analgesia as needed. At 16 p.m. each day the burns on each rat were scored by a laboratory assistant unaware of the treatment selection procedure. This yielded a single mean score for each rat. The scoring scale used was graded from 0 to 5 (0=no mark, 1=diffuse erythema, 2=distinct erythema, 3=score 2 plus wounds or discoloured spots, 4=score 2 plus wounds or discoloured areas covering >50% of burn surface, 5=the whole burn surface necrotic). In group 0, four animals were killed on days 2–3 because of weight reduction. The Kruskal-Wallis analysis was used to evaluate differences between treatment groups at the five days respectively (group 0 excluded). If the p-value obtained was <0.05, correction for multiple comparisons was performed. The study was approved by the animal ethics committee. Results: Mean scores in the four groups are shown in Table 1.
Thus, Hexafluorine treatment was less effective than calcium gluconate gel. Moreover, a consistent trend towards poorer effect of Hexafluorine was observed, both in comparison to calcium gluconate gel and water rinsing alone. Conclusion: Based on these observations, there is no support for replacing water rinsing plus topical calcium with Hexafluorine after skin exposure to HF.
Table 1
46 IATROGENIC INTOXICATIONS—WHAT AND WHY
Seger, DL
Vanderbilt University Medical Center, Nashville, Tennessee, USA
Objective: Iatrogenic disorders may be divided into three categories: complications of procedures (esophageal rupture during gastric lavage), adverse effects of drugs (tardive dyskinesia following drug administration) and intoxications (poisoning induced by a physician's words or actions). Methods: Etiologies of iatrogenic intoxication are reviewed. Specific drugs and agents that have caused iatrogenic intoxications are discussed. Results: An error made by an individual and/or system errors may cause iatrogenic intoxication. In a review of iatrogenic intoxications caused by cytotoxic drugs, the majority of errors were caused by multiple system factors rather than individual responsibility. (1) Homographism, homonymism, similar appearance of drugs, and mislabeling were the most frequent causes of erroneous drug administration. Lack of knowledge about the drug was a significant contributing factor. An earlier study supported these findings. Theophylline toxicity in 27 out of 40 patients was caused by drug administration to the patient while in the emergency department or in the hospital. Failure to recognize signs of toxicity or toxic serum concentrations, administration of high doses to patients with heart failure or liver disease, and drug interactions were the most frequent causes of theophylline toxicity. (2) A number of iatrogenic poisonings and death have occurred from agents that are routinely administered. For example, high-dose adrenaline administration to treat anaphylactic reactions has caused death. Iatrogenic magnesium toxicity has caused morbidity and death. Reasons for the administration of inappropriate magnesium dose may be the availability of a variety of forms, multiple routes of administration (oral or potential), and orders written in various units of measurement. Metoclopramide toxicity is another common cause of iatrogenic drug poisoning. Administration of water for procedures such as whole bowel irrigation and pelvic ultrasound imaging has caused hyponatremia, seizures, and death. Antiepileptic drug intoxication is frequently caused by excess initial dose or increase in incremental dosing. Although iatrogenic ergotism is less frequent, clinical consequences are severe. Conclusion: Due to the potential medical–legal consequences and peer pressure, one could postulate that many cases of iatrogenic poisoning are not published. Therefore, the true incidence of iatrogenic intoxication is unknown. One could further speculate about the adverse events that follow the administration of drugs (such as flumazenil or physostigmine) that have questionable therapeutic efficacy. Perhaps these adverse events should be classified as “iatrogenic intoxications”.
References: 1. Zernikow, B.; Michel, E.; Fleischhak, G. et al. Accidental Iatrogenic Intoxications by Cytotoxic Drugs. Drug Safety 1999, 1, 57–74. 2. Schiff, G.; Hegde, H.; LaCloche, L. et al. Ann. Int. Med. 1991, 114, 748–53.
47 FOLLOW-UP OF 600 IATROGENIC OVERDOSE CASES IN NEONATES AND SMALL INFANTS
Lübke G, Bunjes R, Brockstedt M
Poison Center Berlin, Berlin, Germany
Introduction: In the treatment of neonates as well as of premature and young infants naturally only very small drug amounts are required. Adequate drug preparations are not routinely available. In order to obtain correct dosages, one or even several consecutive dilution steps are necessary. As a consequence, medication errors due to false dilution are rather common. This leads often to the administration of a tenfold overdose—less frequently 20 to 200 times the required dose is given. Objectives: Berlin Poisons Center is contacted fairly frequently in the course of such intoxications. Since we began to follow up all those cases by repeated phone contacts we were able to collect sufficient data on more than 600 medication errors with approximately 100 different drugs, mainly in premature infants. This ever increasing data pool gives us more and more often the opportunity to assess the risk to the patient on an individual basis, to predict the probable clinical severity of the intoxication and to provide specific recommendations as to surveillance and therapy. Case series: The individual risk due to substances administered fairly often in overdose such as theophylline (>70 cases), digoxin (>30 cases), chloral hydrate (>25 cases) or indomethacin (>15 cases) or lipid emulsions (>15 cases) can be assessed rather accurately. Even where only anecdotal experience exists to date (one or very few cases), some practical advice can often be given by taking into consideration the experience gained with poisonings in children or adults. Due to preexisting disease and concomitant therapy some significant clinical signs of poisoning (e.g. respiratory depression caused by opioid overdose in a premature receiving artificial ventilation) can occasionally not be classified. Nevertheless the experience gained during the last decade permits rather often to discard obviously unnecessary invasive diagnostic and therapeutic measures. Typical examples are parenteral overdosage of an aminoglycoside antibiotic (no treatment versus hemodialysis), theophylline (prophylactic anticonvulsant medication and repeated administration of activated charcoal versus hemoperfusion) or vancomycin (strict surveillance of renal function versus hemoperfusion). Results: The vast majority of these patients seems to tolerate a single overdose—tenfold or even higher—with little or no clinical symptoms. This may, at least in part be attributed to the fact, that the iatrogenic intoxication do normally occur in the setting of an intensive care unit. It must never be ignored, that some drugs (e.g. propafenon, chloramphenicol) may lead to life-threatening symptoms and even death.
48 A TEN YEAR REVIEW OF IATROGENIC INTOXICATIONS
Ramón MF, Larrotcha C, Ballesteros S, Martínez-Arrieta R, Cabrera J
Servicio de Información Toxicológica de Madrid. Instituto Nacional de Toxicología, Spain
Background: Iatrogenic events can be a source of intoxications in our community. We examined the calls associated with iatrogenic intoxications made to our PCC by health care personnel. Methods: From January 1991 to December 2000, all iatrogenic intoxications in our Service were tabulated. We studied two causes of iatrogenic poisoning: exposure route and dose confusion. For each case age, gender, hospital or health care unit calls, and severity of clinical manifestations were compiled. Therapeutic drugs were classified by the Pharmaceutical Specialities Catalogue. Results: We registered 158 cases, of which 50.6% were exposure route errors. Calls related to this type of error were from hospitals in 91.2% of occasions; adults comprised 65% of cases, children 31.2%, the rest was unknown; 61.2% were male, 33.7% female and the rest unknown. The exposure routes implicated were: intravenous 47.5%, oral 35%, intramuscular 1.2%, subcutaneous 1.2%, inhalation 1.2% and others 13.7%. Therapeutic drugs involved were: dermatological 31.2%, respiratory 22.5%, antibacterial and vaccines 12.5%, neurological 11.2%, gastrointestinal and metabolism 8.7%, genitourinary 3.7%, antineoplastic 3.7%, haematological 2.5%, cardiovascular 2.5% and other agents 1.2%. An almost equal number (49.3%) of iatrogenic intoxications were dose errors. Of these 70.5% were hospital calls and 29.5% from other health care staff. Adults represented 51.3% and children 48.7% of cases. Male were 60.2%, female 37.1% and the rest unknown. The exposure routes were intramuscular 30.8%, intravenous 27%, oral 25.7%, subcutaneous 9% and other routes of exposure 7.7% of cases. Therapeutic drugs observed were: antibacterial and vaccines: 27%, neurological 25.7%, antineoplastic 19.2%, haematological 7.7%, gastrointestinal and metabolism 5.4%, respiratory: 3.8%, dermatological 2.6%, cardiovascular 2.6%, genitourinary 2.6%, antiprotozoaan 2.6% and others agents 1.3% of cases. Asymptomatic or mildly affected patients were 63.8% and moderate and/or severe 36.3% when there was an error of exposure route. This compared with 55.1% asymptomatic and/or mild and 44.9% moderate and/or severe due to an error in the dose. Conclusions: Iatrogenic intoxications due to exposure route errors were slightly more frequent than dose errors. Both types of poisoning often resulted in moderate or severe outcome especially those due to errors in dose. The medications more frequent involved were vaccines, dermatological (disinfectants), respiratory (bronchodilators) and neurological (sedatives and local anaesthetics) agents. PCCs have an important role to detect such events and to provide information on the toxicity of common drugs in overdose or administered by an unprescribed route.
49 ERRORS IN DRUG-THERAPY REPORTED TO A NATIONAL POISONS INFORMATION CENTRE
Curjuric I, Guirguis M, Kupferschmidt H, Meier-Abt PJ
Swiss Toxicological Information Centre, and Division of Clinical Pharmacology and Toxicology, University Hospital, Zurich, Switzerland
Objective: Errors in medicine have received increased awareness in recent years. This is especially true for errors caused by medical professionals. In contrast, errors caused by non-professionals have received less attention. Therefore, the aim of this study was to compare frequency, type and severity of reported errors in drug-therapy between medical professionals (physicians, nurses, pharmacists) and non-professionals. Methods: We reviewed retrospectively all calls to the Swiss Toxicological Information Centre between January 1997 and June 2001 for errors in drug-therapy and analysed these cases with regard to frequency, responsible party, type of error, involved ATC-drug-class and clinical outcome. We defined three types of errors: type 1, wrong patient/right drug; type 2, right patient/wrong drug; and type 3, right patient/right drug/wrong application (e.g. wrong dose, route of administration). Data on the outcome were based on feedback-reports from physicians. The clinical outcome was graded into asymptomatic, minor, moderate, severe, lethal. Results: Among 38,535 cases with drug exposition, we found 2297 (6%) errors in drug-therapy. Among all errors (2297=100%), 912 cases (40%) were reported by physicians and 1385 (60%) by non-physicians. Medical professionals caused errors in 556 (24%) and non-professionals in 1707 (74%) cases. In 34 (2%) cases the origin of the errors remained unknown. Following types of errors were identified in professional (P; 556=100%) and non-professional (NP; 1707=100%) groups: Type 1 errors, P 20 (4%), NP 50 (3%); type 2 errors, P 126 (23%), NP 461 (27%), and type 3 errors P 361 (64%), NP 1086 (64%). In P 49 (9%)/NP 110 (6%) cases it remained unclear whether the patient or the drug was confounded. Type 3 errors (P; 361=100%/NP; 1086=100%) concerned wrong doses P 269 (75%)/NP 889 (82%), wrong route of administration P 63 (17%)/NP 158 (15%) and others P 29 (8%)/NP 39 (3%). In the P-group most errors were made with drugs acting on the central nervous system (44%) followed by anti-infective (17%) and cardiovascular drugs (7%). In the NP-group central nervous drugs were involved in 24% of errors followed by respiratory (22%) and anti-infective (8%) drugs. In 379 feedback-reports the clinical consequences of the errors were qualified as asymptomatic (P 117; NP 92), minor (P 51; NP 57), moderate (P 23; NP 21), severe (P 12; NP 4), lethal (P 0; NP 2). Conclusions: The majority of drug therapeutic errors were caused by non-professionals (74%). In medical professionals and non-professionals types of errors, drugs involved and clinical consequences of drug therapeutic errors were surprisingly similar although the two lethal cases occurred exclusively in the medical non-professional group.
50 TOXIC DRUG INTERACTIONS
Snook CP
Iceland Poison Information Centre, Landspitali-University Hospital-Fossvogur, Reykjavik, Iceland
Objective: Drug interactions play a significant etiologic role in clinical toxicology and more so as the number of elderly patients increases in European countries. Elderly patients are typically prescribed multiple medications in the context of decreasing rates of drug clearance. The incidence of drug–drug interactions increases from 3–5% in patients taking a few drugs to 20% in those receiving 10–20 drugs. Drug interactions can either be pharmacokinetic if what is affected involves absorption, distribution or elimination of the drugs in question or pharmacodynamic if the effect relates to agonism and antagonism at drug receptors. A drug's ability to act as substrate or inhibitor for P-glycoprotein drug transport has recently been found to be of importance in pharmacokinetic drug interactions. Toxicity from drug interaction often relates to involvement of a agent with a low therapeutic index. Discussion: As our understanding of the nature of drug–drug interactions and the genetic basis of individual variability advances, it is important that this information be effectively used in the prevention and treatment of toxic drug–drug interactions. In some cases, drug–drug interactions can be manipulated to achieve greater therapeutic benefit with fewer untoward side effects. Recent advances in the understanding of cytochrome P450 (CYP)-mediated biotransformations allow prediction of a drug's interactions during the development phase. It is important also to assess potential risk from such interactions as most are not harmful to the patient. Recent reports have detailed the drug–drug interactions of clinical significance for a number of therapeutic classes. For the proton pump inhibitors, as one example, the most important interaction in clinical terms is the 25–50% reduction in clearance of diazepam due to inhibition of CYP2C19 by omeprazole. Recent reviews have detailed significant drug interactions among antiepileptic, antiarrhythmic, antibiotic and drugs used in diabetics. The CYP isoenzymes involved in important cardiovascular drug–drug interactions include CYP3A, CYP2D6, CYP1A2, CYP2C19, and CYP2C9. CYP3A and CYP1A2 show a continuous distribution of enzymatic activity within populations, with most individuals having intermediate activity. CYP2C19, CYP2C9 and CYP2D6, on the other hand, are significantly affected by genetic polymorphism with varying frequencies in different ethnic groups. A drug's potency and specificity of CYP enzyme inhibition is also important to the severity of its drug interactions. As one example, itraconazole, a potent and specific CYP3A inhibitor, can produce torsades de pointe or rhabdomyolysis in combination with astemizole or simvastatin respectively, while the less potent and specific CYP3A inhibitor, cimetidine, does not. Conclusion: As clinical toxicologists, we play an important role in minimizing health risks from toxic drug interactions, both by educating health professionals and the public in their prevention and by recognizing and treating them in patients.
51 MEDICAL MISTAKES: 5 KEY STEPS TO BETTER POISON CONTROL RESPONSES
Woolf A
Children's Hospital; Department of Pediatrics, Harvard Medical School; and the Massachusetts/Rhode Island Poison Control Center, Boston, Massachusetts, USA
Objective: Ludmerer has hypothesized that medical mistakes are increasing, due to a decline in the quality of the training of health professionals, a deterioration in time spent in careful reflection about clinical decisions, and the subversion of patient care to the exigencies of medical economics. The objective of this study was to analyze a poison control center's operations in order to identify key roles for toxicologists in the effective management and prevention of medical mistakes. Methods: The production model of poison control was analyzed to reveal nodal intercepts in the diagnosis and management of medical mistakes. Poison control center reporting on medical mistakes at the NACCT meetings between 1995–1999 was analyzed with regard to six categories: epidemiology, case reports or case series, toxicology errors, the toxic effects of drug–drug interactions, risk analyses, and herbal/dietary supplements errors or adverse effects. Results: Of all 1074 NACCT abstracts published between 1995–1999, as many as 18% annually involved reports of medical mistakes and/or adverse drug reactions. The majority concerned cases or case series of medical errors and adverse drug reactions, although appreciable numbers of toxicology errors in management, drug–drug interactions, and cases of the toxicity herbal therapies were also reported. In the context of such experience, 5 key functions in the production model of poison control were defined: leadership, surveillance, training, quality improvement, and research. Leadership suggests a top-down commitment to proactive change and includes the provision of excellent care for the victims of such mistakes. Surveillance implies better collection of harmonized data. It also suggests the development of systems for timely analysis of data so as to alert health professionals to newly defined hazards. It further suggests the elimination of barriers to the reporting of medical mistakes by all health professionals. Training involves changes in medical education so as to enhance both modeling and mentoring opportunities for students at all levels of medical training. Quality improvement suggests the benchmarking of poison control center practices and the implementation of error-proof systems in all types of health delivery systems. Such quality improvement activities should focus particularly on very frequent, very serious, and problem-prone medical care activities. High hazard medications, such as insulin, digitalis, adrenergic agonists, and heparin, should be targets for improved practices and safeguards against errors. Research anticipates new methods for the reduction of medical errors at every level of patient care, including the substitution of safer but equally effective medications and antidotes. Conclusion: Poison control centers are confronted with medical mistakes in the course of their daily operations. The response of toxicologists to such events should be well-orchestrated so as to limit the injuries related to the toxic reaction. We should as well attempt to forestall future such events through effective quality improvement strategies, new research goals, and innovative educational programs.
52 THE COST-EFFECTIVENESS OF A POISON CENTRE—AN INDEPENDENT STUDY UNDERTAKEN IN SWEDEN
Personne M, Persson H
Swedish Poisons Information Centre, Stockholm, Sweden
Objective: A user group study was performed to assess the impact of the Swedish Poisons Centre on national health economics and to evaluate user satisfaction. Methods: The Swedish Institute for Health Economics (IHE) was asked to design and perform a study on the economic consequences for the society of having access to a poisons centre, and to evaluate customer satisfaction. The study on economics consisted of two parts. One involved 241 randomly chosen hospital doctors contacting the centre with acute inquiries during June–October 2000. A few days later they were asked to complete a questionnaire, reflecting their opinion of the contact (response rate 83%). The other part addressed the general public, and 347 private citizens phoning the centre during four different days in June–October 2000 were contacted afterwards by an interviewer (response rate 74%). Only cases where the poisons centre considered visit to a health care facility unnecessary were selected in this group. Results: Among hospital doctors 85% would have spent more time (estimated to 30 minutes) on searching for information (marginal cost per hour=329 SEK) and 15% judged that the hospital stay would have been prolonged with one day in the absence of a poisons centre advice. During 1999 there were 8,314 calls from hospital doctors and the cost for an extra day in an acute clinic is 4,606 SEK. Hence, the total savings in the hospital setting are estimated to 7 million SEK. As for the general public 37% would have gone straight to a hospital (cost per consultation 1,070 SEK) and 7% would have turned to their general practitioner (cost per consultation 650 SEK), if no poisons information service had been available. During 1999 the centre received 34,594 inquires from the general public where no health care intervention was deemed necessary. Therefore the savings for this group amounted to approximately 15 million SEK. Some additional economic savings were not accounted for in this study, e.g. costs for travelling and lost income. Further reduction of hospital costs would have occurred if a prolonged hospital stay would have meant treatment in an intensive care unit rather than in an acute clinic (as used in the above calculations). More than 97% of both medical professionals and general public were found to be satisfied with the reception and information provided by the poisons centre. Conclusion: The total estimated savings for the society by approximately 22 million SEK well exceed the 20 million SEK required for running the centre (10 SEK≈1 US$). The full report is published in Läkartidningen 2001, 98, 2926–2930.
53 HEALTH CARE COST EFFECTS OF PUBLIC USE OF A NATIONAL POISON INFORMATION CENTRE
Fehr M, Kupferschmidt H
Swiss Toxicological Information Centre (STIC), and Division of Clinical Pharmacology and Toxicology, University Hospital, Zürich, Switzerland
Objectives: Under increasing financial pressure poison control centres have to demonstrate their cost effectiveness in order to keep proper funding. The STIC is the only Poison Control Centre (PC) in the country and serves a population of seven million. In 2000 it received a total of 30,935 calls, 61.3% of which came from the public, 29.9% from physicians, and the remainder from veterinarians, pharmacists and various organisations. The aim of the study was to assess cost sparing effects in the public health care system of the public use of the PC. Methods: A survey was performed by sending out a questionnaire to the public callers during February 2001. Calls on non-toxicology issues and calls from abroad were excluded. Besides questions concerning health care cost effects, the questionnaire included also questions covering user satisfaction and demographic data. The questionnaires were sent out one working day after the call took place. The callers were asked 1) what they would have undertaken if the PC had not been available, 2) whether they had already contacted the health care system (visited or telephoned emergency medical services, a physician or an emergency department), 3) if they had been advised by the PC not to contact the health care system (HCS), and 4) whether they did contact the HCS after the call to the PC. Results: During the study period a total of 1336 calls from the public were answered. A total of 1047 questionnaires were sent out to the callers. The rate of return was 85% (n=888); 813 answers could be analysed (Table 1).
Of 401 primary callers to the PC, 93% could be prevented successfully from contacting the HCS unnecessarily. The extrapolated annual number of these cases is 5000. Conclusions: The services provided to the public by a poison centre do effectively decrease the cost in the HCS by preventing unnecessary measures in non-toxic exposures. Therefore the accessibility of a PC to the public should be broad, and the threshold to call a PC should be low by educating people about the services of the PC.
Table 1. Answers (n=813) (Abstract 53)
54 PEDIATRIC OCCUPATIONAL TOXICOLOGY: CHEMICAL HAZARDS OF CHILD LABOR
McGuigan M
Long Island Regional Poison and Drug Information Center, Mineola, New York, USA
The practice of using children in the workplace has a long history in many countries. Working children, like working adults, may be exposed to a variety of potentially toxic chemicals in the workplace. While there are few objective facts relating to the types, severity, and outcomes of exposures of working children to toxic substances, concern about the risks to the health of these children has been raised. Evaluation of the risks is difficult, requiring knowledge and understanding of pediatrics, occupational/preventive medicine, clinical toxicology, and socio-economics. This paper will discuss the differences between children and adults, that may affect the risks and toxic manifestations that working children may experience and provide an approach to the medical management of these children. Significant differences can be attributed to growth and development, diet and the physical environment, the parameters used to assess toxicity (differences in quantity and quality), and biochemical and physiological responses. Primary data on pediatric workplace exposures are essentially nonexistent so it is necessary to extrapolate from pharmacological and physiological principles to try to understand the potentially hazardous and harmful effects of toxic exposure in working children. The manifestations of toxic exposure depend on several related factors: the toxic substance and its kinetics, the underlying health of the victim, and developmental differences and changes. Each of these issues will be discussed and examples provided to illustrate how and why children are different from adults. It is not enough simply to understand the issue, it is necessary to develop an approach to evaluation and management of these cases. One approach would be to follow the injury prevention model. Primary prevention aims at preventing exposure to the toxic substance. In order to do this, it is necessary to shift the societal balance between perceived benefits and perceived risks of toxic child labor activities. One step would be to identify those occupations which expose children to hazardous chemical and provide information about their dangers or risks to healthcare workers, parents, the general public, employers, governmental agencies, and the public media. Secondary prevention focuses on identifying those children who have been exposed before clinical damage has occurred. Based on the local area occupations, a list of acute and chronic effects related to specific commonly used chemicals can be generated. Each working child should be evaluated medically every six to twelve months or whenever the opportunity arises. Tertiary prevention seeks to identify and treat the clinical effects resulting from exposure to toxic chemicals. Children who have physical complaints during or following work should be evaluated by a health care worker. Pediatric health care professionals and poison information centers have a critical role to play. Any documented exposures should be reported to a centralized repository with close links to the communities in order to collect, analyze, identify sentinel events, and disseminate relevant demographic and epidemiologic data.
55 ASSESSMENT OF RISKS FROM ENVIRONMENTAL EXPOSURE: PRACTICAL IMPLICATIONS IN CLINICAL TOXICOLOGY
Zilker Th
Toxikologische Abteilung II. Med. Klinik, Klinikum r. d. Isar der Technischen Universität München, Germany
Risk assessment from environmental toxins is very difficult and arbitrary. The reasons for this are that no human data after subchronic and chronic exposure are available for most environmental pollutants. Some support stems from occupational medicine but usually only for chemical agents that are out of use due to their recognised toxic effects. Although toxicologists still believe that the dose makes the poison, in a dose response correlation and a threshold, this concept is not true for carcinogenic chemicals. This creates enormous problems as none of us would like to die from cancer. The more we are able to measure chemicals in the environment and in human materials the more frightening it is. This has forced politicians to calm down the fears of the population and to force scientists to give them limits for environmental toxins were no adverse effects can be possible. These limits are dependent on the political opinions, the possibility of measurement and on the technology to keep a specific chemical agent low without hampering industry and economy. As soon as a limit is defined it has legal implications and if somebody does not stick to it he will be sued. To define limits it is necessary to find an Acceptable Daily Intake (ADI). It is assumed that this ADI does not lead to any harm in a whole lifetime. Starting point for this ADI is the No-Observed Adverse-Effect Level (NOAEL). This is the level of a chemical which in a human or in animal experiments does not show any physiological effects. To find acceptable limits a factor of uncertainty has to be installed. This is due to the uncertainty of transferring animal data onto humans because of differences between species, different susceptibility of individuals and a higher susceptibility of risk groups. The factor of uncertainty is not scientifically well founded. In practice the factor of uncertainty (FU) is 10 if there are clear human data available, 100 if animal long term investigations are known, 1000 if arbitrary animal data are available and 10 000 if long persistence chemicals with a high assumed toxicity are involved. For risk groups a factor of 5 is multiplied with the factor of uncertainty. Herewith the limits can be calculated in case the bioavailability of a certain agent is known by the formula: ADI=NOAEL:FU. It is more difficult to define limits for carcinogenic agents as there is no NOAEL for such chemicals. Theoretically one molecule of such an agent can induce cancer. The only possibility is to find the incidence of cancer at a certain exposure over a certain time. In humans this is only possible if disaster has already struck like after asbestos fibre exposure or dioxin exposure. If the incidence of cancer after the exposure to 20 Mill. fibres of asbestos/m3 over 1 year is 2% and after 40 Mill. fibers 4% an extrapolation on a linear basis can be made. The gradient of this line is the Unit Risk (UR). The carcinogenic risk is calculated by the multiplication of UR with the exposure to the pollutant. For agents which may induce cancer in a less obvious way it is impossible to create human data by epidemiological means. The total mortality rate of cancer in a European population is 1:4. To be able to find statistical significance it would be necessary to compare 1 Mill. unexposed persons to 1 Mill. exposed persons which is impossible. Therefore animal exposure data are inevitable. This is were the HERP-Index by Ames comes in. The HERP-Index is the relation of the human exposure dose to the rodent potency dose. The human exposure dose is the dose of a special carcinogenic agent that is taken in per day. The rodent potency dose is the dose that creates 50% more cancer in rodents in excess of the spontaneous cancer development in this species. If this index is low the risk to develop cancer in humans is low. Clinical toxicologists who are involved in the diagnosis and treatment of patients are confronted by their patients with the idea that low level exposure makes them sick. There are many medically qualified advocates of this theory. As many laboratories are able to measure chemicals from the environment down to very low levels, susceptible persons feel sick as soon as anything is measured in their homes, in their urine, blood or hair. What the people suffer from, though their symptoms are very unspecific, is called Environmental Illness (EI). We have tried to find a deeper understanding of this undefined modern disease by doing 3 studies. First a representative survey in the German population to find the incidence of EI. Second a retrospective follow up study with 100 patients as far back as 10 years ago. Third together with a psychiatrist a prospective controlled study comparing chemically exposed workers with patients suffering from EI. Results: 1. Study: 84% of the whole population have the same symptoms as patients who believe they suffer from EI. 32% of the population think that their complaints are caused by environmental sources. 0.5% suffer from what could be called “EI”: 2. Study: Looking into the development of the patient's history and the helpfulness of the interventions they had taken to improve their complaints we found out that psychotherapy helped the most, but only 1/3 of the patients went for it. 3. Study: 117 outpatients with EI were compared with 59 workers in a semiconductor industry. Those who were working in industry had less and different physical complaints, had fewer psychiatric diseases and had more abnormalities in the toxicological analyses. Conclusion: There is no relation between exposure to chemicals and EI. EI patients suffer from psychiatric diseases mainly somatization disorder. From a psychoanalytic standpoint this is projection. Projection is the process whereby what is inside is misunderstood as coming from the outside. Projection can become very aggressive and approaches paranoia. The conflict that is inside somebody's soul is projected onto the environment. This allows factors external of the self to be made responsible for not feeling well.
56 LEVELS OF MERCURY EXPOSURE IN DENTISTS
Schach-Boos V,1,2 Jahanbakht S,1,2 Livardjani F,1 Flesch F,1 Jaeger A,3 Haïkel Y4
1Centre Antipoison, Hôpital Civil, Strasbourg, 2AD Scientifique, Strasbourg, 3Réanimation médicale, Hôpital de Hautepierre, Strasbourg, 4Faculté de Chirurgie Dentaire, HUS, Strasbourg, France
Objectives: Mercury amalgams have raised much controversy since their use as dental filling material. Mercury can be released in the form of vapor and/or solid from the moment the amalgams are made until they are destroyed. The amount of mercury absorbed by amalgam carriers has been assessed but it has seldom been evaluated for dentists. The aim of the study was to estimate the potential amount of mercury absorbed by dentists at the work-place. Methods: Mercury concentrations in the air were measured directly with a portable device using the cold vapor atomic absorption spectrophotometry technique. For mercury analysis in urine a Perkin Elmer flow injection system was used. Results: 1) Mercury in the air. During amalgam treatments the mercury concentrations in the patient's oral cavity ranged from 81.1 to 326.2 μg/m3. The concentration varied with the type of treatment, the highest level being observed during amalgam polishing. During amalgam treatments the concentration of mercury in the air inhaled by the dentists was mean 2.70 μg/m3. During the other periods the concentration was mean 0.70 μg/m3 The air concentration depended also on the type of treatment: it was highest during the amalgam hardening and during the removals of amalgams. 2) Mercury in the urine. In 59 dentists and 41 assistants mercury was measured in urine (one urine sample or a sample of 24 h). The mean mercury urinary concentration was 2.91±2.34 μg/L in the group of dentists and 1.90±1.40 μg/L in the group of assistants. Conclusion: Our results show that the mercury concentrations in the air inhaled were below the occupational exposure limit values (50 μg/m3 in France, 25 μg/m3 according to WHO) but could be above the limit for a year-long exposure (1μg/m3 according to WHO). The mercury concentrations in urine were also below the level considered as normal (<5 μg/m3) but, nevertheless, higher than the concentration usually observed in non-exposed persons (<0.5 μg/m3). The dentists are at higher risk than the assistants. Mercury amalgams are undoubtedly a source of pollution in the dentist's work-place. Preventive measures should be focused on the major causes of the mercury release in the air.
57 PARACETAMOL POISONING—EARLY DETERMINANTS OF POOR PROGNOSIS AND THE NEED FOR HEPATIC TRANSPLANTATION
Jones AL
National Poisons Information Service (London), Guy's and St Thomas' NHS Trust, London, United Kingdom
Introduction: Paracetamol is the commonest cause of acute liver failure (formerly termed fulminant hepatic failure) in the UK. Between early paracetamol poisoning and acute liver failure (ALF) lies a continuum between which the mechanisms and clinical manifestations are different. The mechanisms of toxicity in late paracetamol poisoning, particularly ALF are not well understood but many include the production of cytokines and free radicals, and may involve the action of caspases.1 ALF tends to evolve quickly and decisions about care, prognosis or transplantation must therefore be made quickly. Estimating the severity of the patient's condition is important, because if liver transplantation is necessary, it must be undertaken quickly and urgent transport of patients to centres where liver transplantation is available is essential before they deteriorate.2 A major problem for the physician is that transplanted patients have a 60–80% 5 year survival rate i.e. not 100% and in addition a significant number of ALF patients will survive without a transplant.3,4 Therefore patient selection before it is too late for the patient to get a donor liver, but not so early that they have been transplanted unnecessarily is extremely difficult.2–4 Intracerebral pressure monitoring: A common cause of death from ALF is cerebral oedema due to raised intracranial pressure,2,5 which usually occurs in patients with Grade IV coma, although it may occur rapidly in patients with lesser degrees of encephalopathy. Intracranial pressure may increase rapidly and waiting for clinical signs of cerebral oedema such as pupillary abnormalities, bradycardia, hypertension and opisthotonus may result in brain death before treatment can be initiated.6,7 Intracerebral pressure monitoring should ideally therefore be undertaken in all patients with acute liver failure who require mechanical ventilation and all patients fulfilling criteria for transplantation (e.g. using a Camino monitor).6–7 Coagulation tests: The prothrombin time is the best single marker of hepatic function and prognostic indicator to date.8,9 92% of patients whose peak prothrombin time is greater than or equal to 180 seconds died.9 Of 42 patients with a continuing rise in prothrombin time between days 3 and 4 after overdose, 93% died compared with 22% of those in whom the prothrombin time fell.9 Our clinical experience indicates that once a patient's PTR starts to improve, their other clinical parameters start to improve i.e. they begin to “turn the corner”. Reduction of Factor V concentrations to <30% if more than 30 years old or <20% if less than 30 years old may also be of prognostic value.10 Factor V is a prognostic indicator in ALF with a positive predictive value of 0.73, if combined with degree of encephalopathy.11 This is less effective than the well-validated King's criteria. Factor VII/Factor V ratios can also be used as a prognostic indicator in paracetamol poisoning, the ratio being <30 in patients surviving paracetamol overdose.12,13 A factor V of <10% on admission predicted adverse outcome in 10 of 11 fatal cases (91% sensitivity).12 Immunological tests and acute phase response: Gc globulin scavenges actin released from dead hepatocytes. Using a cutoff level of serum Gc globulin of 100 mg/L, a lesser value correctly predicted non survival in 100% of patients and survival in 53% of patients with ALF i.e. acute liver transplantation should be considered in ALF patients with Gc-globulin <100 mg/L.14 A total Gc-globulin level <120 mg/l on day 2 of hospitalisation is a good predictor of later encephalopathy in patients with paracetamol poisoning with ALT>1000 iu/l but not ALF yet.15 Higher hepatocyte growth factor in serum (10.1 vs 4.3 ng/ml) was seen in ALF patients who died.16 No significant association was found between either TNF A or B genotype in patients with ALF and parameters for multiple organ failure. The tumour necrosis factor B1B1 genotype was significantly underrepresented in patients developing severe encephalopathy. The association is independent of HLA class II allele DRB1*3 which is closely linked to the TNFB locus. The apparent protective effect of this genotype may be related to TNF alpha production is sepsis.17 In ALF the Systemic Inflammatory Response Syndrome, whether or not precipitated by infection, is implicated in the development of encephalopathy, reducing the chances of transplantation and conferring a poorer prognosis.18 Metabolic profiling: Early metabolic acidosis, due to lactate has long been reported to be associated with high plasma paracetamol concentrations and subsequent significant hepatic injury.19–20 Hyperlactaemia, with or without significant acid-base disturbance is common following paracetamol overdose, particularly in those severely poisoned.21–22 Renal phosphate loss (and serum phosphate concentration) correlates with degree of hepatic damage, though can occur in its absence.23 The 14C-aminopyrine breath test, carried out within 24–36 hours of an overdose of paracetamol was predictive of the degree of liver injury.24 Multivariate prognostic scoring systems: The O'Grady criteria, incorporating assessment of coagulopathy and cerebral oedema provides the best practical guide to date to decide when a patient should be listed for transplantation i.e. when the prognosis is poor enough to justify its use.2,8,25 A positive predictive value of 92% is reported,11 though others have found a positive predictive value of 88%, a negative predictive value of 65% and a predictive accuracy of 71%.26 The O'Grady criteria include; a) arterial blood pH<7.3 or H+>50 mmol/L or b) PTR>100 secs and serum creatinine>300 μmol/L in patients with Grade III or Grade IV encephalopathy. It is important to avoid any sedative drugs and also not to give clotting factors unless there is a life threatening bleed, a need to place an intracerebral pressure monitor or the PTR has reached at least 100 seconds i.e. the patient has reached transplant criteria already, otherwise the O'Grady criteria are skewed and prognostic value will be lost. Bernal has produced a modified O'Grady criteria which indicates that if serum lactate is >3 at 4 hours or >3.5 at 12 hours, this increase the positive predictive value of the O'Grady criteria.27 The finding of a high APACHE II or III score can be used to indicate the need for liver transplantation.4,28 Specialist liver scores may be unfamiliar in the general intensive care setting and APACHE scoring may expedite appropriate transfer to a liver unit. Prediction of hepatic encephalopathy in paracetamol overdose can be achieved by using the log 10 of hrs since overdose, log 10 of plasma coagulation factors on admission and platelet count hrs from overdose.29 This has a positive predictive value of 88% and a negative predictive value of 90%. Again, it is useful in deciding on transfer of high risk patients to a liver intensive care unit. Conclusions: The management of late paracetamol poisoning, in particular acute liver failure, is complex. There is no completely reliable prognostic test for individual patients with paracetamol overdoses. To date the modified O'Grady criteria have the best positive predictive value and the widest use. However, lack of fulfillment does not predict survival.30
References: 1. Blatzka, M.E.; Wilmer, J.L.; Holladay, S.D.; Wilson, R.E.; Luster, M.I. Role of Proinflammatory Cytokines in Acetaminophen Hepatotoxicity. Toxicol. Appl. Pharmacol. 1995, 133, 43–52. 2. Fellay, M.; Kehtari, R. Insuffisance Hépatique Fulminante et Subfulminante. Rev. Med. Suisse. Romande. 1996, 116, 483–491. 3. Caraceni, P.; Van Thiel, D.H. Acute Liver Failure. Lancet 1995, 45, 163–169. 4. Bernal W.; Wendon, J.; Rela, M.; Heaton, N.; Williams, R. Use and Outcome of Liver Transplantation in Acetaminophen-Induced Acute Liver Failure. Hepatology 1998, 27, 1050–1055. 5. Ware, A.J.; D'Agostinio, A.N.; Coombes B. Cerebral Edema: A Major Complication of Massive Hepatic Necrosis. Gastroenterol 1971, 61, 877–884. 6. Ellis, A.; Wendon, J. Circulatory, Respiratory, Cerebral and Renal Derangements in Acute Liver Failure: Pathophysiology and Management. Semin. Liver Dis. 1996, 16, 379–388. 7. Jones, A.L. Recent Advances in the Management of Late Paracetamol Poisoning. Emergency Med. (Austr) 2000, 12, 14–21. 8. O'Grady, J.G.; Alexander, G.J.M.; Hayllar, K.M.; Williams, R. Early Indicators of Poor Prognosis in Fulminant Hepatic Failure. Gastroenterology 1989, 97, 439–445. 9. Harrison, P.M.; O'Grady, J.G.; Keays, R.T.; Alexander, G.J.; Williams R. Serial Prothrombin Time as Prognostic Indicator in Paracetamol Induced Fulminant Hepatic Failure. Br. Med. J. 1990, 301, 964–966. 10. Bernuau, J.; Rueff, B.; Benhamou, J.P. Fulminant and Subfulminant Liver Failure: Definition and Causes. Semin. Liv. Dis. 1986, 6, 97–106. 11. Izumi, S.; Langlet, P.G.; Wendon J.; Ellis, A.J.; Pernambuco, R.B.; Hughes, R.D.; Williams R. Coagulation Factor V Levels as a Prognostic Indicator in Fulminant Hepatic Failure. Hepatol. 1996, 23, 1507–1511. 12. Pereira, L.M.; Langley, P.G.; Hayllar, K.M.; Tredger, J.M.; Williams R. Coagulation Factor V and VIII/V Ratio as Predictors of Outcome in Paracetamol Induced, FHF: Relation to Other Prognostic Indicators. Gut 1992, 33, 98–102. 13. Bradberry, S.M.; Hart, M.; Bareford, D.; Jones, A.F.; Vale, J.A. Factor V and Factor, VIII:V Ratio as Prognostic Indicators in Paracetamol Poisoning. Lancet 1995, 346, 646. 14. Schiodt, F.V.; Bondesen, S.; Petersen, I.; Dalhoff, K.; Ott, P.; Tygstrup, N. Admission Levels of Serum Gc-Globulin: Predictive Value in FHF. Hepatology 1996, 23, 713–718. 15. Schiodt, F.V.; Ott, P.; Tygstrup, N.; Dahl, B.; Bondesen, S. Temporal Profile of Total, Bound and Free Gc-Globulin After Acetaminophen Overdose. Liver Transpl. 2001, 7, 732–738. 16. Hughes, R.D.; Zhang, L.; Tsubouchi, H.; Daikuhara, Y.; Williams, R. Plasma Hepatocyte Growth Factor and Biliprotein Levels and Outcome in Fulminant Hepatic Failure. J. Hepatol. 1994, 20, 106–111. 17. Bernal, W.; Donaldson, P.; Underhill, J.; Wendon, J.; Williams, R. Tumor Necrosis Factor Genomic Polymorphism and Outcome of Acetaminophen (Paracetamol) Induced Acute Liver Failure. J. Hepatol. 1998, 29, 53–59. 18. Rolando, N.; Wade, J.; Davalos, M.; Wendon, J.; Philpott-Howard, J.; Williams, R. The Systemic Inflammatory Response Syndrome in Acute Liver Failure. Hepatology 2000, 32, 734–739. 19. Flanagan, R.J.; Mant, T.G. Coma and Metabolic Acidosis Early in Severe Acute Paracetamol Poisoning. Hum. Toxicol. 1986, 5, 179–182. 20. Record, C.O.; Iles, R.A.; Cohen, R.D.; Williams R. Acid–Base and Metabolic Disturbances in Fulminant Hepatic Failure. Gut 1975, 16, 144–149. 21. Gray, T.A.; Buckley, B.M.; Vale, J.A. Hyperlactaemia and Metabolic Acidosis Following Paracetamol Overdose. Q. J. Med. 1987, 65, 811–821. 22. Murphy, N.D.; Kodakat, S.K.; Wendon, J.A.; Jooste, C.A.; Muiesan P.; Rela M.; Heaton, N.D. Liver and Intestinal Lactate Metabolism in Patients with Acute Hepatic Failure Undergoing Liver Transplantation. Crit. Care Med. 2001, 29, 2111–2118. 23. Jones, A.F.; Harvey, J.M.; Vale, J.A. Hypophosphataemia and Phosphaturia in Paracetamol Poisoning. Lancet 1989, 2, 608–609. 24. Saunders, J.B.; Wright, N.; Lewis, K.O. Predicting Outcome of Paracetamol Poisoning by Use of 14C-Aminopyrine Breath Test. Br. Med. J. 1980, 280, 279–280. 25. O'Grady, J.G.; Wendon, J.; Tan, K.C.; Potter, D.; Cottam, S.; Cohen, A.T. et al. Liver Transplantation After Paracetamol Overdose. Br. Med. J. 1991, 303, 221–223. 26. Anand, A.C.; Nightingale, P.; Neuberger, J.M. Early Indicators of Prognosis in Fulminant Hepatic Failure: An Assessment of the King's Criteria. J. Hepatol. 1997, 26, 62–68. 27. Bernal, W. et al. Prognostic Value of Serum Lactate in Addition to O'Grady Criteria. Lancet (in press). 28. Mitchell, I.; Bihari, D.; Chang, R.; Wendon, J.; Williams R. Earlier Identification of Patients at Risk from Acetaminophen-Induced Acute Liver Failure. Crit. Care. Med. 1998, 26, 279–284. 29. Schiodt, F.V.; Bondesen, S.; Tygstrup, N.; Christensen E. Prediction of Hepatic Encephalopathy in Paracetamol Overdose: A Prospective and Validated Study. Scand. J. Gastroenterol. 1999, 34, 723–728. 30. Shakil, A.O.; Kramer, D.; Mazariegos, G.V.; Fung, J.J.; Rakela J. Acute Liver Failure: Clinical Features, Outcome Analysis and Applicability of Prognostic Criteria. Liver Transpl. 2000, 6, 163–169.
58 INDICATION OF LIVER TRANSPLANTATION (LTX) BY AMANITA INTOXICATION
Ganzert M, Felgenhauer N, Zilker Th.
Toxikologische Abteilung der, I.I. Medizinischen Klinik, Klinikum rechts der Isar, Technische Universität München, Germany
Objective: Improvement of liver transplantation technique makes LTX an option in therapy of severe Amanita intoxication. Because amatoxin-induced liver damage recovers completely in survivors criterias are needed which indicate the outcome in an early stage. Methods: To find criteria for LTX in Amanita intoxication we examined 140 cases. A group of 33 severe cases were compared with 20 fatal cases. The severe cases were characterized by a rise of the ALT above 560 U/l and a decline of the thromboplastin time below 46%. Following laboratory parameters characterizing hepato- and nephropathy were used to discern the two groups: ALT, bilirubin, thromboplastin time and creatinine. For each parameter for each day after ingestion the sensitivity and specifity of fatal outcome was determined. As cutoff values we used the upper limit of normal range for creatinine and the mean value of all cases for the other parameters. Results (see Table 1): The transaminases and the bilirubin do not predict the outcome. A creatinine value which stays in the normal range after the second day indicates a non fatal course. Any elevation above 1.2 mg/dl indicates a fatal outcome. The day to day variation of creatinine-sensitivity is caused by early death and by the variation of the start of the creatinine increase. In 5 cases this increase commences before the third day, in 11 cases between the third and the sixth day. The second important parameter is the thromboplastin time, if it is below 35% at the fifth day under moderate substitution of clotting factors; all fatal cases show a thromboplastin time value below 35% at the fifth day. Conclusion: Whenever the creatinine value is below 1.2 mg/dl after the second day and the thromboplastin time stays over 35% beyond the fifth day no liver transplantation is needed. All other cases have to be transmitted to a transplantation centre.
Table 1. Sensitivity and Specificity Related to Fatal Outcome of ALT, Bilirubin, Thromboplastin Time and Creatinine from Day 2 to 5 After Ingestion (Abstract 58)
59 RENAL AND LIVER TRANSPLANTATION FOR TOXIN-INDUCED ORGAN FAILURE
Tracey JA,1 Casey PB,1 Cunningham P,2 Counihan A,2 Fleming J,3 Hickey D,2 Hegarty J3
1National Poisons Information Centre Beaumont Hospital, 2Renal Transplant Unit, Beaumont Hospital, 3Liver Unit, St Vincent's Hospital, Dublin, Ireland
Objective: To determine the number of renal and liver transplants performed in Ireland for drug or toxin-induced organ failure. Methods: Retrospective review of records held by the renal and liver transplant co-ordinators on patients requiring transplantation for drug or toxin-induced organ failure. All cases of acute and chronic toxicity since the start of the national renal and liver transplant programmes in 1987 and 1993 respectively were included. Results: A total of 1686 renal and 209 liver transplants were performed. 14 (0.8%) patients received renal transplants for nephropathy secondary to drugs or toxins. Renal failure was caused by acute toxin ingestion in only 2 cases: an unidentified mushroom in 1 case and ethylene glycol in the second. In the remaining 12 cases renal failure was caused by chronic exposure to pharmaceuticals or chemicals. 7 patients developed renal failure secondary to cyclosporine A therapy following previous heart, lung or heart/lung transplants, 2 following therapy with gold salts, 1 following lithium therapy, 1 due to occupational exposure to mercury and 1 due to an unknown toxin. Four of these patients died: 1 from carcinoma, 1 from rejection of the heart/lung transplant and 2 from renal failure. Of the surviving cases, 1 patient has deteriorating renal function (creatinine 400), 1 suffered rejection 5 years after the transplant and 8 have good renal function. 34 (16.3%) of the 209 liver transplants were for toxin induced organ failure; 8 for drug induced liver failure (7 paracetamol overdose and 1 ibuprofen induced) and 26 for alcoholic liver disease (3 of these cases also had hepatitis C, 1 alpha1 antitrypsin deficiency and 1 haemachromatosis). 1 patient subsequently required two further liver transplants because of chronic rejection and 2 patients required a second transplant because of hepatic artery thrombosis. 12 of these 34 liver transplants recipients died: 3 from cardiac arrest within days of the transplant, 3 disease and in 3 cases the cause of death could not be ascertained. Conclusions: Less than 1% of renal transplants from multi-organ failure, 1 from acute pylenephritis, 1 from sepsis secondary to hepatic ischaemia, 1 from graft vs. host were for drug or toxin induced renal failure. Only two of these 14 renal transplants were required because of acute toxicity. The remainder were for chronic toxicity, most commonly cyclosporine A toxicity in recipients of heart, lung or heart/lung transplants. A higher proportion (16.3%) of liver transplants were for drug or toxin induced organ failure, predominantly alcoholic liver disease and paracetamol overdose.
60 ORGAN DONATION AFTER FATAL ACUTE POISONING
Hantson Ph
Department of Intensive Care Medicine, Cliniques St-Luc, Université catholique de Louvain, Brussels, Belgium
Objective: Due to the increasing gap between the number of patients awaiting organ transplantation and the number of available grafts, policies of organ donation have been reconsidered. The use of “marginal” donors is discussed in most of the centers. They may include a minority of patients who died from acute poisoning. Experience indicates that, in selected cases, the organs from such donors function as well in recipients as in those from more conventional sources. The objective of this presentation is to present the experience of an university hospital in this field. The problem of brain death diagnosis will be discussed and some criteria of organ procurement without unacceptable risks may be proposed. Material and Methods: From January 1989 to December 1997, 908 kidney transplantations, 894 adult and child liver transplantations, 39 pancreas transplantations and 197 heart transplantations were performed at Cliniques St-Luc, a 900-bed teaching hospital with a 42-bed Intensive Care Unit (ICU); lung transplantations (n=27) were performed in an affiliated hospital beginning in 1991. During the same period (1989–1997), 864 organs were procured from 293 donors within our organ procurement area (±1.5 million inhabitants). It was remarkable to note that of the 293 donors, 21 (7%) had developed brain death after acute poisoning. The toxic substances involved were: methaqualone (n=1), benzodiazepines (n=3), tricyclic antidepressants (n=2), barbiturates (n=1), insulin (n=2), carbon monoxide (n=3), cyanide (n=1), paracetamol (n=1), methanol (n=7). On the whole, 39 kidneys were procured, 6 hearts, 2 lungs, 9 livers and 2 pancreas. General criteria for organ donation were applied, and also more specific criteria according to the nature of the toxins. Results: The survival rate at 1 year and 5 years was 100 and 88% in the kidney group, 67 and 67% in the liver group, 100% and 100% in the pancreas group, 50 and 33% in the heart group, 100 and 100% in the lung group. Six grafts were lost (acute or chronic rejection) in the kidney group, 2 in the liver group and 1 (but only after 8 years) in the pancreas group. The analysis of immediate mortality (n=4) showed no association with toxic graft origin. Outcome comparison versus a non-poisoned donor group is difficult in a single-center study due to the small number of observations. Discussion: Organ procurement from poisoned donors remains a poorly documented topic. Experience in most countries is limited. The data obtained from organ procurement organizations in Europe (Eurotransplant, France, United Kingdom, Spain) or in North America indicate that poisoned patients in these different countries represent usually less than 1% of all the organ donors. Several reasons could be identified. First of all, the problem of brain death diagnosis after acute poisoning remains difficult. Indeed, brain death is an uncommon issue after acute poisoning. Brain death is defined in most countries by the complete and irreversible loss of all brain and brainstem function. The diagnosis of brain death relies mainly on clinical criteria but the clinical pattern is not sufficient to confirm brain death in all cases. Confirmatory tests are required when misleading conditions are present (hypothermia, sedative drugs,…). The electroencephalogram is by far the most used confirmatory test in the brain death diagnosis. However, this technique is very sensitive to hypothermia, drugs, and metabolic disorders and therefore has the same limitation as the clinical examination. We propose to use multimodality evoked potentials (MEPs); they combine the use of brainstem auditory evoked potentials (BAEPs), flash visual evoked potentials (VEPs) and median somatosensory evoked potentials (SEPs). They are easily and rapidly recordable at the patient's bedside and evaluate the brainstem as well as the cerebral cortex. They are less influenced by hypothermia or by central nervous system acting drugs. A second reason that could explain the reluctance to consider organ donation is the insufficient knowledge of the action of the toxins on organs function. The routine biological data and the morphologic analysis at the time of organ harvest are extremely helpful to exclude organ dysfunction. The risk of toxin transmission to the recipient can be minimized by a careful analysis of the toxicologic data. In the literature, the largest experience has been collected with kidney donation. The results of organ transplantation are similar to those observed in a non-poisoned donor population. Heart donation from poisoned donors has been poorly documented and it is obvious that the heart is extremely sensitive to hypoxic or ischemic injury. Some toxins clearly preclude heart donation: tricyclic antidepressants, cocaine,… Exposure to some toxins remains controversial. For example, carbon monoxide poisoning is a relative contraindication to heart donation as the heart is a target organ in carbon monoxide poisoning and failures have been reported with grafts originated from carbon monoxide poisoned donors. Heart donation after fatal methanol poisoning can also be debated. Positive experience has been reported after insulin or cyanide poisoning. Liver donation is possible in selected cases and successful liver allografts have been reported after poisoning by benzodiazepines, methaqualone, barbiturates, tricyclic antidpressants, insulin, carbon monoxide, cyanide, methanol, cocaine and lead. Literature data concerning lung or pancreas donation are virtually non-existent. Conclusion: Organ donation after fatal poisoning has been poorly studied. Our experience together with the existing literature data suggests that organ donation is feasible in selected cases without unacceptable risks. Reference: Hantson, P.; Vekemans, M.C.; Squifflet, J.P.; Mahieu, P. Outcome Following Organ Removal from Poisoned Donors: Experience with 12 Cases and Review of the Literature. Transpl. Int. 1995, 8, 185–189.
61 LETHAL PLANT EXPOSURES REPORTED TO POISON CENTERS: PREVALENCE, CHARACTERIZATION AND MECHANISMS OF TOXICITY
Krenzelok EP
Pittsburgh Poison Center, Children's Hospital of Pittsburgh; Schools of Pharmacy and Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
Objectives: Plant alkaloids and derivatives have played important roles in ancient and contemporary medicine. The Ebers Papyrus, which has origins to approximately 1600 BC, contains hundreds of prescriptions and various concoctions, many of which utilized plant components known to be associated with significant toxicity. The infamous death of Socrates in 399 BC, is attributed to the ingestion of a beverage that contained poison hemlock (Conium maculatum). Ancient Romans allegedly assassinated political adversaries with poisonous plants. History has painted a rather reprehensible picture of plants, portraying many as being associated with significant morbidity and mortality. Based upon published data from the American Association of Poison Control Centers Toxic Exposure Surveillance System (AAPCC TESS), this morbid embellishment is not justified. The objective of this presentation is to identify the plants that have been associated with a fatal outcome in AAPCC TESS, discuss those most commonly associated with a fatal outcome, create a perspective about plant toxicity and to examine the mechanisms of toxicity. Methods: The annual report of AAPCC TESS is published in the American Journal of Emergency Medicine. Each report provides a profile of all fatalities submitted by American poison centers. These reports (1983–2000) were reviewed systematically to identify the total number of exposures reported to poison centers, the number of plant exposures and the specific plant exposures that resulted in a fatal outcome. Results: Over 18 years 29,172,989 exposures were reported by poison centers and 1,709,805 (5.9%) involved exposure to plants. During the same period 10,931 fatalities occurred and 30 involved exposure to plants (0.3%). Twenty-four (Table 1) different botanical species or derivatives were implicated in the fatalities. Discussion: There are a number of plant species that have been identified in the literature as being associated with fatal outcomes. Some such as Taxus species are more prominent problems in veterinary medicine. Exposure to plants that contain typical and atypical cardiac glycosides including Digitalis purpurea and Thevetia peruviana have been documented to produce fatal outcomes. Ricinus communis and Abrus precatorius have received recent attention as bioterrorism agents since they are the source of the highly toxic toxalbumin. Many plants (e.g., Convallaria majalis, Nerium oleander, Solanum dulcamara, Hydrangea species, Rhododendron species) have been vilified due to inaccurate characterization in the literature or based upon isolated case reports. In 18 years of AAPCC TESS reports none of these plant species were associated with a fatal outcome. Fatal outcomes are most commonly associated with the intentional abuse or misuse of botanical agents. Cicuta species accounted for 23.3% of the botanical related fatalities. Commonly referred to as “water hemlock” (beaver poison, children's bane, cowbane, death-of-man, musquash root, poison parsley), four species predominate: C. bulbifera L., C. douglassii, C. maculata L., C. virosa L. Cicuta species thrive in wet habitats. The geographical distribution includes most of North America and Europe. Genus Oenanthe is common in Europe and contains similar toxins. Cicuta produces cicutoxin and cicutol which are complex 17 carbon linear structures as well as a number of acetylenic compounds. Cicutoxin is a potent central nervous system (CNS) stimulant that produces violent seizure activity. The CNS effects of cicutoxin are similar to those of picrotoxin, a known inhibitor of GABA. Severe gastrointestinal symptoms, diaphoresis, salivation and skeletal muscle stimulation may precede the seizure activity. Conium maculatum (“poison hemlock”) is often confused with Cicuta maculata and Cicuta species, due to similarities in their common and botanical names and the “Queen Ann's Lace” flowering appearance of the plants. Other common synonyms for Conium maculatum include: spotted hemlock, stinkweed, St. Bennett's weed, poison parsley, fool's parsley, carrot fern and winter fern. The plant is distributed widely throughout North America, with the exception of desert regions, and Europe. Conium maculatum contains a variety of volatile pyridine alkaloids, predominated by the presence of coniine, N-methylconiine and gamma-coniceine. Coniceine is significantly more toxic than coniine and is thought to be the precursor to coniine. The toxic activity of the alkaloids is similar to that of nicotine. Large doses produce nonpolarizing neuromuscular blockade which may result in respiratory depression and death. Datura stramonium or “jimsonweed” (datura, devil's apple, devil's trumpet, Indian apple, Jamestown weed, loco seed, stinkweed, thornapple) and other Datura species (D. discolor Bernh., D. ferox L., D. inoxia Mill., D. metel L., D. quercifolia Kunth, D. wrightii Regel) have extensive distribution throughout North America. Several species are indigenous to Europe and Asia. The majority of toxic effects may be attributed to the tropane alkaloids L-hyoscyamine and scopolamine. These alkaloids are potent antagonists of acetylcholine at muscarinic receptors and produce the well-described anticholinergic toxidrome. While morbidity is significant, fatalities are rare and may be the consequence of hyperthermia, seizures and/or arrhythmias. Pennyroyal oil is derived from Mentha pulegium and Hedeoma pulegioides. Often referred to as American pennyroyal, squaw mint and mosquito, the primary geographical distribution of the plants is eastern North America. Pennyroyal oil use is increasing due to its role in alternative medicine. The ketone pulegone is the primary toxin and small amounts are capable of producing severe hepatotoxicity. Conclusions: Most plant exposures have a favorable outcome and fatalities are rare. However, it is important to be aware of which plants have toxic potential and their mechanisms of toxicity.
Table 1. Botanicals Implicated in Fatalities (AAPCC TESS 1983–2000) (Abstract 61)
62 SNAKE ENVENOMATION: IS IT A EUROPEAN TOXICOLOGICAL PROBLEM?
Barelli A,1 De Giacomo 1 Russo A,2 Gargano F,1 Dannaoui B,3 Della Puppa T,4 Assisi F4
Poison Centre, 1Catholic University School of Medicine, 2Università La Sapienza, Roma; 3Toxicology Unit and Poison Control Centre, Azienda Ospedaliera Careggi, Firenze; 4Poison Centre, Osp. Niguarda, Milan-Italy
Objectives: To describe the prehospital and hospital care of snake envenomations. To describe the epidemiology of snake envenomations in Italy and Sri Lanka. Background: 3000 species of venomous snakes exist throughout the world, of which 15% are known to be poisonous to humans. Estimates of deaths each year from snakebite range from 30,000 to 110,000 worldwide (out of 300,000–400,000 venomous snakebites). Most victims are males aged 18–28 years. Snake venom consists of a complex mixture of chemical substances, mainly proteins, with enzymatic and spreading activity. Neurotoxic, haemolytic, pro-coagulant and cytotoxic effects are usually present. Because most venom components appear to bind with multiple physiological receptor sites, the classification of snake venoms as “neurotoxins”, “haemotoxins” and “cardiotoxins” should be considered arbitrary and superficial. The venom is mostly injected subcutaneously, occasionally intramuscular or intravenous injection occurs. The venom of most vipers (crotalids) contains toxic protein compounds which produce local and systemic effects. Local effects include fang marks, immediate burning pain, edema, erythema or ecchymosis. If untreated, edema progresses quickly and may involve the entire extremity. Fang marks can suggest the type of snake but do not provide any definitive identification. Bites by nonvenomous snakes usually show multiple teeth marks. Coral snake (elapid) venom contains primarily neurotoxic components which produce neuromuscolar blockade; the lack of significant proteolytic enzyme activity accounts for minimal or absent pain and swelling seen at the bite site. The clinical manifestations of envenomation vary hugely between individual patients, being influenced by factors such as body weight, amount of venom injected, age and state of health of the patient, time elapsed since the bite and site of the bite. The size and maturity of the snake and the time since it last bit will influence the severity of envenomation. Myolysis is typical in sea snake and tiger snake envenomations, while death adder and coral snake venom is predominantly neurotoxic. Rattlesnake envenomations may induce a wide range of coagulation abnormalities. Systemic symptoms and signs may include nausea, vomiting, diaphoresis, fever, generalized weakness, paresthesias, muscle fasciculation, altered mental status, hypotension and shock. Epidemiology, Italy: The Poison Centres of Milan, Rome and Florence manage more than 90% of calls in Italy. Data concerning snake envenomations managed by these Poison Centres are summarised in table 1. Epidemiology, Sri Lanka: hospital admissions and deaths from snake bites (1987–1996) in Sri Lanka are shown in table 2. Discussion: Before treatment is begun, it must be established whether the snake was venomous and whether envenomation occurred. “Dry bites” (snake bites without envenomation) occur in about 20 to 30% of pit viper bites and in about 50% of coral snake bites. The injured part should be immobilized in a functional position just below heart level. Jewelry and constrictive clothing should be removed in anticipation of swelling. The victim should avoid exertion and be reassured, kept warm and transported to the nearest ED as quickly and as safely as possible. Lymphatic constriction bands and pressure-immobilisation methods may inhibit the spread of venom but may increase local necrosis. Negative pressure extraction devices, applied directly over fang marks, may be of value in pit viper bites if applied within a few minutes of the bite and continued for 30 to 60 minutes. Tourniquets, incision and suction, cryotherapy, electric shock, alcohol, stimulants, aspirin, and various folk and herbal remedies are contraindicated. A fondly held folk treatment is the well known “black stone” (piedra nigra), a small piece of animal bone which has been charred in a fire, apparently able to adsorb and neutralize the venom from the site of the bite. General support should be given to airway, breathing and circulation, following the ALS guidelines if indicated. Antivenom remains the key aspect of treatment for moderate and severe envenomation. Identification of the offending snake is necessary for selection of the most specific antivenom and alert clinicians to particular features of envenomation by that type of snake. Venom Detection Kits can be used in conjunction with other information such as clinical presentation, geographic area, identification of the snake by the zoo staff, herpetologists or other experienced snake handlers. If a reliable identification is not possible, a polyvalent snake antivenom should be considered. The effectiveness of antivenom is time- and dose-related; antivenom is most effective within the first 4 h and less effective after 12 h. Because most commercially approved antivenoms are equine derived, hypersensitivity reactions can occur. The issue of premedication prior to the administration of antivenoms has been controversial. Some studies have shown that premedication with subcutaneous adrenaline reduces the rate of allergic reactions. Antivenom ovine Fab are also available (Viper, Crotalus, etc); clinical trials have shown that they are safe and effective with a lower incidence of immediate and delayed hypersensitivity reactions. Snake bites are not a major toxicological problem in Italy, as far as mortality is concerned. As for morbidity, snake bites are a problem and Poison Centres must deal with them in a proper way. It is believed that 2–3 people in Sri Lanka die from snake bite every day, and this country has the highest death rate from snake bite in the world.
References: Seth, A.K.; Varma, P.P.; Pakhetra, R. Randomised Control Trial on the Effective Dose of Anti-Snake Venom in Cases of Snake Bite with Systemic Envenomation. J. Assoc. Physicians India 2000, 48, 756. Galli R. The Antivenin Is Safe, but Its Future Is Uncertain. West J. Med. 2001, 175 (2), 91–92. Saadeh, A.M. Case Report: Acute Myocardial Infarction Complicating a Viper Bite. Am. J. Trop. Med. Hyg. 2001, 64 (5–6), 280–282. Persson, H. Envenoming by European Vipers Antivenom Treatment Influence on Morbidity. Przegl. Lek. 2001, 58 (4), 223–225.,
Table 1. Viper Bites in Italy (Abstract 62)
Table 2. Snake Bites in Sri Lanka (Abstract 62)
63 ADMINISTRATION OF CROTALIDAE POLYVALENT IMMUNE FAB (OVINE) FOLLOWING TIMBER RATTLESNAKE ENVENOMATION: A CASE SERIES
Gunn SK, Burkhart KK, Berger DR, Donovan JW
Pennsylvania State University, Hershey, Pennsylvania, USA
Objectives: Crotalidae Polyvalent Immune Fab Ovine (CroFab) (Altana, Inc.) became available for the treatment of crotalid snake envenomations in October 2000. This antivenin contains a mixture of monospecific Fab fragments (FabAV) against the Eastern Diamondback (Crotalus adamanteus), Western Diamondback (C. atrox), Mojave (C. scutulatus) and Cottonmouth (Agkistrodon piscivorus) snakes. To our knowledge, there have been no reports of its use following envenomation by the timber rattlesnake, Crotalus horridus horridus. Thrombocytopenia has been problematic and unresponsive to Antivenin (Crotalidae) Polyvalent (ACP), Wyeth-Ayerst Laboratories.1 We present a three case series of FabAV use following timber rattlesnake envenomation. Case Series: Three male patients ages 53, 30 and 36 were all bitten on their hands. All three patients developed thrombocytopenia (nadirs 20, 14 and 5×103/L). Two of the three patients also demonstrated a coagulopathy; prolonged prothrombin time, decreased fibrinogen, and elevated d-dimer. Patient 1 received 34 vials of FabAV. Patient 2 received six vials ACP prior to transfer and then 26 vials FabAV. Patient 3 received 18 vials FabAV. He developed recurrence and received 4 vials ACP as a medication error followed by 4 more vials FabAV. The medication error was secondary to the pharmacy sending the ACP rather than the FabAV product to the Emergency Department. In cases 1 and 3 the thrombocytopenia resolved within three hours of completion of the FabAV loading dose. In case 2, where the patient received six vials of (ACP) prior to initiating FabAV therapy, the thrombocytopenia failed to resolve; on day 9 it had slowly increased to 54×103/L. The coagulopathies also resolved following a total of 18 and 14 vials FabAV, respectively for patients 1 and 3. The thrombocytopenia and coagulopathy, however, recurred within 70 to 95 hours of the FabAV dosing. Additional FabAV reversed the coagulopathy, but not the thrombocytopenia. Conclusion: FabAV as initial antivenin was effective in reversing thrombocytopenia and coagulopathy in two patients. It was not effective in the patient who was given ACP first. Unfortunately, 2–3 days later without additional FabAV the thrombocytopenia and coagulopathy recur. The thrombocytopenia then appears to be refractory to additional antivenin. While hematologic abnormalities respond rapidly to initial therapy, recurrence and diminished effectiveness of subsequent FabAV appears similar following timber rattlesnake envenomation as compared to other crotalid snakes as described in the clinical trials.2
References: Bond, G.R. et al. Thrombocytopenia Following Timber Rattlesnake Envenomation. Ann. Emerg. Med. 1997, 30, 40–44. Boyer, L.V. et al. Recurrence Phenomena After Immunoglobulin Therapy for Snake Envenomations: Part 2. Guidelines for Clinical Management with Crotaline Fab Antivenom. Ann. Emerg. Med. 2201, 37, 196–201.
64 VIPERA BERUS BITES IN SWEDEN DURING ONE YEAR—EPIDEMIOLOGY AND MORBIDITY IN 231 CASES
Karlson-Stiber C, Salmonson H, Persson H
Swedish Poisons Information Centre, Stockholm, Sweden
Objective: To determine the morbidity and hospitalization rate due to Vipera berus envenoming in Sweden 1995 and to compare with a similar study from 1975. Methods: In Sweden a national register makes it possible to identify all patients with a certain diagnosis code. All case records with a relevant code during 1995 were requested. Epidemiological data, symptoms and treatment were documented and analyzed. The severity of envenoming was assessed on admission to hospital (initial severity grading) using the Poisoning Severity Score. In addition, a severity grading was made of the overall clinical course (maximum severity grading). Results: In total 231 patients were treated as in-patients in Swedish hospitals because of V. berus bites 1995. Most bites occurred along the coasts in the southern half of the country. Bites were recorded from April to September with a peak in the middle of the summer. Hospitalization of children below the age of 10 was more frequent than in all other age groups. The incidence of bites was slightly higher in males. Bites on the lower extremity were most common (63%). Severity grading could be assessed in 229 patients. The maximum severity grades were distributed as follows: none 11%, minor 46%, moderate 29% and severe 13%. The highest proportion of severe envenomings was observed in children (42%) and in the elderly (32%). Nine patients initially graded as minor or moderate, subsequently met the criteria for severe envenoming. The most common symptoms of systemic poisoning were gastrointestinal disturbances together with hypotension/shock. Leucocytosis and thrombocytopenia were common laboratory findings in significant poisoning. Local swelling involving the whole extremity occurred in 28% and oedema involving the trunk in 5%. Late symptoms included pulmonary oedema, heart infarction, compartment syndrome and persistent local oedema. Corticosteroids, antibiotics and antihistamines were commonly administrated regardless the severity of symptoms. Antivenom was given in 42 patients. No side-effects were reported. The median duration of hospital stay was 1, 1, 3, and 4 days respectively, in the four severity groups. In 1975 the average time in hospital was 7.6 days for patients with severe symptoms (antivenom was not available in Sweden at that time). Conclusion: The incidence of V. berus bites leading to hospitalization in Sweden was 2.6 per 100,000 inhabitants. This is in accordance with a similar study from 1975. The mortality rate is low and the time spent in hospital due to adder bite has been reduced during the last 20 years. However, complications still occur, and the need for close observation and adequate interventions (e.g. antivenom treatment) must be emphasized.
65 BITES BY EXOTIC SNAKES IN SWEDEN 1990–2000
Karlson-Stiber C, Persson H, Wernell I
Swedish Poisons Information Centre, Stockholm, Sweden
Background: In the middle of the 1990s the Swedish Poisons Information Centre noted a clear increase of inquiries concerning bites by exotic snakes (venomous and non-venomous). This phenomenon initiated a survey (1995), in collaboration with the Swedish Society of Herpetology, concerning the holding of different venomous snakes in private homes as well as in commercial terrariums. On the basis of the data from this survey guidelines were established for the supply of antivenoms in Sweden. Later (2001) one more survey was done, this time in collaboration with another society of herpetology. Objective: To elucidate possible consequences of holding venomous exotic snakes, data collected by the Poisons Centre have been investigated. Methods: Inquiries concerning bites by exotic snakes 1990–2000 and all hospital case records sent to the Poisons Centre during the same period were studied. The severity of poisoning was assessed using the Poisoning Severity Score (J Toxicol Clin Toxicol 1998, 36:205–13). Results: The total number of inquiries concerning bites did slowly increase 1993–1998. Thereafter, however, the curve shows a declining trend. Concerning venomous snakes, the frequency of bites has stabilized on a lower level (about 20 cases/year) than in the middle of the nineties. In total 74 hospital case records were received 1990–2000. The offending snakes were, in order of frequency, different species of Agkistrodon (contortrix, bilineatus, rhodostoma), Trimeresurus albolabris, Vipera (ammodytes, xanthina, wagneri, palaestinae, mauretanica), Crotalus (viridis, polystictus, lepidus, atrox and “unknown”), Naja (melanoleuca, mossambica, haje, annifulera, kaouthia, siamensis and a cross-breed), Bitis (arietans, gabonica), Sistrurus (miliaris, catenatus), Dendroaspis (polylepis, viridis) and Atheris nitschei. Severity grade was None in 16%, Minor in 58%, Moderate in 22% and Severe in 4%. Symptoms of systemic toxicity were observed in 6 patients (8%). Antivenom treatment was given in 19 (26%) of the cases, acute adverse reactions was seen in one of them and serum sickness in four. Compartment syndrome developed in three patients, one of whom had sequelae (an extension defect of a finger). All patients but two were men. There was an overweight for bites occurring Friday–Sunday, late hours. Conclusion: A great number of different species of venomous snakes from all continents (125 according to the two surveys) are held in Sweden. Although many of these are highly venomous, the occurrence of severe envenoming was low in this case series. However, bites constitute a challenge to the medical service as well as to the Poison Centre. Complete antivenom supply is difficult to achieve, to a great deal because of financial considerations.
66 SNAKE BITES IN GERMANY: EXPERIENCE OF THE MUNICH POISON INFORMATION AND TREATMENT CENTRE—PRESENTATION OF A DATA BASE ON ANTIVENOM STOCKING
Zilker Th, Felgenhauer N, Gerber-Zupan G, Kleber JJ
Toxikologische Abteilung der II. Med. Klinik, Klinikum r.d. Isar TU München, München, Germany
In the Poison Centre in Munich a data base is available which allows to find all antivenoms on stock in Germany, Austria and Switzerland, From other European countries the important stockholders are also in that list (Belgium, Denmark, Greece, UK, Italy, Croatia, The Netherlands, Poland and Sweden). This data base can be searched for antivenoms against scorpion and fish sting, spider and snake bites. It can tell where and how much of each antivenom is on stock, the person's telephone number in charge and the expiring data of the antivenom. All stockholders are reminded three months in advance by us about the expiring date of their antivenom and to tell us how much of the antivenom has been ordered again. In a case of a poisonous animal bite or sting the Munich centre can tell the caller where and how much of the necessary antivenom is available closest to the residence. The data base contains 89 sites stocking 84 different antivenoms. We had to treat 106 bites by snakes of the Viperidae, 33 by Crotalidae and 8 by Elapidae family. Bites by the following species happened in the snake's natural habitat: 76 Vipera berus, 14 Vipera ammodytes, 1 Vipera latasti, 1 Vipera lebentina, 2 Vipera palestinae, 1 Vipera xanthina. Viperidae bites happened at home in 6 cases of Bitis sp., 2 of Cerastes sp., 2 of V. russeli, 1 of Echis sp. All bites by Crotalidae and Elapidae happened at home: 10 by Crotalus sp., 10 by Trimerusurus sp., 8 by Agkistrodon rhodostoma, 4 by Sistrurus sp., 1 by Bothrops sp. Elapidae, 7 by Naja sp., 1 by Dendroaspis sp. In the Viperidae group 72/106 (68%) showed local reaction, 9/106 (8%) clotting disorder, none neurological impairment, 8 cardiovascular involvement 8/106 (7%). In the Crotalidae group 25/33 (76%) had local reaction 8/33 (24%) clotting disorder, none neurological and none cardiovascular impairment. In the group of Elapidae 5/8 (63%) exhibited local reaction, none clotting dysfunction 2/8 (25%) neurological involvement and 1/8 (13%) cardiovascular impairment. In some cases our data base was useful in getting the antivenom, especially in one case when a tiger snake antivenom was flown in from London to Cologne. Mostly the antivenom was supplied from our centre. We had two spectacular cases when a Naja naja bite was saved by our antivenom in Salzburg (Austria) and a clotting disorder following a bite by Hoplozephalus sp. was controlled by our Tiger snake antivenom in Lund (Sweden). In conclusion it can be said: Crotalidae bites show mostly severe local reaction, Viperidae bites lead besides the local reaction more often to cardiovascular impairment. Elapidae bites do not always exhibit neurological but quite often local reaction.
67 EXOTIC VENOMOUS PETS ENVENOMATIONS: EXPERIENCE OF THE POISON CENTRE OF MARSEILLE BETWEEN 1997 AND 2001
De Haro L, Hayek-Lanthois M, Arditti J, Valli M
Poison Centre of Marseilles, Hôpital Salvator, Marseilles, France
Objective: The fashion of exotic animals maintained as pets is increasing in France. Venomous species are imported from various tropical countries. Several snakes are locally bred, with creation of hybrid specimens which toxicity is unknown. In order to evaluate the problems due to these poisonous pets, the poison centre of Marseilles studied the cases of envenomation of exotic animal bites or stings between 1997–2001. Case Series: 49 cases of envenomation were observed during this period, consisting of 20 snakes, 16 fishes, 10 spiders, 1 scorpion and 2 marine invertebrates. The snakes belonged to different families: crotalids (genus Crotalus, Sistrurus and Trimeresurus), viperids (genus Bitis, Echis and Vipera, both families responsible for extensive swelling and coagulation disturbances), elapids (Naja, Dendroaspis which bites induce severe neurological signs) and colubrids (3 cases of Lampropeltis bites with pain, oedema and lymphangitis). 12 of the 20 patients bitten by snakes needed Intensive Care Unit management, and 5 received specific antivenom treatment. The antivenom was necessary but not available in southern France for 6 other cases. Sequels (finger necrosis, muscle damages) were observed in 5 cases (Sistrurus, Echis and 3 Trimeresurus bites). All other patients recovered after 1 to 7 days in the hospital. The site of the fish stings had severe pain and local swelling (stings by Pterois sp., Siganus sp. and cat-fishes, bite by the eel Echidna nebulosa). One case, due to Amazonian Stingray Potamotrygon histrix, had extensive swelling with malaise, headache, and tremor. For the spiders, pain and lymphangitis appeared in the 9 tarantulas envenomations, and one patient bitten by a black widow from Madagascar presented a severe latrodectism (diffuse muscle pain and contractions, blood pressure disturbances). The Centruroides scorpion sting and the 2 marine invertebrates dermal contacts induced only local signs. For the fish, the marine invertebrates and the arachnids envenomations, the patients were treated in an emergency unit. There was one hospitalization due to the black widow (treatment: infusions of calcium gluconate). Conclusion: Exotic pets can be dangerous for their owners and family. Antivenom made in foreign countries are not often available in France. Furthermore, French medical doctors are not trained to manage such poisoned patients, and the cost of such pathology is very high for the society (problem of antivenom transport).
68 AUSTRALIAN SCORPION STINGS: PROSPECTIVE STUDY OF 56 CONFIRMED STINGS FROM FORMALLY IDENTIFIED SCORPIONS
Isbister GK,1 Volschenk ES,2 Harvey MS3
1Discipline of Clinical Pharmacology, University of Newcastle Australia. 2Curtin University of Technology, Bentley, Westerm Australia. 3Western Australian Museum, Perth, Australia
Objective: There is little information on scorpion stings in Australia. This study was done to determine the clinical effects and circumstances of confirmed scorpion stings. Methods: Subjects were recruited prospectively from February 2000 to May 2001 from calls to two Australian poison information centres. Subjects were included if they had a confirmed sting and the scorpion was caught. The scorpion was identified by an arachnologist. All subjects were followed for at least 1 week. Results: Of 110 enrolled subjects, 56 were included, 19 males and 37 females, with a mean age of 32 (SD 19.5; Range 1–71). The scorpions were from 3 families:: Buthidae (47), Bothruiridae (5, all Cercophonius sp.) and Urodacidae (4, all Urodacus sp.). The majority of stings (68%) were by the small scorpions, Buthidae: Archisometrus spp., including 3 species (A. marmoreus. A. sp. nov. O, A. nigrescens), 49 stings (88%) occurred indoors and 40 stings (71%) between 1800 and 0800. Stings were most commonly on distal limbs in 35 cases (63%). Pain occurred in all cases, with a median time of 2 hours (I.Q. 1–4 hrs), was severe in 41 cases (73%) and radiated in 10 cases (18%). The commonest local signs were red mark/redness (35), tenderness (24) and puncture marks (11). Numbness and paraesthesia were uncommon local effects occurring in 3 cases each, and another 2 with both. Systemic effects occurred in 5 cases, all mild (nausea, headache, malaise). There were no deaths and no major systemic effects. There were no cases of hypersensitivity reactions (95% C.I. 0–6.4%), despite a history of minor bee allergy in 3 patients. Less severe effects were observed for the larger scorpions Urodacus sp., compared to Archisometrus sp. Conclusion: Scorpion stings in Australia do not appear to cause severe or life-threatening effects like other parts of the world, even in children. The major problem is pain that rarely lasts longer than 4 hours, but may initially be very severe.
69 HIGH RISK FOR SYMPTOMS FROM USE OF WATER CONTAMINATED WITH CYANOBACTERIAE IN SAUNA
Hoppu K, Salmela J, Lahti K
Poison Information Centre, and The Finnish Environment Institute, Helsinki, Finland
Objective: It is known that under special circumstances Cyanobateriae (blue-green algae) toxins can cause severe poisoning. Less clear is the association between low levels of exposure, as encountered in daily life, and the often vague symptoms accredited to this exposure. We performed a pilot-study to investigate the feasibility of approaching this question from calls received by a poison centre. Methods: All calls received during the summer of 1999 concerning humans with symptoms suspected to be caused by exposure to water contaminated by Cyanobacteria were included in the study. At the initial contact, permission was asked to make a follow-up call for a structured interview within one week. The location where the contaminated water came from was recorded. Data on toxin-producing Cyanobacteriae detected in water at the actual time of exposure was retrieved from the Finnish Environmental Institute and local environmental health authorities. Results: Thirty-five calls with 48 persons exposed were included. Seventeen of the subjects were under 6 years of age, 20 6–15 years old and 11 16 years or older. The route of exposition was dermal in 25 (52%), dermal+gastrointestinal in 13 (27%), gastrointestinal in 4 (8%) and dermal+respiratory in 6 (12.5%) cases. Gastrointestinal symptoms were reported by 30 (62.5%), dermal symptoms, HEENT symptoms and fever by 20 (41.7%) persons. Neurological symptoms were reported by 3 (6.3%) and musculoskeletal symptoms by 2 (4.2%)persons. The probability of symptoms being caused by Cyanobacteria was classified as unlikely in 8 cases (17%), possible in 22 (46%) and likely in 18 (38%). The probability of the water causing the exposure having contained toxic Cyanobacteriae was classified to be unlikely in 4 cases (8%), possible in 29 (60%) and likely in 8 (17%), insufficient information in 7 cases (15%). The associations between the route of exposure, the symptoms and the probabilities mentioned were generally weak. However, all 6 persons exposed through dermal+respiratory route had symptoms. In 5 the symptoms were classified as likely to be caused by Cyanobacteriae toxins. All these exposures had occurred in sauna, where the water had been used not only for washing but also thrown on the heated stones to produce the hot steam called “löyly”. Boiling does not inactivate Cyanobacteriae toxins and animal studies have shown inhalation exposure to be one order of magnitude more toxic than gastrointestinal exposure. Conclusion: Use of water contaminated with Cyanobateriae in sauna may increase the risk of developing symptoms and should therefore be avoided.
70 AACT/EAPCCT POSITION PAPER ON URINE ALKALINIZATION
Proudfoot AT,1 Krenzelok EP,2 Vale JA,1 with the assistance of Bradberry SM,1 Harrison WH,1 Watt BE1
1National Poisons Information Service (Birmingham Centre) and West Midlands Poisons Unit, City Hospital, Birmingham, United Kingdom. 2Pittsburgh Poison Center, Children's Hospital of Pittsburgh, University of Pittsburgh Schools of Pharmacy and Medicine, Pittsburgh, Pennsylvania, USA
Definition: Urine alkalinization is a treatment regimen that increases poison elimination by the administration of intravenous sodium bicarbonate to produce urine with a pH≥7.5. The term urine alkalinization emphasizes that urine pH manipulation rather than a diuresis is the prime objective of treatment; the terms forced alkaline diuresis and alkaline diuresis should therefore be abandoned. Methodology: Using a methodology agreed and published by the American Academy of Clinical Toxicology (AACT) and the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT), all relevant scientific literature was identified and reviewed critically using agreed criteria. A draft Position Paper was then produced and, to allow participants to comment on the draft, a summary of the Position Paper was presented at the North American Congress of Clinical Toxicology in October 2001; a further presentation will be given at the EAPCCT Congress in May 2002. In addition, the draft will also be subjected to detailed peer review by an international group of clinical toxicologists chosen by the AACT and EAPCCT. Rationale: Most drugs are weak electrolytes that, at physiological pH, exist partly as undissociated molecules. The extent of dissociation is a function of the ionization (acid dissociation) constant (Ka) of the drug and the pH of the medium in which it is dissolved. Ionization (dissociation) constants are expressed in the form of their negative logarithms (pKa). Hence, the stronger an acid, the lower its pKa; conversely, the stronger a base, the higher the pKa. The relationship between pKa and the proportion of total drug in ionized form is represented by the Henderson-Hasselbalch equation. When pH=pKa, the concentrations of ionized and non-ionized drug are equal. Cell membranes are more permeable to substances that are lipid soluble and in the non-ionized, rather than the ionized form. The rate of diffusion from the renal tubular lumen back into the blood is decreased when a drug is maximally ionized and increased if the drug is non-ionized. As the ionization of a weak acid is increased in an alkaline environment, manipulation of the urine pH potentially can enhance renal excretion. For an acidic drug, there is a greater degree of ionization at pH 8 than pH 7.4. Thus, elimination of a weak acid by the kidneys is increased in alkaline urine. Since pKa is a logarithmic function then, theoretically, a small change in urine pH could have a disproportionately larger effect on clearance, especially for those drugs that have pKa values close to blood pH. For each change in urine pH of one unit, there is theoretically a 10-fold change in renal clearance, whereas at best the renal clearance of a reabsorbed drug varies directly with the urine flow rate. The effectiveness of urine alkalinization depends on the relative contribution of renal clearance to the total body clearance of active drug. If only 1% of an ingested dose is excreted unchanged in the urine, even a 20-fold increase in renal clearance will have no clinically significant effect on the total clearance. Review: Salicylate: Vree et al.,1 conducted a randomized crossover study in six volunteers who were administered sodium salicylate 1.5 g orally and were then subjected to urine alkalinization (mean urine pH 7.67±(SD) 0.65) or urine acidification (mean urine pH 5.54±0.57). The mean peak salicylate concentration was 93.3±(SD) 18.6 mg/L and 109.8±17.8 mg/L (NS) respectively. The mean elimination half-life during urine alkalinization (2.50±(SD) 0.41 hr) was significantly less (p=0.0156) than the mean elimination half-life during urine acidification (3.29±0.52 hr). The mean total body clearance was increased significantly (p=0.041) during urine alkalinization (2.27±(SD) 0.83 L/hr) compared to urine acidification (1.38±0.43 L/hr). Prescott et al.,2 studied six patients with a mean admission plasma salicylate concentration of 439±(SD) 86 mg/L who were given sodium bicarbonate 225 mmol and potassium 60 mmol in a fluid load of 1.5 L (mean urine pH 8.1±(SD) 0.5) and sixteen patients with a mean admission plasma salicylate concentration of 328±(SD) 57 mg/L who received only oral fluids and who acted as a control group (mean urine pH 6.1±0.4). There was a highly significant correlation (r=+0.82; p<0.001) between urine pH and log salicylate clearance. Patients receiving urine alkalinization had a significantly greater (p<0.05) mean renal salicylate clearance (23.5±13.7 mL/min) than the control group (1.4±(SD) 1.4 mL/min). In addition, in those patients undergoing urine alkalinization, a significant (p<0.05) decrease in the mean plasma elimination half-life 4–16 hr (9.0±(SD) 6.1 hr) compared to the control group (29.4±7.6 hr) was reported. These data show that urine alkalinization enhances salicylate clearance. However, as the conclusions of the study were based on only six patients, there were insufficient data to determine if urine alkalinization had an impact on patient morbidity. Phenobarbital: Using a randomized crossover design, Frenia et al.,3 compared urine alkalinization with multiple-dose activated charcoal (MDAC) in enhancing phenobarbital elimination in 12 volunteers who were administered phenobarbital 5 mg/kg intravenously. In the urine alkalinization phase, the urine pH was maintained between 7.5–8.0. Urine alkalinization reduced significantly (p=0.013) the phenobarbital elimination half-life (47.24±(SD) 42.04 hr) compared to the control group (148.1±332.1 hr) and increased significantly (p<0.001) the mean total body phenobarbital clearance (8.29±(SD) 8.62 mL/kg/hr) when compared to controls (2.79±(SD) 9.69 mL/kg/hr); MDAC was superior (19.95±11.55 mL/kg/hr), however, to urine alkalinization (p<0.0005) in increasing phenobarbital elimination. Ebid and Abdel-Rahman4 also described the impact of urine alkalinization (the urine pH was maintained between 7.5–8.0 and the urine volume was not less than 3–6 mL/kg/hour) and MDAC on phenobarbital elimination. In each group there were ten male patients poisoned with phenobarbital (the mean plasma phenobarbital concentration in the two groups was 100.6±12.6 mg/L and 103.2±12.2 mg/L respectively). Compared to urine alkalinization (81.1±(SD) 14.6 hr), MDAC reduced significantly (p<0.05) the mean phenobarbital elimination half-life (38.6±6.6 hr) and increased significantly (p<0.05) the mean total body clearance of phenobarbital (10.8±(SD) 1.8 mL/kg/hr) compared to urine alkalinization (5.1±0.9 mL/kg/hr). With MDAC, the mean durations of assisted ventilation (40.2±(SD) 12.5 hr), intubation (29.7±(SD) 10.3 hr) and coma (24.4±(SD) 9.6 hr) were significantly shorter (p<0.05) than in the group treated with urine alkalinization (79.4±20.9 hr; 54.2±12.8 hr; 50.6±12.5 hr respectively). Although this study did not include a control group, urine alkalinization did not appear to increase phenobarbital clearance (mean 6.34 mL/min) significantly compared to reported endogenous clearances (4 mL/min5) and was less effective than MDAC. 2,4-D and Mecoprop: In a 39-year-old male poisoned severely with 2,4-D and mecoprop6,7, the renal 2,4-D clearance corrected for urine flow (adjusted to 1 mL/min) was directly proportional to urine pH (r=0.99) and clearance was estimated to increase almost five-fold for each unit increase in urine pH. The authors cited a mean corrected renal clearance of 0.28 mL/min over the urine pH range 5.1–6.5 and 9.6 mL/min over the pH range 7.55–8.8. At pH 5.1 and pH 8.3 the uncorrected renal clearances were 0.14 mL/min and 63 mL/min. The plasma half-life of 2,4-D was approximately 219 hours before urine alkalinization and 3.7 hours over the period 96–112 hours post ingestion when the urine pH exceeded 8.0. A substantially increased 2,4-D clearance was achieved only when the urine pH exceeded 7.5 and was accompanied by a urine flow rate of the order of 200 mL/hour. The maximal uncorrected 2,4-D renal clearance of 63 mL/min at pH 8.3 would have required a urine flow rate of approximately 600 mL/hr. In these circumstances 2,4-D clearance compared favorably with that achieved with hemodialysis (56.3–72.9 mL/min8), whereas the effect of urine alkalinization alone without high urine flow is markedly less efficient than hemodialysis as a means of removing 2,4-D. The renal mecoprop clearance corrected for urine flow (adjusted to 1 mL/min) was directly proportional to urine pH (r=0.94) and clearance was estimated to double for each unit increase in urine pH. The authors cited a mean corrected renal clearance of 0.38 mL/min over the urine pH range 5.1–6.5 and 2.08 mL/min over the pH range 7.55–8.8. The plasma half-life of mecoprop was shortened from 39 to 14 hours with urine alkalinization. Chlorpropamide:Neuvonen and Karkkainen9 investigated the effect of urine alkalinization and urine acidification on chlorpropamide kinetics in a randomized cross-over study in which each treatment modality was applied to six volunteers at two to three week intervals. Before each regimen, chlorpropamide 250 mg was administered orally. In the urine alkalinization phase sodium bicarbonate was administered orally between one and 64 hours after chlorpropamide dosing, to achieve and maintain a urine pH of 7.1–8.2. Urine alkalinization reduced significantly (p<0.001) the chlorpropamide AUC0–72, AUC0–∞ and the chlorpropamide elimination half-life (12.8±1.1 hr) compared to control (49.7±(SEM) 7.4 hr), and increased significantly (p<0.001) the total chlorpropamide clearance from 104±(SEM) 13 mL/hr (control) to 363±22 mL/hr. The mean chlorpropamide excretion over 72 hours was significantly greater (p<0.001) in those volunteers undergoing urine alkalinization (213±(SEM) 11 mg) than in the control group (50.9±7.3 mg) and in those subjected to urine acidification (3.5±0.52 mg). Although urine alkalinization increased chlorpropamide elimination, the administration of dextrose alone is effective treatment in the majority of cases of chlorpropamide poisoning, which is now a relatively uncommon intoxication. Conclusions: On present evidence, while urine alkalinization increases the elimination of salicylate, phenobarbital, 2,4-D, mecoprop and chlorpropamide, with the exception of salicylate poisoning, urine alkalinization cannot be recommended as sole therapy in cases of poisoning due to these agents as MDAC is superior in the case of phenobarbital, supportive care is invariably adequate in the case of chlorpropamide and a substantial diuresis is required in addition in the case of chlorophenoxy herbicides to achieve clinically important herbicide elimination.
References: 1. Vree, T.B.; Van Ewijk-Beneken Kolmer, E.W.J.; Verwey-Van Wissen, C.P.W.G.M.; Hekster, Y,A. Effect of Urinary pH on the Pharmacokinetics of Salicylic Acid, with Its Glycine and Glucuronide Conjugates in Human. Int. J. Clin. Pharmacol. Ther. 1994, 32, 550–558. 2. Prescott, L.F.; Balali-Mood, M.; Critchley, J.A.J.H.; Johnstone, A.F.; Proudfoot, A.T. Diuresis or Urinary Alkalinisation for Salicylate Poisoning? Br. Med. J. 1982, 285, 1383–1386. 3. Frenia, M.L.; Schauben, J.L.; Wears, R.L.; Karlix, J.L.; Tucker, C.A.; Kunisaki, T.A. Multiple-Dose Activated Charcoal Compared to Urinary Alkalinization for the Enhancement of Phenobarbital Elimination. J. Toxicol. Clin. Toxicol. 1996, 34, 169–175. 4. Mohammed Ebid A-HI, Abdel-Rahman, H.M. Pharmacokinetics of Phenobarbital During Certain Enhanced Elimination Modalities to Evaluate Their Clinical Efficacy in Management of Drug Overdose. Ther. Drug. Monit. 2001, 23, 209–216. 5. Hardman, J.G.; Limbird, L.E.; Molinoff, P.B.; Ruddon, R.W.; Gilman, A.G. Goodman and Gilman's The Pharmacological Basis of Therapeutics, New York: McGraw-Hill, 1996. 6. Park, J.; Darrien, I.; Prescott, L.F. Pharmacokinetic Studies in Severe Intoxication with 2,4-D and Mecoprop. Proc. EAPCCT Meet, 1977, 18, 154–155. 7. Prescott, L.F.; Park, J.; Darrien I. Treatment of Severe 2,4-D and Mecoprop Intoxication with Alkaline Diuresis. Br. J. Clin. Pharmacol. 1979, 7, 111–116. 8. Durakovic, Z.; Durakovic, A.; Durakovic, S.; Ivanovic, D. Poisoning with 2,4-Dichlorophenoxyacetic Acid Treated by Hemodialysis. Arch. Toxicol. 1992, 66, 518–521. 9. Neuvonen, P.J.; Kärkkäinen, S. Effects of Charcoal, Sodium Bicarbonate, and Ammonium Chloride on Chlorpropamide Kinetics. Clin. Pharmacol Ther. 1983, 33, 386–393.
71 FEASIBILITY STUDY ON ACTIVATED CHARCOAL GIVEN PREHOSPITAL BY EMERGENCY MEDICAL SYSTEM (EMS) IN ACUTE INTOXICATIONS
Alaspää AO,1 Kuisma MJ,1 Hoppu K,2 Neuvonen PJ3
1Helsinki City EMS, 2Poison Information Centre and 3Department of Clinical Pharmacology, Helsinki University Central Hospital, Helsinki, Finland
Background and objectives: Activated charcoal (AC) decreases considerably the absorption of many toxic drugs when given early enough, preferably within 30–60 min after their ingestion. In order to shorten the lag time in the administration of AC, all basic life support (BLS) units of the Helsinki EMS were guided to give AC (50 g AC as water suspension to adults) to acute intoxication patients who fulfilled predetermined criteria, e.g. ingestion of highly toxic drugs (such as calcium channel blockers, digoxin, opioids, SSRIs with moclobemide), ingestion of other toxic agents adsorbable to charcoal within previous 2 hours, or signs of serious poisonings (deep unconsciousness, hypotension/cardiac arrhythmia). We studied the feasibility and time lag in the administration of AC during the first year of the new treatment policy, starting from the 1 April 1999. Methods: The time points of the emergency calls, arrival of the BLS unit to the patient, administration of AC, and other treatments were exactly recorded. The amount and time point of ingested drugs and other compounds, medical status and other relevant information were also recorded on site by the BLS unit. Preliminary data will be reported here. Results: During the 12 months (1.4.1999–31.3.2000) Helsinki EMS system was activated in about 2000 cases because of a suspected intoxication. AC was given prehospitally to about 500 patients: AC was drunken by the patient (90% of cases) or given (after tracheal intubation) via nasogastric tube (10% of cases). About 10% of the patients, who had an acute intoxication and in whom AC was indicated (“intention to treat”), either refused or were unable to drink AC or they did not receive AC because of technical problems. The median time interval from poison ingestion to phone call (to the emergency centre) was 60 min and the median time lag from the call to administration of charcoal was less than 30 min. No serious complications associated with the administration of AC were observed. Conclusion: This preliminary data suggests that the prehospital administration of AC by BLS units to the patients who have a criteria-based acute intoxication is feasible and safe. This kind of treatment may be the method of choice to minimise the delay in the AC administration.
72 A RANDOMISED CLINICAL TRIAL OF ACTIVATED CHARCOAL FOR THE ROUTINE MANAGEMENT OF ORAL DRUG OVERDOSE
Cooper GM,1,3 Le Couteur DG,2 Richardson D,3 Buckley NA1,3
1Department of Clinical Pharmacology and Toxicology The Canberra Hospital, Woden, Australian Capital Territory, Australia. 2Centre for Education and Research on Aging, University of Sydney, Concord Repatriation General Hospital New South Wales, Australia. 3Canberra Clinical School, University of Sydney, Woden, Australian Capital Territory, Australia
Objective: While it is known that activated charcoal may have a role in the management of oral drug overdose it is questionable that it has a role in the routine management of overdose. This trial addresses the issue of the routine use of activated charcoal and it effect on patient outcomes (e.g. length of stay, adverse effects). Method: We conducted an open randomized clinical trial at the Canberra Hospital Emergency Department. We attempted to recruit all patients who presented following an oral overdose. Excluded from the trial were those subjects who presented within one hour of an ingestion of a highly lethal substance (e.g. large amounts of tricyclics, antineoplastics, aspirin and cardioactive agents) and subjects less than 16 years old. Patients were randomized to either activated charcoal or no decontamination. All patients received routine supportive therapy. This trial was approved by the ACT Health and Community Services Ethics Committee. Results: The trial recruited 330 patients over a 16 month period. Analysis of the trial indicates no difference between treatment and no treatment in terms of clinical outcomes such as length of stay [15.1±30.0 hrs (AC) vs. 10.9±13.9 hrs (control) (p=0.09)] or mortality. The incidence of vomiting and complications was similar in the two groups. Conclusion: Our clinical trial suggests that routine administration of charcoal following oral overdose does not alter patient outcome. This does not exclude a role for its use within one hour of a significant highly lethal drug ingestion. This supports the recent AACT/EAPCCT guidelines that suggest activated charcoal should not be given routinely and administered only within one hour of ingestion of a substantial amount of a toxic substance known to bind activated charcoal.
References: Chyka, P.A.; Seger, D. AACT/EAPCCT Position Statement: Single-Dose Activated Charcoal. J. Toxicol. Clin. Toxicol. 1997, 35, 721–741.
73 DO WE HAVE TO CLEAR PLASMA OR CELLS FROM TOXICANT? A LESSON FROM LITHIUM POISONING
Mégarbane B, Baud F
INSERM U26, Hôpital Fernand Widal and Réanimation Médicale et Toxicologique, Hôpital Lariboisière, Paris, France
Objectives: Enhancement of toxicant elimination is a classical conventional method proposed in acute poisonings. Lithium (Li) poisoning can generate severe and prolonged neurological complications, due to central nervous system. Extra-renal elimination methods may enhance Li elimination; however, to date, no randomized study proved clearly their benefit for making clinical signs regress. The objectives of this study were to analyze the dialysis criteria used by the physicians in charge of the poisoned patients and to evaluate its possible benefits and effects on Li kinetics. Methods: Retrospective data collection of patients admitted to our intensive care unit (ICU), from 1992–2000 for acute intentional self-poisonings or therapeutic overdoses; descriptive analysis (median, extremes); Li kinetic study (Kinetica® Software); subgroup comparisons using Chi2 and Mann–Whitney tests; multivariate analysis with logistic regression and odds-ratio determination. Results: Forty-eight patients were included: 14M/34F, age: 47 years [21–78], SAPS II score: 29 [6–102]—acute self-poisoning (10%), acute intoxication upon chronic Li therapy (63%) and therapeutic overdose (27%)—stage I (38%), II (31%) and III (31%) of the Hansen and Amdisen classification. On admission, 46% had a Glasgow Coma Score=12, 8% were shocked and 33% were mechanically ventilated. The median plasma Li concentration was 2.82 mEq/L [0.46–10.0] on admission and increased to 3.02 mEq/L [0.55–12.40]. 5/48 patients (10%) were dialyzed (2 with hemodialysis and 3 with continuous veno-venous hemodiafiltration). The median length of ICU stay was 5 days [1–40]. 2/48 patients (4%) died. The median plasma Li peak was significantly different according to the poisoning type (p=0.01). The supposed ingested dose (p=0.04), the delay between admission and ingestion (p=0.004) and the plasma creatinine concentration on admission (p=0.004) were significantly different between the subgroups of patients according to their plasma Li level (< or =3 mEq/L). There was a significant decrease in plasma elimination half-life following dialysis. Subgroup comparison according to dialysis shows the results in Table 1. By a multivariate analysis, only a plasma Li maximal concentration=3 mEq/L was predictive of dialysis requirement (OR=5.6). Conclusion: Dialysis is not a priority in the management of Li poisoning. Indications are based on clinical (worsening of neurological signs and renal failure), and kinetic criteria (poisoning profile and plasma Li concentration). Clearing plasma from Li is not a guarantee to obtain any favorable clinical results. The brain–blood barrier contributes to the separation of Li biophase from blood compartment, rendering the efficacy of Li plasma instead of cells clearing very illusive.
Table 1. (Abstract 73)
74 HYDROXOCOBALAMIN VERSUS CONVENTIONAL TREATMENT IN CYANIDE POISONING
Mégarbane B, Borron SW, Baud F
Réanimation Médicale et Toxicologique and INSERM U26, Université Paris VII, Hôpital Lariboisière, Paris, France
Objectives: There are a large number of conditions resulting in exposure to cyanide or cyanogenic compounds including domestic exposure (residential fires), occupational exposure to hydrogen cyanide, cyanide salts or nitriles, drug poisonings (such as nitroprussiate) and plant intoxications (such as cassava). However, therapeutic strategies of this rare poisoning should take into account the most common cause of cyanide poisoning in western countries, i.e. smoke inhalation resulting from residential fires, which always results in a poly-intoxication including carbon monoxide. Our objective was to discuss the different therapies proposed in cyanide poisoning and compare conventional treatments to hydroxocobalamin. Methods: A review of the literature on cyanide poisoning treatments. Results: The main clinical features of pure cyanide poisoning include: dyspnea, restlessness, transient hypertension, mental confusion, coma, seizures, respiratory arrest, cardiovascular collapse with a paradoxical normal heart rate, and eventually cardiorespiratory arrest. The biological hallmark of cyanide poisoning is lactic acidosis. In fire victims without severe burns, a plasma lactate concentration greater than or equal to 90.1 mg/dL (10 mmol/L) is a sensitive and specific indicator of cyanide intoxication. Significant cyanide poisoning may also induce hyperglycemia, increased serum CPK activity, and acute renal failure. Conventional treatment of cyanide poisoning includes supportive treatment, decontamination, and specific treatment. Supportive treatment: Basic life-support of cyanide poisoning includes -1- the immediate administration of high flow of oxygen, -2- the protection of the airways, -3- cardiopulmonary resuscitation. Advanced life support includes endotracheal intubation in comatous patients, controlled ventilation in apneic patients, the administration of anti-epileptic drugs in case of seizures, continous epinephrine infusion to correct cardiovascular collapse, sodium bicarbonate to correct deep metabolic acidosis. There is a large number of case reports supporting the assumption that this supportive treatment is efficient in cyanide poisoning. However, regarding the classification of treatment in clinical toxicology, either toxicodynamics or toxicokinetics, we should consider that supportive treatment belongs to toxicodynamic treatment aiming at correcting signs and symptoms of cyanide poisoning but without any effect on the time-course of cyanide in the body. Decontamination: Decontamination should be adapted to the condition of cyanide poisoning: either by ingestion, by inhalation, or even by cutaneous absorption. Decontamination should never postpone supportive treatment. Specific treatment: Numerous antidotes are available to treat cyanide poisoning. Regarding the classification of treatment in clinical toxicology: Oxygen counteracts the action of cyanide at the mitochondrial level and, thus, should be considered a toxicodynamic treatment. Both experimental and clinical data support the efficiency of oxygen in cyanide poisoning. Other antidotal treatments include: Sodium thiosulfate, methemoglobin forming agents, and cobalt compounds. These treatments act by complexing (methemoglobin forming agents) or transforming cyanide into non-toxic stable derivatives (thiosulfate and cobalt compounds). Thus, these drugs belong to toxicokinetic treatment. All these antidotes are efficient. However, regarding the main clinical condition of cyanide poisoning, i.e. smoke inhalation, we should take into account not only for the efficiency but also for the -1- delay in onset of antidotal activity, and -2- the safety of the antidotal treatment. Sodium thiosulfate is both efficient and safe but there are some question about the delay in the onset of its antidotal effect. Methemoglobin forming agents are potent antidotes. However, due to the transformation of hemoglobin into methemoglobin, these drugs impair the transport and delivery of oxygen to tissues. Experimental data showed, in rats poisoned with carbon monoxide and cyanide, that this treatment increases the mortality rate of the poisoned animals. Cobalt derivatives (Cobalt EDTA and hydroxocobalamin) act immediately. Cobalt EDTA is a very potent antidote. However, numerous side-effects have been reported limiting its use to evidenced cyanide poisoning. Hydroxocobalamin is less potent than cobalt EDTA on a molar basis (1/2). However, in a prospective study, hydroxocobalamin was shown to be safe in fire victims with or without cyanide poisoning, the lone side-effect was a deep red coloration of the skin and urine. Conclusion: Therapeutic strategy of cyanide poisoning is based on -1- the removal of the patient from the source of cyanide and -2- supportive treatment. However, both experimental and clinical data support the assumption that antidotal treatment is beneficial in cyanide poisoning. Numerous case reports suggest that the early use of antidotes to cyanide alleviates the need of supportive treatment. In patients with suspected cyanide poisoning, we recommend the use of hydroxocobalamin as first-line antidote according to its safety. In massive cyanide poisoning, the potency of hydroxocobalamin is limited and the continuous infusion of sodium thiosulfate should also be recommended.
75 PUMPING UP THE “NATURAL” WAY: A REVIEW OF PERFORMANCE-ENHANCING SUPPLEMENT USE IN THE UNITED STATES
Haller CA
University of California, San Francisco, Calif. Poison Control System, San Francisco Division, San Francisco, California, USA
Background: Drug use to improve athletic performance has occurred since ancient times. Today, most collegiate and professional sports associations prohibit use of performance-enhancing drugs such as sympathomimetics and anabolic steroids. However, a number of dietary supplements available in the U.S. are promoted to increase muscle mass, burn fat, and improve exercise tolerance, and are permitted by some sports organizations. The use of supplements such as herbal stimulants, trace minerals, amino acids, and hormone precursors appears to be widespread, even encouraged, among amateur and professional U.S. athletes, as well as law enforcement, fire-fighting and military occupations. Consumers of these products often rely on anecdotal “locker-room” reports of beneficial effects, and rarely consult a physician for advice prior to use. A number of recent, well-publicized deaths of American football players has drawn attention to the potential dangers of sports supplements and raised important public health concerns about the unrestricted availability of these products that are exempt from U.S. Food and Drug Administration (FDA) regulation. Methods: A systematic review of the English language scientific literature and the World Wide Web was conducted to identify the most commonly used performance-enhancing dietary supplements in the United States. The pharmacology and toxicology of several popular sports products, including creatine, ephedrine/caffeine “stacks,” chromium picolinate, and androstenedione was investigated. Relevant medical literature citations were reviewed for: 1. Epidemiological surveys on patterns of use; 2. Scientific evidence supporting product claims; and 3. Published case reports of serious toxicity associated with performance enhancing supplements. Recent cases involving sports supplements at the California Poison Control System, San Francisco division were identified to illustrate potential health risks related to their use, and to provide guidelines for diagnosis and treatment of acute and chronic toxic effects. Results: Performance-enhancing supplement sales account for one-fourth of the nearly $17 billion U.S. annual dietary supplement market. Surveys on use show that performance-enhancing supplements are used by more than 40% of NCAA athletes, with creatine being the most common supplement. An estimated 16% of high school athletes and 5% of children aged 12 to 17 years also use creatine. Polysupplement use occurs in at least one-third of creatine users. Ephedrine use ranges from about 4% of NCAA athletes to 25% of U.S. male gymnasium clients. Sex steroid precursors such as androstenedione and 19-norandrostenedione are banned by many sports organizations, but use in private gyms ranges from about 3% in women to 18% in men. Clinical studies involving small numbers of subjects have largely been equivocal, with many demonstrating no benefits of sports supplement use in improving strength or body composition, and a few studies showing modest benefits. Although more than half of users are older individuals and adolescents, study subjects have mostly been young adults <30 years of age. Nearly 1400 adverse events and 80 deaths associated with ephedrine/caffeine-containing supplements have been reported to the FDA, and several cases are described in the medical literature. Results of searches of the World Wide Web and MEDLINE for efficacy claims, scientific studies and adverse health effects are summarized in Table 1. Conclusion: Sports supplements are widely used by U.S. athletes of all ages. Few studies have provided evidence that these supplements are efficacious in their purported claims, and large-scale safety studies are non-existent. Several commonly used supplements can cause potentially serious adverse health effects. Further research is needed to clearly establish the risks-versus-benefits of performance-enhancing supplements, including potential risks associated with polysupplement use, and use by younger and older athletes.
76 MULTIPLE MDMA TOXICITY: A CASE SERIES WITH TOXICOKINETIC DATA
Greene SL,1 Dargan PI,1 O'Connor N,2 Jones AL1
1National Poisons Information Service (London)1 and Emergency Department,2 Guy's and St Thomas’ NHS Trust, London, United Kingdom
Objective: There have been no published series illustrating ecstasy (MDMA) toxicity in patients who have ingested ecstacy while in the same environment. We present clinical and toxicokinetic data for 7 patients presenting with evidence of ecstasy toxicity following acute ingestion. Case Series: 7 patients were treated in 2 different hospitals after ingesting varying amounts of ecstasy in the same nightclub. Clinical and toxicokinetic data are shown in the table. All patients ingested ecstasy within a 2-hour period and presented to the Emergency Department 6 to 8 hours later. 3 patients presented with clinical and biochemical features of severe ecstasy toxicity. One patient died in the Emergency Department within an hour of presentation, and one after 4 days. The third patient recovered after 5 days in ICU. Serum MDMA concentrations (GC) were obtained in 6 of the patients 4 to 8 hours after ingestion. No other amphetamines were identified in patient's serum. As shown in the table, high serum MDMA concentrations correlated with severe clinical and biochemical features including coma, hyperpyrexia, cardiovascular compromise, acidosis and hyperkalaemia. Conclusion: We report a series of patients presenting with a range of clinical and biochemical features following ingestion of varying amounts of ecstasy in the same environment. Severe MDMA poisoning was associated with higher serum MDMA concentrations (Table 1).
Table 1. Clinical and Toxicokinetic Data in Seven Patients with MDMA Toxicity (Abstract 76)
77 LIQUID ECSTASY INTOXICATION (GHB): A NEW CAUSE OF COMA OF UNKNOWN ORIGIN
Nogué S, Miró O, Espinosa G, To-Figueras J, Alicarte J, Munné P
Intensive Care, Toxicology Unit and Emergency Department. Hospital Clínic, Barcelona, Spain
Objectives: Liquid ecstasy (gamma-hydroxybutyrate, GHB) is a relatively new synthetic recreational drug introduced in European countries in recent years. Our Emergency Department (ED) treats an increasing number of patients with GHB overdose. We present the epidemiologic, toxicological, clinical and outcome data of such patients. Methods: Between April 1, 2000 and June 30, 2001, we saw 104 patients whose chief complaint was directly related to the ingestion of liquid ecstasy. Data were collected from interview with the patients and/or accompanying persons, and the results of blood and urine analysis were also compiled. Urine toxicological screening in urine was performed in nearly all cases and, included GHB for the terminal 6 months. Results: Mean age was 23±5 years (range 17–39), 64% were males, 84% of cases were seen on the week-end with 63% between midnight and 8 a.m. All patients complained of some impairment of consciousness at the scene of drug consumption. At the ED, 74% had a Glasgow Coma Score (GCS) of 12 or less. Of the 104 patients (16%) showed a deep coma (GCS 3 or less) and 6 (6%) required brief mechanical ventilation due to severe hypoventilation and blood oxygen desaturation. No patient presented with hemodynamic instability. Other remarkable findings were poorly or non-reactive mydriasis in 48%, vomiting in 17%, sinus bradycardia in 15%, generalized seizures in 3%, mild hypokalemia in 20%, and moderately increased serum creatinekinase in 19%. Seventy-three percent of patients had also consumed alcohol and 61% had simultaneously used other illicit drugs (amphetamines 43%, cocaine 25%, ketamine 8%, cannabis 3%, benzodiazepines 3%, lysergic acid 1% and opiates 1%). In 14% of cases naloxone and/or flumazenil was employed on empirical basis before obtaining the history of liquid ecstasy consumption, but no adverse or beneficial responses were observed. Outcome was good in all cases: complete recovery of consciousness was achieved 42±39 minutes after ED arrival (range: 1–180), and 90% left the ED in less than 6 hours. Conclusion: Toxicology and emergency room personnel must be aware of the frequency of liquid ecstasy ingestion. Since it is not detected by most drug screening tests, a high degree of suspicion is needed to diagnose this increasingly frequent cause of coma at both the scene of drug consumption and in ED patients.
78 OBIDOXIME PLASMA LEVELS IN ORGANOPHOSPHATE POISONED PATIENTS
Thiermann H,1 Worek F,1 Szinicz L,1 Haberkorn M,2 Eyer F,2 Felgenhauer N,2 Zilker Th,2 Kiderlen D,3 Krummer S,3 Eyer P3
1Institut für Pharmakologie und Toxikologie, SanAkBw, Muenchen, Germany; 2Toxikologische Abteilung der Technischen Universität Muenchen, Muenchen, Germany; 3Walther-Straub-Institut Institut für Pharmakologie und Toxikologie, München, Germany
Objectives: Oximes are used to reactivate inhibited acetylcholinesterase (AChE) in patients intoxicated with organophosphorus compounds (OPs). For this purpose, oximes are to be administered at appropriate dose as soon as possible and as long as reactivation can be anticipated. From experimental studies on human erythrocytes (Erys) it was expected that adequate reactivation may occur with about 10 μM obidoxime in vivo. In adults, this plasma level should quickly be achieved by administration of a 250 mg i.v. bolus, followed by infusion of 750 mg/day. To investigate, whether this regimen fits the anticipated oxime levels, plasma concentrations of obidoxime were determined in patients with severe (need for artificial ventilation) OP-poisoning. Methods: During a clinical trial on 34 OP-intoxicated patients, obidoxime concentrations were determined by HPLC. Activity of Ery-AChE was determined by a modified Ellman method. For assessment of reactivatability patient's Erys were incubated with obidoxime and for determination of inhibitory material donor Erys were incubated with patient's plasma. Results: In view of the individual courses 2.9±1.7 g obidoxime were administered to the patients. Usually, steady-state concentrations ranged between 10 and 20 μM. However, in one patient, 100 kg body weight, steady-state concentration amounted to 5.9 μM only. In 3 patients obidoxime plasma concentrations increased to 26, 30 and 40 μM during renal insufficiency, while in one patient, who was repetitively dialysed, steady-state concentration could be kept at about 20 μM. Cardiac insufficiency occasionally lead to an increase of obidoxime concentrations, with a sharp peak in one patient at 37 μM (blood pressure 70/43 mmHg). After subsiding the infusion, the elimination of obidoxime could be described with a two compartment model (r2=0.993, CV 0.8%, n=28). The elimination half-lives were 2.1 and 14.6 hours. Powerful reactivation of inhibited AChE could be achieved with obidoxime. However, complete reactivation was only possible at low poison load. In patients, poisoned with umpteen times lethal OP-doses, effective obidoxime levels had to be maintained long enough, to make reactivation possible when the poison burden was dropping and AChE was not aged completely. Regarding safety of obidoxime, we found that possible treatment-related signs of cholestatic icterus and slight increase of creatinine, both reversible, are tolerable. Conclusions: The proposed regimen is appropriate to achieve the anticipated obidoxime plasma levels in intoxicated patients. Dose correction in extremely heavy patients should be considered.
79 EFFECTIVENESS OF OXIME THERAPY AND CLINICAL DATA OF THIRTY-FOUR ORGANOPHOSPHATE INTOXICATIONS
Haberkorn M,1 Eyer P,2 Eyer F,1 Thiermann H,3 Felgenhauer N,1 Zilker Th
1Toxikologische Abteilung, Klinikum r.d. Isar, Technische Universität, München, Germany. 2Walther-Straub Institut, LMU München. 3Institut für Pharmakologie und Toxikologie, SanAkBw, München, Germany
Objectives: The treatment of acute intoxications with organophosphorus compounds (OP) is still a major problem in clinical toxicology. The efficacy of oxime therapy was monitored by analysis of the erythrocyte-AChE status and electrophysiologically by neuromuscular transmission studies indicating a reactivation of the endplate AChE. Data of the clinical course were evaluated. Case Series: 34 cases of severe OP poisoning were divided into 3 major groups of different OPs. There were 13 cases of parathion (diethyl-OP) intoxications, 12 cases of oxydemeton-methyl (dimethyl-OP) intoxications, 6 cases of dimethoate (dimethyl-OP) intoxications and 3 others. Obidoxime was given as an i.v. bolus 250 mg followed by continuous infusion at 750 mg/d. 85% of the patients in the parathion group were found unconscious on the spot and one third had to be resuscitated, while 33% were unconscious in the two other groups and did not require resuscitation. The mean heart rate prior to atropine treatment was also significantly lower in the parathion group (38/min) compared to 78/min in the oxydemeton-methyl group and 106/min in the dimethoate group. The duration at ICU was between 5 and 46 days (mean 17.5) in the patients surviving. The most common complications irrespective of the poison were pneumonia (27 cases), circulatory insufficiency (27) and acute renal failure (24). The mortality was 38% in the parathion group and only 8% in the oxydemeton-methyl group. Despite control of the cholinergic crisis, 5 patients in the parathion group and only one in the oxydemeton-methyl group died between day 17 to 38, mainly due to pulmonary insufficiency. In 12 out of 13 parathion cases sustained reactivation of the erythrocyte-AChE was achieved along with normalization of the neuromuscular function, compared to 2/6 in the dimethoate group and 2/12 in the oxydemeton-methyl group. Conclusion: The onset of intoxication with parathion is much more precipitous and mortality is higher than in cases with dimethyl-OP's. Notwithstanding, diethylphosphorylated AChE can be mostly (>90%) reactivated by obidoxime, while dimethylphosphoryl-OP's lead to rapid ageing of the inhibited AChE and oximes are only effective when administered early in cases of low poison levels. Neuromuscular transmission and erythrocyte-AChE activity are reliable parameters of cholinergic function and hence of the oxime effectiveness. Reactivation to about 20% of normal erythrocyte-AChE activity parallels normalization of the neuromuscular function. Under obidoxime treatment, fatalities were no longer due to inadequate cholinergic function but resulted from late organ failures.
80 ENDOSCOPICAL DIAGNOSTICS OF ACETIC ACID LESIONS OF ESOPHAGUS AND STOMACH
Sentsov VG, Kirichenko AV, Leiderman IN, Teriaev AD
City Toxicological Center, Ural State Medical Academy, Yekaterinburg, Russia
Objectives: From 1996 to 2000 we undertook endoscopic investigation in 615 patients with acetic acid lesions of the oesophagus and stomach. Emergency fibrogastro-duodenoscopy gave us the possibility to confirm or exclude the presence of corrosive destruction of the stomach, and to determine correctly the extent of this chemical destruction to predict the future development of the lesions and possible serious complications. There were no complications during these emergency endoscopic procedures. Methods: 407 patients with acute acetic acid poisoning were investigated (66.2% from all poisonings by aggressive chemical fluids). 186 (46.4%) men and 218 women were included into the study. In 224 cases the poisonings were suicide attempts. The age range of the patients was between 15 and 92 years old. The average volume of acetic acid ingested was about 54.7 ml. 173 of the patients were investigated within 12 hours, 99 within 12 to 24 hours, 68 from 24–48 hours and 59 after 48 hours. The primary bleeding site was found in 80 patients (19.7%). The main results are detailed in Table 1. Results: The symptoms of lesions were absent in 27 patients. Conclusion: The first-degree lesions were characterized by acute inflammation with oedema, hyperaemia and aggregations of mucosa and layers of destroyed epithelium. Additions of fibrin aggregates, erosions and mild bleeding characterized the second-degree lesions. Progression to the third-degree lesions led to the findings of ulcers, severe fibrin clots and sites of acute necrosis. In difficult cases we provided the dynamical endoscopic investigation on 5–6 day after poisoning. 42 (10.3%) patients died. The development of oesophageal and gastric stenosis was found in 11 patients (2.7%).
Table 1. The Symptoms of Oesophagus and Stomach Lesions in Acetic Acid Poisoned Patients (Abstract 80)
81 CHEST X-RAY INTERPRETATION FOLLOWING DRUG OVERDOSE
Kelly CA,1 Din S,2 Bateman DN
1National Poisons Information Service, Edinburgh Centre, Royal Infirmary of Edinburgh, Scotland, United Kingdom. 2Medical School, University of Dundee, Scotland, United Kingdom
Objectives: Many patients admitted to critical care facilities following drug overdose have evidence of chest X-ray abnormality and are treated as presumed aspiration pneumonia with antibiotic therapy. Our objective was to determine if evidence of sepsis was present and if the use of antibiotic therapy was appropriate. Methods: Retrospective review of case records was undertaken for all patients with a history of drug overdose admitted to the intensive care (ITU) or medical high dependency unit (HDU) from January 1999 to December 2000. If the chest X-ray was reported as being abnormal the following data were collected: fever (temperature ≥38°C), peak white blood count, microbiology results, Glasgow coma score on admission, witnessed vomiting or seizure activity at any time and the need for intubation or ventilatory support. Results: Data were analysed for 41 patients with a mean age of 41.2±2.8 years. 22 patients were intubated and treated with intermittent positive pressure ventilation and 2 non-invasively with continuous positive airway pressure ventilation (CPAP). 11 patients (26.9%) had bilateral infiltrates on admission chest X-ray, 8 patients (19.5%) had basal changes and 8 patients (19.5%) had lobar collapse. 14 patients (34.1%) had a normal admission chest X-ray but subsequently developed pulmonary infiltrates and clinical evidence of sepsis. Median Glasgowcoma score was 4 on admission and witnessed vomiting or seizures occurred in 5 patients (12.2%). 3 patients (7.3%) were febrile within 24 hours of admission and a further 17 (41.4%) became febrile at a later stage. Mean white blood count was 15.3±0.8×109/L, blood cultures were positive in 5 patients (12.2%) and organisms were grown in sputum culture in 13 patients (31.7%). Antibiotic therapy was started within 24 hours in 30 patients (73.2%). Conclusions: Chest X-ray abnormalities occurring after drug overdose are assumed to be due to aspiration pneumonia, often with no evidence of vomiting. A large number of patients are treated with antibiotics in the absence of clinical evidence of sepsis. Bilateral pulmonary infiltrates may represent non-cardiogenic pulmonary edema which will usually respond to CPAP. Lobar collapse is often secondary to retained secretions and mucus plugging and is best treated with chest physiotherapy or suction bronchoscopy. Alternative diagnoses other than aspiration pneumonia should be considered in overdose patients without signs of sepsis presenting with chest X-ray abnormalities. Our data also suggest that the risks of hospital acquired infection following intubation should not be underestimated.
82 WITHDRAWN
83 ELEMENTAL MERCURY POISONING IN A FAMILY OF SEVEN: IT STILL HAPPENS
Velez LI, Shepherd JG, Cottrell C, Rivera W, Keyes DC, Benitez FL
Division of Emergency Medicine, The University of Texas Southwestern Medical Center and the North Texas Poison Center, Dallas, Texas, USA
Objectives: Symptoms related to mercury exposures vary depending on the type of mercury, the duration, and degree of exposure. This case report provides an example of chronic elemental mercury intoxication. Neurological and psychological symptoms predominate following this type of exposure, although renal toxicity may also occur. The long-term consequences of elemental mercury exposure in children are uncertain. Case Report: In June 2000, a 3 y/o Hispanic girl was referred for admission due to 6 months of progressive neurologic symptoms. On initial exam, the patient was found to be tachycardic and hypertensive. She was thin, ataxic, mute, hypotonic, and drooling. The patient had a tremor that was abolished during sleep. Diagnostic testing included CT and MRI of the brain, EEG, abdominal US, CXR, UGIS, and laboratories, including blood levels for heavy metals. All tests were within normal limits, except for the blood mercury level, which was reported as 295 mcg/L (normal range<10 mcg/L). After consultation with the Regional Poison Center, the index patient began inpatient chelation therapy with oral DMSA. Since the history was suspicious for an environmental exposure, the family was tested. All other 6 family members had elevated mercury levels (range: 286–2940 mcg/L). Her family was removed from the home and also started on DMSA. The source of the mercury was a plastic container left by the previous tenant, which the children had been playing with and spilled over the carpet. In total, the index patient and her family received three rounds of oral chelation therapy between June and October of 2000. Although the patient did not undergo neuropsychiatric testing, her family reported that her behavior gradually returned to baseline. The family arranged for decontamination, aided by the local HazMat team and moved back in the house. The industrial hygienist from the health department visited the home 3 months later and found that mercury was detectable at levels ranging from 0.009–0.017 mg/M3. Conclusions: A high index of suspicion, a thorough environmental history, and appropriate laboratory data can aid in the diagnosis of mercury poisoning. In a patient with tremor, personality changes, and unusual neurologic symptoms with an insidious onset, physicians should include mercury intoxication in the differential diagnosis. Poison Control Centers can help coordinate the environmental investigation and refer the health care provider to any appropriate resources.
84 CLINICAL LEAD INTOXICATION DUE TO CONTACT OF GUNSHOT BULLET WITH CEREBRAL SPINAL FLUID
Madureira PR,1 De Capitani EM,1 Vieira RJ,1 Sakuma AMA,2 Toledo AS,1 Mello SM1
1Poison Control Centre—Hospital de Clínicas—Universidade Estadual de Campinas—Campinas, São Paulo, Brazil; 2Labroratory of Atomic Absorption Spectrometry—Instituto Adolfo Lutz—São Paulo, Brazil
Objectives: Lead intoxication due to retained gunshot bullets is a well known clinical problem that has previously been described in the literature. Risk factors for this occurrence are mainly related to any contact of the lead bullet with joint fluid or cerebral spinal fluid (CSF). The acidic pH of these fluids solubilize lead which then migrates from the bullet via blood to reach target organs. Treatment of these cases requires chelation therapy during symptomatic periods and definitive surgical removal of the bullet as a potential source of lead. The aim of this paper is to describe a clinical case of lead intoxication due to retained gunshot bullet which was in contact with CSF. Only one similar case was found in a literature review. Case report: A male 42 year old real estate businessman was hit by a gunshot bullet in 1992 during a hold up. Bullets were localised in the abdomen and right leg, and a laparotomy was necessary for small intestine segment resection. Six years later, he developed intense lumbar back pain, when doctors realised the source of pain was the retained bullet between L5 and S1. At that time he was submitted to laminectomy in an attempt to excise the bullet, without success. In April 2001 (around 9 years after the accident) the patient had his first episode of abdominal pain. Next month he had an arthrodesis of L5-S1. He developed intense abdominal pain after the surgical procedure and lead poisoning was diagnosed. He was then treated with calcium gluconate initially, and calcium versenate for 5 days with good response. ALA in urine was measured before chelation therapy, resulting 122 mg/L, and 8.9 mg/L after that. Two weeks later he showed recurrence of symptoms when ALA was 55 mg/L. A further cycle of calcium versenate was prescribed with good symptomatic response. For three months he was fine. During that period there was remarkable dispersion of lead from the bullet with precipitation of it throughout the sciatic nerve sheath as seen at sequenced radiographs. Conclusion: Due to failure to remove the gunshot bullet during surgical procedure, because of the risk from surgery of neurological sequelae, long term oral chelation therapy seems to be the treatment of choice in this case. DMSA has good penetration in CNS and has a low incidence of adverse effects during short term therapeutic protocols. However, the high costs of this compound, and unknown side effects during long-term administration raise concerns. Renal and neurological monitoring must also be performed during follow up to detect organ dysfunction due to lead.
85 LEAD TOXICITY DUE TO USE OF AN AYURVEDIC COMPOUND
Traub SJ, Hoffman RS, Nelson LS
New York City Poison Control Center, New York, New York, USA
Objective: Traditional remedies are routinely used both for health maintenance and the treatment of illness. Many of these compounds contain agents that are undisclosed and potentially toxic. We report a case of severe lead toxicity due to the use of an ayurvedic compound from India. Case Report: A 40 year-old woman presented to the Emergency Department complaining of 2 months of abdominal pain which had become progressively worse over the two weeks prior to presentation. The pain was poorly localized, and associated with occasional vomiting. She took no prescription medications, but admitted to consuming several tablets of an “herbal medication” that she had obtained in India to treat her abdominal pain. Vital signs were: pulse, 87/minute; blood pressure, 125/91 mm/Hg; respiratory rate, 12/minute; temperature, 98.8°F. The abdominal examination was unremarkable. The neurologic examination was notable only for mild confusion. The remainder of the physical examination was unremarkable. Serum electrolytes and liver function tests were normal; plain abdominal radiography and serum pregnancy testing were also negative. The complete blood count indicated a normal white blood cell count but a mild anemia (hematocrit 34%). The patient was admitted for further evaluation. On hospital day 2 she suffered 2 generalized tonic–clonic seizures. Her initial laboratory tests were reviewed, and basophilic stippling was noted on a smear of the peripheral blood. This prompted evaluation of her blood lead level, which was 98 mcg/dL. She underwent chelation with British Anti-Lewisite (BAL) and Ethylene Diamine Tetraacetic Acid (EDTA) for 3 days. Her abdominal symptoms and neurologic findings resolved during this period. She was discharged to complete a 19-day course of DMSA and instructed to discontinue the ayurvedic medication. Repeat lead levels were 50 mcg/dL after 5 days of chelation, 42 mcg/dL after 15 days of chelation, and 41 mcg/dL one week after completion of chelation. She had no residual neurologic dysfunction. Laboratory analysis of her ayurvedic medication revealed that it was 4.4% lead by weight. Conclusion: Traditional ethnic remedies are a potential cause of lead toxicity, and a high index of suspicion is necessary to make the diagnosis. This patient developed severe lead toxicity, including abdominal pain, diastolic hypertension, and encephalopathy. This case demonstrates that lead toxicity due to the use of traditional ethnic remedies may be profound, in patients who are unaware that they are poisoning themselves.
86 EPIDEMIOLOGY AND TREATMENT OF CARBON MONOXIDE POISONING IN LJUBLJANA, SLOVENIA
Brvar M,1 Jamšek M,1 Možina M,1 Horvat M,2 Gorjup V2
1Poison Control Centre, University Medical Centre, Ljubljana, 2Centre of Intensive Care Medicine, University Medical Centre, Ljubljana, Slovenia
Objective: The aim of the study was to investigate the epidemiology, treatment and outcome of carbon monoxide (CO) poisoning in the Ljubljana region (Slovenia). Methods: A retrospective study was carried out of CO-poisoned patients admitted to the Poison Control Centre and Centre of Intensive Care Medicine of the University Medical Centre Ljubljana, between January 1, 1990 and December 31, 1999. Results: 143 CO-poisoned patients were admitted to the University Medical Centre in Ljubljana over the 10 year period, an annual rate of 2.4 cases per 100,000 population. Of these patients 72% were male and 28% were female. 25% were suicide attempts and 75% were unintentional poisonings. 65 patients had unequivocal loss of consciousness at the scene. On admission to the emergency department 7 patients were unconscious, 4 patients had focal neurological deficits, 13 patients had myocardial ischaemia and 5 patients metabolic acidosis (pH<7.20). 38% of the patients had an initial carboxyhemoglobin level of more than 25%, and 12% of the patients more than 40%. The carboxyhemoglobin level did not correlate with clinical findings. 50% of the patients met the criteria for hyperbaric oxygen according to the European Consensus Conference on Hyperbaric Medicine, but only 7 patients (5%) were treated with hyperbaric oxygen therapy while 136 (95%) received 100% normobaric oxygen. The indications for hyperbaric oxygen therapy were unconsciousness on admission to the emergency department or persistent neurological deficits after 24-hours treatment with 100% normobaric oxygen. The patients were transferred for hyperbaric oxygen therapy to Austria, because there is no centre with hyperbaric facilities in Slovenia. All of the hospitalised CO-poisoned patients survived. 139 patients (97%) were asymptomatic and 4 patients (3%) had headache or vertigo at hospital discharge. Neuropsychological testing and follow-up to determine delayed sequelae were not performed. Conclusions: No CO-poisoned patient died after arrival at our hospital, despite very strict criteria for hyperbaric treatment. The annual number of CO deaths will not decrease when the hyperbaric chamber is built in Ljubljana next year. In the future we should harmonize our selection criteria for hyperbaric oxygen according to the European Consensus Conference on Hyperbaric Medicine and arrange for neuropsychological testing and follow-up examinations.
87 PROGNOSTIC FACTORS IN CARBON MONOXIDE ACUTE POISONINGS NEEDING MECHANICAL VENTILATION
Bilbault P, Schlossmacher P, Méziani F, Delabranche X, Kummerlen C, Rémy C, Assemi P, Schneider F, Lutun P, Martinet O, Tempé JD, Jaeger A
Service de Réanimation Médicale, Hôpital de Hautepierre, Strasbourg, France
Objectives: Carbon monoxide (CO) poisoning is still the most frequent and severe domestic poisoning in Europe. The aim of the study was to determine the prognosis factors in CO poisoned patients admitted in the ICU for neurological and/or cardiovascular and/or respiratory failure. Methods: In a retrospective study of patients poisoned by CO admitted between 1986 and 1999, we compared 2 groups of patients, with and without mechanical ventilation. The following parameters were collected: age, SAPS II, GCS, hypotension, initial HbCO level, smoke inhalation, treatment by hyperbaric oxygen (HBO), mortality and sequelae. Results: From the 997 patients included in the study 34 (3.41%) were admitted in the ICU for mechanical ventilation (MV). The characteristics of the 2 groups are indicated in Table I.
In the group of patients treated by MV, seven died (20%): 5 from cerebral death after cardiac arrest, 1 from septic shock, and 1 from ventricular fibrillation with underlying cardiac disease. Sequelae included skin burns (8 cases), neurological (3 cases) and respiratory (2 cases) disturbances. The analysis of the areas under the ROC curves showed that 4 parameters were related with a bad prognosis: an initial GCS<3, an initial systolic blood pressure<90 mmHg, a metabolic acidosis with a BE<7 mmol/l and a SAPS II>40. Conclusion: This study shows that CO poisonings needing MV are rare but associated with a high mortality, especially if the poisoning is due to smoke inhalation. Four criteria which can be collected during the first 24 hours are factors of a bad prognosis.
Table 1. (Abstract 87)
88 DELAYED STATUS EPILEPTICUS AFTER A SUSTAINED RELEASE BUPROPION OVERDOSE
Velez LI, Delaney KA, Rivera W, Shepherd JG, Benitez FL
Division of Emergency Medicine, The University of Texas Southwestern Medical Center and the North Texas Poison Center, Dallas, Texas, USA
Objective: The antidepressant Bupropion inhibits CNS reuptake of dopamine and norepinephrine. Symptoms following acute overdose include mainly tremors, seizures, hallucinations, and tachycardia. Seizures occur relatively early after acute overdose, in up to 21% of patients. Status epilepticus (SE) has not been reported. We describe a patient who developed status epilepticus more than 32 hours after an ingestion of sustained-release (SR) bupropion. Case Report: A healthy 31 y/o woman was brought to the ED after taking about 30 tablets of Wellbutrin® SR (4.5 g) over several hours. Vital signs on arrival were BP 155/74, HR 169, RR 24, T 38.5°C, sat 98% on RA. She was tremulous, confused, and had hallucinations. The rest of the exam was unremarkable. Initial laboratories included: APAP<1.0 mg/dl, ASA<1.0 mg/dl, ETOH 17 mg/dl, negative urine drug screen, and a normal anion gap. The ECG showed sinus tachycardia with a normal QRS. The patient received activated charcoal and 6 mg of IV lorazepam. Delirium, which persisted during the hospitalization, was treated with haloperidol. Thirty-two hours after admission, the patient had 4–5 seizures over 30 minutes. The seizures ceased after 6 mg of IV lorazepam. She was intubated for airway protection and received fosphenytoin. The patient had no further seizures and was extubated within 24 hours. Conclusion: This case indicates a risk of delayed onset of seizures after ingestions of SR bupropion when delirium fails to improve. Aggressive decontamination is indicated in such cases, which may include repeated doses of activated charcoal and/or whole bowel irrigation.
89 ABNORMAL MAGNETIC RESONANCE IMAGING AND EVOKED POTENTIALS FINDINGS IN A CASE OF VALPROIC ACID-RELATED HYPERAMMONEMIC ENCEPHALOPATHY
Hantson Ph,1 Wittebole X,1 Grandin C,2 Guérit JM3
1Department of Intensive Care Medicine, 2Department of Neuroradiology, 3Laboratory of Neurophysiology, Cliniques St-Luc, Université catholique de Louvain, Brussels, Belgium
Background: Hyperammonemic encephalopathy is a rare but possible complication of valproic acid treatment or overdose. We report a case documented by unusual magnetic resonance imaging (MRI) and evoked potentials (EP) findings. Case Report: A 48-year-old man with a history of recurrent epilepsy was admitted in a regional hospital for generalized seizures. He was chronically treated by phenytoin, and carbamazepine. He was initially treated by intravenous valproic acid (10 mg/kg as a loading dose, followed by the administration of 1 mg/kg/hr). After the postictal period, the patient remained poorly reactive to painful stimuli and did not regain consciousness. Localized seizures of short duration were still observed clinically and confirmed by EEG recording. Brain computed tomography did not reveal any new lesion. The neurologic condition still worsened by the fourth hospital day (areactive coma, areactive dilated pupils and absence of corneal reflex). The patient was then referred to our hospital for further investigation. On admission, he was deeply comatose (Glasgow Coma Scale 3/15). Among brainstem reflexes, only corneal and cough reflexes were obtained with difficulty. Status epilepticus was ruled out by an EEG recorded over several hours. We tested the hypothesis of metabolic encephalopathy in relation with valproic acid therapy. Indeed, blood arterial ammonia concentration was 125.6 μmol/l (<58.7) and peaked up to 411 μmol/l 24 hours later. Blood valproic acid level was within therapeutic range. The administration of valproic acid was immediately discontinued and levocarnitine supplement (100 mg/kg/day) was given intravenously. Multimodality (visual, somatosensory and brainstem auditory) evoked potentials (EP) were recorded at the bedside. In accordance to the clinical findings, severe brainstem dysfunction was also assessed by EP examination. A first MRI was performed the day after admission. The MRI changes were consistent with bilateral and diffuse cytotoxic edema affecting bilaterally not only the cortical areas, but also the basal ganglia and the brainstem. Such findings could not only be explained by prolonged seizures. Brainstem reflexes reappeared after 7 days together with a regression of EP abnormalities. Concerning MRI, we observed on two consecutive examinations a fading of the lesions previously described in the grey matter, basal ganglia, brainstem and cerebellum. Localized epileptic activities were still recorded on the EEG. Final outcome was poor. Discussion: Valproic acid-related encephalopathy is usually completely reversible after drug discontinuation. This observation suggests that cytotoxic edema, involving cortical areas and brainstem, may accompany valproic acid-related hyperammonemic encephalopathy.
90 A CASE OF SEVERE POISONING CAUSED BY 5-MEO-DMT
1Nyman T, 1Hoppu K, 2Koskinen R, 2Harjola V-P, 3Kuisma MJ
1Poison Information Centre, and 2Department of Medicine, Helsinki Emergency Medical Services, Helsinki University Central Hospital, Helsinki, Finland
Objective: 5-methoxy-n,n-dimethyltryptamine (5-MeO-DMT) is a hallucinogenic indolealkylamine structurally related to serotonin, psilocybin and LSD. It is smoked, taken orally, or injected. Hallucinogenic dose i.v. is said to be 6 mg. 5-MeO-DMT is easily obtained from Internet. Usually 5-MeO-DMT and related tryptamine derivatives don't cause severe poisonings. Typical symptoms in overdose include agitation, hyperactivity, salivation, mydriasis, tremors, mild increase in temperature, hypertension and tachycardia. We present a case where a patient developed status epilepticus after ingesting 5-MeO-DMT. Case Report: A 18-year-old man ingested about 200 mg of 5-MeO-DMT, which he had purchased via the Internet. About 3.5 hours later emergency services were called because he was found lying on the ground unable to get up. He was conscious, remembered his home address but not where he was, and had muscular hypotonia. He was transported to hospital where 1 hour later he developed first tremors at his jaws and little later started convulsing. He was first treated with diazepam i.v. (4×10 mg). When the convulsions continued and the oxygen saturation started to decrease, he was intubated and given thiopental (300 mg). The patient was transferred to a tertiary hospital and during transportation the convulsions continued and he received additional thiopental. He was connected to a ventilator. The patient was drooling, sweating, had dilated pupils with an elevated body temperature (38°C). The convulsions ceased within a couple of hours and the patient could be extubated. About 8.5 hours after the ingestion of the drug he was asymptomatic. He was observed until the next day. He confirmed the dose and substance he had taken. He had used it also several times before in different doses and in pursuit of the best dose took this time a high dose. These types of drugs are easily obtained from the Internet. They may contain other substances and contaminations, which can cause unexpected symptoms. The strength of the drug obtained may vary and together with the often diffuse instructions for dosing obtained from Internet may lead to unexpected effects or like in this case to experimenting with high doses. It is also possible that the responses to a substance vary individually. In this case no other plausible explanations than the high dose of 5-MeO-DMT emerged for the symptoms observed. Conclusion: 5-MeO-DMT overdose may cause convulsions that can progress to status epilepticus.
91 MASSIVE GABAPENTIN AND PRESUMPTIVE QUETIAPINE OVERDOSE
Spiller HA, Dunaway MD, Cutino L
Kentucky Regional Poison Center, Louisville, Kentucky, USA
Background: Previous reports of gabapentin overdose have described mild symptoms of somnolence, ataxia and slurred speech. Quetiapine has produced a false positive for cyclic antidepressants on immunoassay drug screens. Quetiapine overdose is associated with coma, QTc prolongation and hypotension. Case Report: A 61 year old female with a history of severe COPD, type 2 diabetes and previous recent suicide attempts was found unresponsive by family after ingesting up to 180 300 mg capsules (54 gm) of gabapentin. The patient's home medications were buproprion 150 mg, klonopin 1 mg, clopidogrel 75 mg, gabapentin 300 mg, glyburide 10 mg, losartan 50 mg, metoprolol 100 mg and quetiapine 200 mg. Upon arrival in the ED she was responsive to pain, with shallow and irregular respirations. Vital signs were HR 82, BP 68/40. Pupils were dilated at 6 mm with minimal response to light. She was intubated and placed on mechanical ventilation. Dopamine at 10 mcg/kg/min was effective in restoring her blood pressure. She received activated charcoal via gastric tube. Laboratory results were pH 7.39, pCO2 33, pO2 657 and O2 sat 99%. WBC was 12.2. All other labs including liver and renal function were normal except a glucose of 205 mg/dl. A urine drug screen was negative for cocaine THC, amphetamine, barbiturate, benzodiazepines, opiates, PCP and tricyclic antidepressants. A serum drug screen was qualitatively positive for tricyclic antidepressants by immunoassay. ECG showed sinus rhythm with ST wave changes in the inferior lead, a QTc of 470 msec and QRS of 80 msec. A gabapentin concentration result drawn at the time of arrival in the ED was 104.5 mcg/ml (therapeutic range 4–8.5 mcg/ml). Eight hours post arrival the patient vomited and may have aspirated. She subsequently developed fever and elevated WBC count and clindamyocin and lentamicin were initiated. Ten hours post arrival the patient was awake and could recognize staff. Over the subsequent next 10 hours the patient was weaned from the ventilator and extubated. The patient made an uneventful recovery and was discharged 4 days post ingestion to a psychiatric facility. Discussion: Peak serum concentrations of gabapentin of 62 mcg/ml, 60 mcg/ml and 44.5 mcg/ml have previously been associated with lethargy, ataxia and mild hypotension. Quetiapine has been reported to produce a false positive for cyclic antidepressants on immunoassays drug screens. It appears the serious clinical effects noted in this patient were secondary to the presumptive quetiapine and that gabapentin even with massive ingestion produces only mild symptomatology. Conclusion: We report a case of massive gabapentin and presumptive quetiapine overdose with the highest recorded serum gabapentine concentration that was associated with coma, respiratory depression requiring mechanical ventilation and hypotension.
92 GABAPENTIN WITHDRAWAL PRESENTING AS STATUS EPILEPTICUS
Barrueto F, Jr.,1 Green J,2 Howland MA,3 Nelson LS1
1NYU–Bellevue, New York City Poison Control Center, New York, New York, USA. 2Staten Island University Hospital, Dept. of Internal Medicine, Staten Island, New York, USA. 3St. John's University College of Pharmacy, New York, New York, USA
Objective: Gabapentin withdrawal syndrome is described previously as irritability, agitation, tachycardia and diaphoresis. There are no reports of abrupt withdrawal of gabapentin causing seizures in a patient without a pre-existing seizure disorder. We are reporting the first case of status epilepticus secondary to gabapentin withdrawal. Case Report: A 34-year-old male with lumbar disc disease and surgery had been placed on 8000 mg of gabapentin daily for chronic back pain. He had been on that dose for the past nine months. He had no medical history, no history of seizure disorder and was on no other medications. The day prior to admission the patient developed nausea and vomiting and was unable to take any of his gabapentin. The next day, the patient had a witnessed seizure at home. Diazepam, 10 mg intravenously, was administered for his seizures however; he eventually required intubation and needed phenobarbital and phenytoin to stop his seizures. Vitals signs were temperature 98.8°F, pulse 80, blood pressure 120/80. His physical exam was unremarkable. He was maintained on a lorazepam drip for sedation and control of his seizures. All laboratories were unremarkable. Electrocardiogram showed normal sinus rhythm with normal intervals. Computed tomographic scan of the head was normal. Lumbar puncture was negative. Magnetic resonance imaging of the brain was also negative and electroencephalogram showed no abnormalities. His gabapentin level was less than 0.5 mcg/dL, which was drawn at the time of admission to the Emergency Department. The patient was restarted on his gabapentin at a reduced dose of 800 mg, three times a day. He was extubated on the third hospital day and did well during his nine-day hospitalization. He was discharged home with a completely intact neurological exam. Conclusion: This is the first reported patient whose presentation is most consistent with seizure and status epilepticus secondary to gabapentin withdrawal.
93 ATYPICAL CLINICAL PICTURE AND COURSE OF HEROIN INTOXICATION
Batora I, 1Kukumberg P, Plackova S, Kresanek J
Department of Industrial Medicine and Toxicology, 12nd Department of Neurology, School of Medicine, Comenius University, Bratislava, Slovakia
Objective: Heroin, an opioid derivative, is the most misused illicit drug in Slovakia because of the excitement, euphoria, sensation of wellbeing and alteration of mood that it produces. The rapid onset of these effects with an intravenous bolus is called a “flash” or “rush.” The characteristic syndrome of overdose consists of respiratory depression, impaired consciousness and miosis with pinpoint pupils. The following central nervous impairments after overdose in heroin addicts have been reported in the literature: spongiform leuco-encephalitis, myelopathy, stroke, intracranial hypertension, abnormal movements. Case Report: The patient, a 25 year-old man, with an 8 year history of heroin addiction, was found unconscious lying on the ground. The patient was given oxygen and specific antidote Naloxone immediately in the emergency ambulance with only transient improvement of mental status. Toxicological analysis revealed the presence of large quantities of opiates in urine (>10,000 ng/ml). For the subsequent six days atypical prolonged variable impairment of consciousness (alternating stupor and somnolence) was accompanied by mixed quadriparesis and severe gastroplegia. Laboratory findings: hyperglycaemia, hypokalaemia, leucocytosis and BUN elevation. CT and MR revealed clinically insignificant bilateral frontal hygroma (posttraumatic?). Because of the marked gastroparesis and because extreme fluid loss had resulted in severe dehydration (about 5 L of fluid was aspirated by gastroduodenal tube), the patient was admitted temporarily to the surgical department where oral mannitol and intravenous physostigmine were administered to support gastric motility. The sophisticated neurological examinations of conscious quadriparetic patient including EEG, EMG and various tests of evoked sensory potentials (magnetic-MEP, visual-VEP, brain auditory-BAEP, somatosensoric-SEP) enabled a diagnosis of encephalomyelo-olyradiculoneuropathy to be established. Five month after intoxication the patient was still not able to walk without support. Conclusion: The observed diffuse impairment of the central and peripheral nervous system after acute heroin intoxication, diagnosed as encephalo-myelo-polyradiculoneuropathy in our patient, is apparently extremely rare, and is not listed in toxicological databases (1,2).
References: 1. Micromedex Healthcare Series, Poisindex Toxicology Information, 2001. 2. International Programme on Chemical Safety (IPCS) INTOX. CCOHS 2001.
94 ACUTE VERATRUM TINCTURA POISONINGS
Sentsov VG, Brusin KM, Rokin SR, Novikova OV
Sverdlovsk Regional Toxicological Center, Ural State Medical Academy, Yekaterinburg, Russia
Objectives: Acute veratrum alkaloid poisonings in Russia are closely connected with the use of alcoholic infusions of veratrum containing medicines. Rarely the poisonings are of a criminal nature. From 1993 to 2000 110 patients with acute veratrum poisonings were admitted to Sverdlovsk Regional Toxicological Center. No deaths were recorded. 85.5% of the patients were male and the age range was 30–49 years old. Methods: We recorded the measurements of arterial blood pressure, cardiac output, acid–base balance, cardiac rhythm and ECG, and diagnostic transoesophageal cardiac pacing for these patients. Results: The symptoms of nausea, vomiting, bradycardia and arterial hypotension were recorded in 100% of these patients. Other symptoms occurred frequently—hypersalivation (83.6%), hyperhydrosis (80%), general fatigue (71.8%), sting in the mouth (80%), and oesophagus and stomach pain (71.8%). Rarely seen were diarrhoea (40%) and mental confusion with respiratory failure (28.2%). In all patients the diagnosis of veratrum poisonings was confirmed by the analytical determination of veratrum in urine, using chromatography. The patients were classified into three groups. Mild poisonings (16.4% of patients) were determined when the usage of the drug was about 30–50 ml. These patients had no symptoms of mental confusion, but had nausea and fatigue. Pulse rate was about 59±0.2 min−1 and arterial blood pressure was normal. Moderate poisonings (37.3% of patients) corresponded with ingested doses of 50–100 ml. These patients exhibited mental confusion, general fatigue, hyperhydrosis, stinging in the oesophagus, vomiting and diarrhoea. Pulse was 46±1.1 min−1 and arterial blood pressure normal. During severe poisonings (46.3%; doses greater then 100 ml) some patients had coma with respiratory failure. In all patients we found arterial hypotension (systolic arterial pressure 77±1.5 mmHg, diastolic 39±1.6 mmHg) and pulse was 56±1.1 min−1. In 5.5% of patients substituting atriovenricular rhythm together with sinus node arrest was defined. In 7.3% of patients we found 1st degree atrioventricular block, in 8.2%—2nd degree. The depression of sinus node was marked in 19.1% and sinoatrial block in 59.1% by transesophageal electric stimulation. The principal treatments given were gastric lavage, IV administration of atropine 0.02 mg/kg, and fluid infusions—15 ml/kg/h during first 2–3 h. Such treatment saw the stabilisation of breathing, mental disorders were still evident in 15.5% of patients, vomiting and diarrhoea in 10.9%, nausea in 26.4%, fatigue in 20%, stinging in the mouth in 18.2%, and stinging in oesophagus and stomach in 6.4%. In all patients heart rate increased, but in some cases we recorded re-appearance of rhythm disorders and the need for further atropine administration on the second day after poisoning.
95 ANTICHOLINERGIC POISONINGS ASSOCIATED WITH FENNEL TEA
Plackova S, Kresanek J, Klobusicka Z, Batora I, Kostolanska K
Toxicological Information Centre, Department of Industrial Medicine and Toxicology, Derer Hospital, Bratislava, Slovak Republic
Objective: The effects of anticholinergic substances on the CNS have already been reported, generally in connection with ingestion of Datura stramonium or other plants. We report several cases of anticholinergic poisonings from consumption of Foeniculum vulgare (fennel) tea, contaminated with Datura stramonium seeds. A tea made from Feoniculum vulgare is a good treatment for indigestion, a circulatory stimulant, mild expectorant and the seeds also promote milk flow in breast feeding. Case report: In the Czech Republic in February 2001, it was reported that fennel tea exported from the Slovak Republic was contaminated with Datura stramonium seeds. This information was also published in the Slovak Republic. Our Toxicological Information Centre received 35 telephone calls from people who had drunk fennel tea. 15 people had symptoms of intoxication, 10 of whom were very seriously intoxicated. In most cases, young mothers and their children were adversely affected. They had mydriasis, blurred vision, dryness of the throat and mucous membranes, somnolence and lethargy. In one case there was urinary retention and one person lost consciousness. These symptoms required medical treatment e.g. neurological and ophthalmic examinations, CT scan, and MRI. The diagnoses varied from suspicion of a cerebral tumour to virosis. Only one woman was admitted to hospital due to visual problems. Microscopic examination of the seeds by a botanical expert identified them as Datura stramonium seeds. The association between the Datura contaminated fennel tea and the toxicity it produced was made public. Conclusion: The population should be aware that all herbal remedies have the potential to be misidentified, mislabelled or contaminated.
96 STAR ANISE TOXICITY. AN OUTBREAK OF PEDIATRIC POISONING
Ramón MF, Martínez-Arrieta R, Ballesteros S, Gómez P,1 Garzo C,2 Cabrera J
Spanish Poison Control Centre, Instituto Nacional de Toxicología. 1Centro Nacional de Epidemiología. 2Servicio de Neuropediatría, Hospital Gregorio Marañón. Madrid, Spain
Background: Star anise (Illicium verum Hook) is used as a carminative product. In Spain it has both medicinal and food additive considerations. This herb is imported from oriental countries. Contamination or adulteration with Illicium religiosum has already been described. I. religiosum is a more toxic plant due to its anisatine contents, and lacks any medicinal properties. Both species are difficult to be differentiated by a macroscopical study. The relationship between high doses of star anise and neurological symptoms in children has been known for a long time. Two convulsivogenic components named veranisatines (A and B) have been described. The objective of this analysis is to evaluate an increase in incidence of poisoning in babies after ingestion of star anise contained in bags incorrectly labeled. Methods: From March to November 2001, several cases of star anise poisoning were reported to our PCC. The following data were examined: age, gender, dose, onset of signs and symptoms, clinical manifestations and treatment. Results: There were 20 consults due to star anise poisoning from local hospitals. The age range was 8 days to 3 months. Fifty percent were male and 45% female; 5% unknown. Dose of star anise preparation ranged between 6–7 flowers in 40 mL of water to 2 flowers in 500 mL/day for 6 days or more. The onset of clinical features ranged from 30 minutes to 4 hours after ingestion. The frequency of clinical manifestations was as follows: Neurological: hyperexcitability and/or crying or irritability 100% of cases, nystagmus 80%, hypertonia 35%, tremors 30%, convulsions 25%, arm and leg spasms 25%, hyperreflexia 10%, drowsiness 10%, and disorientation 5%. Gastrointestinal (vomiting or diarrhea) 40%. Other: flushing 15%, pallor 5%, cyanosis 5% and bradycardia 5%. All patients were followed for 3–5 days and the majority were asymptomatic within 24 hours. Four cases required diazepam and/or phenobarbital. The bags were bought at both chemist's and herb shops. The labels did not contain indications of use or administration (infusion, decoction, doses, contraindications, indication, etc.). Conclusion: An outbreak of star anise intoxication with significant neurological symptoms in infants was reported. Our PCC was able to detect sentinel events such as those described here. The authorities have removed the bags containing star anise until correct identification of the specimen is accomplished. Other regulations for herb use and administration should be implemented including special warnings for children and infants.
97 STUDY OF CEREBRAL AND PLASMA KINETICS OF HIGH DOSES OF MIDAZOLAM AND DETERMINATION OF CORRELATIONS WITH RESPIRATORY EFFECTS IN RATS
Mégarbane B, Lesguillons N, Risède P, Monier C, Trout H, Buneaux F, Galliot-Guilley M, Baud F
INSERM U26, Hôpital Fernand Widal and Réanimation Médicale et Toxicologique, Hôpital Lariboisière, Paris, France
Objectives: Although benzodiazepines poisonings are frequent, the exact mechanism of respiratory depression in intoxicated patients is still misunderstood: central nervous system inhibition or peripheral upper air ways obstruction, in relation to bronchial muscle relaxation. The objectives of our study were to determine in rats midazolam kinetics in case of high doses intravenous infusions and to analyze the toxicokinetic–toxicodynamic (TK–TD) relationships concerning its respiratory effects. Methods: We performed a comparative study of blood and striatal kinetics of midazolam using cerebral microdialysis in male Sprague-Dawley rats. A 160 mg/kg-single dose of midazolam was intravenously infused during 20 min. Midazolam concentrations were measured using an inverse polarity high-performance liquid chromatography (HPLC) procedure coupled to ultraviolet detection. Midazolam induced-respiratory effects were studied by a serial determination of arterial blood gases (ABL 300, Radiometer) in catheterized rats. Modeling of midazolam blood and cerebral kinetics was then realized and the kinetics parameters evaluated using Kinetica software. Results: We first assessed the optimal conditions for the assay procedure (detection threshold of 8 ng/ml, quantification limit of 25 ng/ml, appropriate linear calibration graph and reproducibility of measurements with a 5%-coefficient of variation). Midazolam blood kinetics were correctly represented by a bi-exponential model, peaking at the end of infusion and giving a slow elimination rate. A 5 hour’;-elimination half-life was calculated, which represented 10 times the values obtained in pharmacological conditions. Elevation of half-life was explained by a probable saturation of midazolam metabolism during treatments with high doses. Regarding the brain kinetics, the peak was observed 50 min after the end of infusion, with a significant delay compared to the blood kinetics. This delay was explained by a slower distribution phase into the brain, with a time necessary to cross the blood–brain barrier. Respiratory depression, as assessed by an elevation in PaCO2, was more significantly correlated with midazolam cerebral kinetics than with blood kinetics. Conclusion: In Sprague-Dawley rats, respiratory effects of high doses midazolam are correlated with midazolam striatal concentrations determined using cerebral microdialysis. This preliminary study may contribute to better understanding of the mechanisms of high dose benzodiazepines induced-respiratory depression.
98 EFFECTS OF SINGLE INTRAVENOUS HIGH DOSES OF BUPRENORPHINE IN ASSOCIATION WITH PHARMACOLOGICAL DOSES OF FLUNITRAZEPAM ON ARTERIAL BLOOD GASES IN RATS
Mégarbane B, Gueye PN, Pirnay S, Monier C, Risède P, Baud F
INSERM U26, Hôpital Fernand Widal and Réanimation Médicale et Toxicologique, Hôpital Lariboisière, Paris, France
Objectives: Buprenorphine (BUP), a substitution treatment of opiate addiction, largely prescribed in France, may cause severe acute poisoning with coma and respiratory failures, in case of overdose, misuse or association with benzodiazepines, including flunitrazepam (FLU)1. The mechanisms of the interaction between BUP and FLU are still misunderstood. Our objectives were to test the effects of the association of single high doses of BUP with pharmacological doses of FLU on ventilation in rats. Methods: Catheterized restrained male Sprague-Dawley rats were randomized in 4 groups. In the first group, rats received intravenously FLU (2.5 mg/kg)+BUP (30 mg/kg) and in the 3 others, FLU+aqueous solvent, aqueous solvent+BUP or aqueous solvent+aqueous solvent. Effects of treatments were analyzed using clinical parameters (scale of sedation and respiratory rate determination) and measured on serial arterial blood gases obtained over 3 h (Radiometer ABL 300). Baseline values were compared using one-way analysis of variance followed by multiple comparison tests using Bonferroni's correction. In each group, the effect of time was studied using repeated measures ANOVA and Dunnett's multiple comparison tests. Results: There was a significant effect (p<0.001) of combining BUP and FLU on rat ventilation, with a decrease in pH and elevation in PaCO2 values, at all times of measurements following drug infusion. Effects on respiration were absent in rats that received the 2 aqueous solvents. Alteration in pH and PaCO2 were significantly more important (p<0.05) in the group treated with BUP+FLU than in those treated with solvent+BUP or FLU+solvent. Moreover, in these 2 latter groups, effects were limited to the first 5 minutes for BUP and the first 60 minutes for FLU. Infusion of flumazenil (5 mg/kg at 10 min), a benzodiazepine competitive antagonist, gave a significant reduction in the effects of combined FLU+BUP on rat ventilation. Conclusion: Our data show that association of high doses of BUP (30 mg/kg) and pharmacological doses of FLU (2.5 mg/kg) may cause important perturbations on rat arterial blood gases. Respiratory depression, resulting from additive or synergic action of BUP and FLU, seems partially reversed by flumazenil. This study may confirm the deleterious role of benzodiazepines, and especially flunitrazepam, in the appearance of BUP respiratory toxicity and fatalities. Reference: 1. Tracqui A. Buprenorphine-Related Deaths Among Drug Addicts in France: A Report on 20 Fatalities. J. Anal. Toxicol. 1998, 22, 430–434.
99 EFFECTS OF SINGLE INTRAVENOUS DOSES OF NORBUPRENORPHINE ON ARTERIAL BLOOD GASES IN RATS
Mégarbane B, Gueye PN, Risède P, Monier C, Borron SW, Baud F
INSERM U26 and Réanimation Médicale et Toxicologique, Hôpital Lariboisière, Paris, France
Objectives: High dose buprenorphine is used in France as substitution treatment in heroin addiction. To date, many deaths have been reported in addicts using buprenorphine, frequently in case of misuse or association with benzodiazepines. However, experimental data demonstrated the safety of single doses of buprenorphine administered alone in naive rats. Therefore, we tested in rats, the hypothesis of the role of single intravenous doses of norbuprenorphine, the N-dealkyl metabolite of buprenorphine, in producing respiratory depression and fatalities. Methods: Male Sprague–Dawley rats were administered norbuprenorphine intravenously to determine the median lethal dose (LD50), using the up-and-down method. Subsequently, catheterized 3 groups of 10 restrained rats received either the aqueous solvent required to dissolve norbuprenorphine, or 3 or 9 mg/kg of norbuprenorphine intravenously. Serial arterial blood gases were obtained over 3 h. Baseline values were compared using one-way analysis of variance followed by multiple comparison tests using Bonferroni's correction. In each group, the effect of time was studied using repeated measures ANOVA and Dunnett's multiple comparison tests. Results: The LD50 of norbuprenorphine, determined in triplicate, was 10 mg/kg (median of 3 series: 10, 12 and 7 mg/kg). The mean dose received by surviving animals was 11±2 mg/kg. The mean delay of death was 8±4 hours (0.7–48 hours). Deep coma and apnea were the apparent cause of death. Norbuprenorphine produces a rapid, profound and prolonged respiratory depression as demonstrated by the significant increase in PaCO2 (p<0.01) and decrease in arterial pH (p<0.01), at 5, 20, and 60 min after infusion of 3 and 9 mg/kg norbuprenorphine, when compared to the baseline values. A significant delayed hypoxia (p<0.01) was also present. The arterial pH and PaCO2 in the 3 mg/kg and 9 mg/kg norbuprenorphine groups were both significantly lower than in the aqueous solvent at 5 min (p<0.01 and p<0.001 respectively), 20 min (p<0.01) and 60 min (p<0.05 and p<0.001 respectively). The lowest pH values in the aqueous solvent, 3 mg/kg, and 9 mg/kg norbuprenorphine groups were 7.41±0.01 at 90 min, 7.25±0.06 at 20 min, and 7.14±0.07 at 90 min. The greatest PaCO2 values in the aqueous solvent, 3 mg/kg, and 9 mg/kg norbuprenorphine groups were 5.70±0.10 kPa at 90 min, 8.38±0.89 kPa at 20 min, and 9.95±0.96 kPa at 90 min. Conclusion: Our data show that norbuprenorphine alone demonstrate important deleterious effects on arterial blood gases in adult rats. Consequently, norbuprenorphine may play a key role in the respiratory depression associated to buprenorphine overdoses in human fatal cases. However, the mechanism of death remains to be determined.
100 EFFECTS OF SINGLE INTRAVENOUS HIGH DOSES OF BUPRENORPHINE AND MIDAZOLAM ON ARTERIAL BLOOD GASES IN RATS
Gueye PN, Mégarbane B, Borron SW, Monier C, Risède P, Baud F
INSERM U26, Hôpital Fernand Widal and Réanimation Médicale et Toxicologique, Hôpital Lariboisière, Paris, France
Objectives: High dose buprenorphine is used as substitution treatment in human heroin addiction. Deaths have been reported in addicts using buprenorphine, frequently in association with benzodiazepines. The exact mechanism of this association is still not established to date. We observed the effects of buprenorphine and midazolam alone, and in combination on arterial blood gases. Methods: Four groups of 10 catheterized restrained male Sprague-Dawley rats received a parenteral injection of aqueous solvent (water+ethanol+hydrochloric acid), buprenorphine (30 mg/kg i.v.), midazolam (160 mg/kg i.p.), or buprenorphine (30 mg/kg i.v.) plus midazolam (160 mg/kg i.p.). Serial blood gases were obtained over 3 hours (Radiometer ABL 300). Baseline values were compared using one-way analysis of variance followed by multiple comparison tests using Bonferroni's correction. In each group, the effect of time was studied using repeated measures ANOVA and Dunnett's multiple comparison tests. Results: There was a mild but significant effect of buprenorphine alone in comparison with the aqueous solvent on PaCO2 at 60 min (6.24 vs 5.65 kPa, p<0.01). There was also a mild but significant effect of midazolam alone in comparison with aqueous solvent on arterial pH at 90 min (7.33 vs 7.41, p<0.001) and PaCO2 at 60 min (6.52 vs 5.65 kPa, p<0.01). In comparison with either buprenorphine or midazolam alone, the combination of midazolam and buprenorphine produces a rapid, profound and prolonged respiratory depression as demonstrated by the increase in PaCO2 and a decrease in arterial pH with appearance of delayed hypoxia. Conclusion: These data show that high doses of midazolam and buprenorphine alone have limited effects on arterial blood gases in rats while midazolam and buprenorphine appear to act in an additive or synergistic fashion to depress ventilation in rats. However, the exact mechanism of buprenorphine and benzodiazepines interactions remains to be determined.
References: 1. Gueye, P.N. Lack of Effect of Single High Doses of Buprenorphine on Arterial Blood Gases in the Rats. Toxicol. Sci. 2001, 62, 148–154.
101 POISONINGS DUE TO INGESTION OF LITHIUM MEDICATIONS: EXPERIENCE OF THE MARSEILLES POISON CENTRE
De Haro L, Roelandt J, Prost N, Hayek-Lanthois M, Arditti J, Valli M
Poison Centre of Marseilles, Hôpital Salvator, Marseilles, France
Objective: Lithium is used for control of manic-depressive psychosis. This medicine is considered as one of the most dangerous pharmaceuticals responsible for severe poisonings and several deaths. The authors present 306 cases of lithium intoxication observed in the Marseilles Poison Centre between January 1991 and December 2000. Case series: The 306 cases observed during the studied period were categorized into 6 groups. For the first 3, the symptoms were mild: 1)accidental ingestion in children (13 cases, average age 2.3 years; average ingested quantity: 2 pills; the only sign was drowsiness; all patients recovered); 2)mistakes in the dose or the number of ingested tablets (43 cases; average age 55 years; average ingested quantity 3.7 pills; average lithium blood level 1.6 mmol/L; all patients recovered); 3)elevation of lithium blood level due to diuretic therapy (8 cases, average age 65 years; all cases due to a new prescription of diuretic; average lithium blood level 1.45 mmol/L; all patients recovered after stopping one of the 2 treatments). For the next two circumstances, the clinical signs were more severe 4)treated patients who developed renal failure (15 cases; average 59 years; average lithium blood level 2.1 mmol/L; 6 patients managed in intensive care unit (ICU) with 1 death). 5) dehydration in psychiatric patients (35 cases; average age 58 years; average lithium blood level 2 mmol/L 8 patients treated in ICU and 1 death). Finally, the most severe cases were due to suicide attempts. 6) 190 cases; 67% women; average age 43 years; average ingested quantities 25 tablets; co-ingestion of other psychotropics by 76%. Most frequent signs: drowsiness 76%, muscle rigidity 21%, tremor 16%, coma 15%. Treatments: saline enhanced diuresis 93%, gastric emptying 49%, emesis 8%, hemodialysis 5%. The average lithium blood level was 2.35 mmol/L, and 56% of the patients were managed in ICU. 19 patients had cardiac or neurological complications, 4 of these died. Conclusion: The severity of lithium poisoning depends on the circumstances. The ingestion of large quantities of sustained released tablets is the most dangerous situation. Inadvertant ingestion, even by children, is generally less severe.
102 LITHIUM POISONING OR LITHIUM-INDUCED HYPEROSMOLAR ENCEPHALOPATHY?
Botti P, Dannaoui B, Pistelli A
Toxicology Unit and Poison Control Center. Azienda Ospedaliera Careggi, Firenze, Italia
Objectives: Lithium is an effective drug, widely used for treating bipolar affective disorders. It has a narrow therapeutic index with therapeutic levels between 0.6 and 1.2 mEq/L. Lithium toxicity is possible during chronic treatments with the drug. This can include an interstitial nephritis and/or a nephrogenic diabetes insipidus: these conditions are related to lithium concentration. Other conditions such as a diuretic therapy and dehydration may result in lithium intoxication. Intentional acute intoxication can also be observed, as well as chronic toxicity and sub-acute therapeutic overdose during chronic lithium therapy. The aim of our presentation is to retrospectively evaluate lithium intoxication in patients admitted to the Toxicology Unit, Firenze. Methods: 21 patients were admitted to our Unit between 1992 and 2001 with lithium intoxication. The age, sex, daily lithium dose and length of lithium therapy of each patient were recorded on admission. Signs and symptoms of possible renal involvement (hypernatremia, BUN, creatinine plasma increase, history of polyuria and polydipsia), ECG abnormalities and length of stay in hospital were also investigated. Results: our experience relates only to patients with chronic overdose and patients receiving chronic lithium therapy who acutely overdosed. Their symptoms were almost exclusively neurological. ECG and EEG showed only non-specific anomalies (T wave abnormalities, diffuse slowing respectively). On admission 7 of the 21 showed signs and symptoms of nephropathy: they were all receiving long-term lithium therapy and were referred due to dehydration or changes in mental state. This group of patients developed a more severe clinical state, which we believe to be due to hyperosmolar encephalopathy rather than to a direct toxicity of lithium on the CNS. They required a longer period for recovery (14.7 days instead of 4.8). The severity of the clinical status did not seem to be closely correlated to the level of lithium plasma. Only one patient who acutely overdosed (Li 3.93 mEq/L) was hemodialized while other patients were rehydrated and received normal saline infusions. All patients recovered. Conclusions: Our experience shows that the careful control of the level of lithium plasma, together with the careful monitoring of the renal function and of the hydro-electrolytic balance is advisable in patients on chronic therapy.
103 MASSIVE ETHYLENE GLYCOL INGESTION TREATED WITHOUT HEMODIALYSIS
Velez LI, Lynton R, Shepherd G, Keyes DC, Benitez FL
Division of Emergency Medicine, University of Texas Southwestern Medical Center and the North Texas Poison Center, Dallas, Texas, USA
Objective: In 1996, fomepizole (4-MP) was approved by the FDA for the treatment of Ethylene glycol (EG) poisoning. The current recommendation is to give early antidotal treatment with alcohol dehydrogenase blockers and to perform hemodialysis for patients with EG levels>50 mg/dL, or severe acidosis, or renal failure. We present a case of a man who ingested EG and ethanol. He presented early, without signs of acidosis or renal failure, and was treated conservatively with only 4-MP. Case Report: The patient is a 33 y/o man with a history of depression. He was brought in by EMS one hour after the ingestion of 1/2 a gallon of antifreeze and ethanol. On arrival, he was awake, and without complaints or apparent distress. His vital signs were: BP 132/87, HR 95, RR 18, Temp 36.3°C, and pulse oximetry 96%. He denied any other ingestion and refused to answer questions. The rest of the physical examination was unremarkable. Initial laboratories revealed Na of 140 mEq/L, K 3.7 mEq/L, Cl 103 mEq/L, CO2 27 mEq/L, BUN 8 mg/dL, creatinine 0.9 mg/dL. Concurrent ABGs were: pH 7.36, pCO2 51 mmHg, pO2 84 mHg (room air). His measured osmolarity was 446 mOsm (osmolar gap 157), his ethanol level was 84 mg/dL, and his initial EG level was 706 mg/dL. Antidotal treatment with 4-MP was started, and continued for a total of 8 doses. After 72 hours of therapy the serum EG level had decreased to 6 mg/dL. Serial laboratories showed normal anion gap and renal function throughout therapy. After treatment, the patient was transferred to a psychiatric facility for additional in-patient care. Conclusions: This case demonstrates a good outcome in a patient who presented early after an overdose with EG and was only treated with 4-MP. Further studies are needed before current recommendations on the use of hemodialysis for EG poisoning can be changed.
104 DOES LOXAPINE HAVE A DIRECT NEPHROTOXIC EFFECT? A CASE REPORT
Delabranche X, Le Gourrier L, Faye A, Zaghdoudi I, Lutun P, Schneider F, Kintz P, Jaeger A
Réanimation Médicale, CHU, Hôpital de Hautepierre, Strasbourg, France
Objectives: Renal failure has been reported in loxapine and amoxapine overdose. Metabolism of loxapine includes demethylation to its primary metabolite, amoxapine. Occurence of renal failure is mostly associated with hypotension and/or severe rhabdomyolysis. We report a patient who developed acute anuric renal failure without hypotension and before the occurrence of seizure and severe rhabdomyolysis. Case report: A 35 year-old man without previous disease was admitted 10 hours (H10) after the ingestion of 2 to 3 g loxapine. On admission, except an anuria, the clinical examination was normal: GCS=15, pulse rate=71/min, blood pressure=138/75 mmHg, respiratory rate=15/min, temperature=36.8°C, SpO2=98%. ECG was normal. Biological analyses showed: sodium=140 mmol/L, potassium=4.0 mmol/L, creatinine=185 μmol/L, urea=7.0 mmol/L, CPK=501 IU/L. The patient remained anuric despite fluid and furosemide administration. At H18, he developed a generalized seizure treated by clonazepam 2 mg. EEG (H19) showed a slow activity but without paroxysms. The CTscan without contrast agent injection was normal. The acute renal failure (maximum levels: creatinine=663 μmol/L, urea=17.5 mmol/L) needed two 4-hours hemodialyses at H50 and H74. CPK levels remained below 700 IU/L until H64 but, afterwards, increased up to 12600 IU/L at H88. Further evolution showed a progressive improvement of the renal failure (with recovery of diuresis at H130) and a decrease of the CPK to 47 IU/L at H180. The patient was discharged from the ICU on day 8 and from the hospital on day 12 without sequelae. Drug analyses on admission were negative for BZD, barbiturates, TcADs and alcohol. Loxapine plasma concentration was 374 μg/L at H10 and decreased slowly to 86 μg/L at H89 with a calculated plasma half-life of 41 hours. During the first hemodialysis, the hemodialysis clearance was 15.8 ml/min and only 501 μg loxapine were removed. Conclusion: This patient developed after loxapine poisoning an acute anuric renal failure which was present on admission and not associated with hypotension, dehydration and severe rhabdomyolysis. Subsequently, he developed a seizure and a severe rhabdomyolysis. The chronology of the renal failure and of the rhabdomyolysis in this case suggest that loxapine may exert, independently of hypotension, dehydration or rhabdomyolysis, a direct nephotoxic effect whose mechanism remains still unclear.
105 POISONS ADMISSIONS IN EDINBURGH: A DESCRIPTION OF INPATIENT READMISSION PATTERNS OVER 20 YEARS
Oliver JJ, Strachan FE, Laing WJ, Bateman DN
National Poisons Information Service, Edinburgh Centre, Edinburgh, United Kingdom
Objective: Admissions with acute poisoning form a significant component of acute medical hospital cases. Of these, a significant proportion are readmissions. The aim of this project was to retrospectively describe the relationship of gender, deprivation, age and drug taken at first admission, to readmission rate over 20 years, from 1981 to 2000. Methods: A database containing information on all discharges from, and deaths in, the Royal Infirmary of Edinburgh with a diagnosis of poisoning or toxicity in any of 6 diagnostic positions was provided by the Information and Statistics Division of the National Health Service in Scotland. Diagnoses were coded according to ICD 9 codes until March 1996, whilst ICD 10 codes were used thereafter. Data were “linked”, so that records relating to individual patients could be identified. Results: The data extract contained 47,155 admissions relating to 24,933 individual patients. Only those admissions where the patient did not die during their first presentation were used in the analysis of readmission rates (44,020 admissions relating to 24,876 patients (11,027 male, 13,849 female)). Over 20 years 16,173 patients (65%) were admitted on a single occasion, 4968 (20%) on 2 occasions, 1713 (6.9%) on 3 occasions and 702 (2.8%) on 4 occasions. 1320 patients (3%) were admitted on 5 or more occasions. One patient was admitted 181 times. Men (37.7%) were more likely than women (33.3%) to be readmitted at least once. As age at first presentation increased there was a progressive rise in the proportion of patients readmitted at least once (see Fig. 1). The proportions of patients readmitted at least once according to deprivation category (1—most affluent to 8—most deprived) were: 1–35%, 2–29.8%, 3–33.1%, 4–34.5%, 5–36.5%, 6–41.1%, 7–40.1%, 8–28.8%. According to drug taken at first admission the proportion of patients subsequently readmitted at least once were, in descending order: benzodiazepines 43.3%, opiates 41.3%, antipsychotics 37.5%, antidepressants 34%, paracetamol 28.3%, salicylates 28.1%. Conclusion: Linked admission data are valuable in determining the influences on readmission rates of toxicology patients. The results may help in planning future strategies to reduce readmissions.
106 POISONINGS IN NURSING HOME RESIDENCES: WHAT KIND OF AGENTS ARE THE ELDERLY EXPOSED TO AND WHICH PREVENTIVE MEASUREMENTS SHOULD BE TAKEN TO AVOID ACCIDENTS?
Tobback C, Daeleman W
Poison Control Centre, Brussels, Belgium
Objective: In Belgium the Royal decree, June 1999, outlined the job descriptions of the coordinating and advising physician working in nursing home residencies. In addition to their many responsibilities, they are to make sure that the quality of the medical care is guaranteed. Prevention of poisoning is part of these job responsibilities. The aim of this study is 1) Evaluate poisonings in nursing home residences by identifying the agents involved and rank them according to frequency of occurrence, 2) Formulate preventive measurements from these data. Methods: All calls involving poisonings in nursing home residences, registered by the Poison Control Centre between 1997 and 2000, were retrospectively reviewed. Results: The Poison Control Centre received a total of 324 calls concerning poisonings in nursing home residences of which 261 (80.5%) were accidental. The route of exposure was oral in 373 (95.5%). Medications were the main cause in 51% followed by cosmetics 21%, household products 14% and plants 8%. Disinfectants were the highest at 42% compared to other categories (max. 5% per class). Symptoms were present in 25% at the time of the call. Conclusion: Nursing home residents are frequently exposed to medicines, in particular to disinfectants and cosmetics. Most of the poisoning cases were accidental oral exposures. Access to disinfectants, placed on a nursing trolley, should be made more difficult by removing the trolley immediately after use. The choice of less toxic products compared to quaternary ammonium disinfectants should by promoted. To avoid mistakes cosmetics should be placed in the bathroom cupboard, rather than being placed on the dressing table or on the bedside table next to a glass of water or another soft drink.
107 ADOLESCENT EXPOSURES: CHARACTERIZING CALLS TO A LOCAL REGIONAL POISON CONTROL CENTER INVOLVING 10–21 YEAR OLDS
McFee RB, Caraccio TR, Mofenson HC
Long Island Regional Poison Control Center, Winthrop University Hospital, Mineola, New York, USA
Introduction: The health of adolescents has declined over the last thirty years. Unintentional injury, motor vehicle crashes, poisoning, suicide and homicide are the leading causes of death and disability. Despite the fact these events often are associated with medications and illicit substances, the pattern of such activities as represented from a poison control perspective have not been described. Methods: To review and characterize calls placed to a local poison control center by persons 10–21 a retrospective review of all reports to a certified regional poison information center for 1999 was conducted and compared to 1999 American Association of Poison Control Center (AAPCC) data. Data analysis included demographics, intent of call, as well as exposure intent, acuity, severity, and outcome. Results: n=2541. There were 6 information and 2535 (99%) exposure calls. Of 2225 (88%) acute exposures, a significant proportion were suicide attempts 731 (33%) and intentional misuse 401 (14%). Analgesics, anxiolytics, alcohol, psychotropics and illicit substances were most commonly misused or used for suicide attempts. This was consistent with AAPCC data. Most outcomes were minor; hospitalizations and moderate toxicity were associated with co-ingestions involving cocaine, alcohol and amphetamines or analgesics. Suicide attempts, alcohol, and ecstasy use were associated with younger adolescents (ave. 15 yrs). Unintentional or therapeutic errors were often associated with psychotropic medications, including methylphenidate, risperidone, and guanfacine. Conclusion: Alcohol remains an important drug of abuse, and risk factor for adolescent injury. Adolescents commonly misuse medications prescribed for them. Therefore increasing clinical support may be needed. More research to identify and treat adolescents at risk for misusing medications is necessary.
108 POISONING UNDER ONE YEAR OF AGE. A MULTICENTRE STUDY, ITALY 2001
Marchi AG, Renier S, Knezevich M
(IRCCS Burlo Garofolo, Trieste, Italy) and Italian Paediatric Emergency Working Group
Background. Poisoning in children under one year has received little attention due to its low frequency. Multicentre studies can improve information on this topic. Aim. To describe clinical and epidemiological features of toxic exposures in children under one year presenting to hospital emergency departments (EDs). Methods. On behalf of the Istituto Superiore di Sanità, from January 2001, Trieste Centre is co-ordinating a national prospective study of child poisoning. The Paediatric EDs of 14 hospitals are currently involved in this project. Information stored in the project database includes age, sex, location of accident, manner of exposure, route of poisoning, substances involved, time lapse between exposure and ED presentation, symptoms, treatment, decision making at ED and outcome. Severity is assessed according to MSPC score (J. Toxicol. Clin. Toxicol. 1995, 33, 223–231). This presentation deals with poisoning in children under one year registered during the first nine months of 2001. Some data was also compared with that from a multicentre study in 1991–94. Results. From January to September 2001 844 0–14 year subjects were referred to EDs. There were 74 (8.8%) children under one year, 9 at 0–2 months, 9 at 3–5 months, 17 at 6–8 months and 39 at 9–11 months, of whom 53% were male. Exposure occurred exclusively at home from parental error or environmental accident (31 cases), or child self-exposure (43). Tobacco, analgesic drugs, cosmetics, home detergents, carbon monoxide and gastrointestinal drugs accounted for 61% of these. Exposure by ingestion occurred in 64 cases and by inhalation in 10. Early ED referral was frequent (32 cases<1/2 hour, 16<1 hour, 11<3 hour). 21 children with non-toxic exposures were sent home without treatment. The other 53 were admitted to hospital for therapy which may have prevented symptoms in 35 of them. Symptoms were mild in 9 children and moderate to severe in 9. Amongst the latter, cyanosis, apnoea, respiratory distress, central nervous system disturbances and prolonged vomiting requiring intravenous perfusion were observed. Outcome was good, no deaths occurred. In 2001 as compared to 1991–94 there was an increase in both poisoning in children under one year and in exposure to pharmaceutical products. A better therapeutic approach was noted and less children were treated. Gastrointestinal decontamination was carried out mainly with activated charcoal. Conclusions. The increase in poisoning in children under one year is deserving of concern. Poisoning severity was low to moderate in most of the cases as the exposures were accidental. Nevertheless, half the children required treatment and three out of four of these were admitted. This study shows that information on poisons prevention for parents needs to be improved to prevent exposure of children under one year to toxic substances in the home.
109 CHILDHOOD POISONING IN ZIMBABWE
Tagwireyi D, Ball DE, 1Nhachi CFB
Drug and Toxicology Information Service, and 1Department of Clinical Pharmacology, University of Zimbabwe, Harare, Zimbabwe
Objective: Data relating to poisoning in most developing countries is often patchy,1 with most published information on poisoning being from developed countries. This is particularly true for poisoning in children who are normally the victims of accidental exposure. In view of this, we describe the toxicoepidemiology of poisoning in children admitted to eight major referral hospitals in Zimbabwe. Methods: A retrospective review of all cases of poisoning admitted to eight major referral hospitals in Zimbabwe was conducted for the period January 1998–December 1999 using methods already described.2 All cases of poisoning occurring in children under 12 years were selected and analysed. Results: There were a total of 761 cases of paediatric poisoning admitted to the study hospitals. The median age was 2 years (range 7 days–11 years). Over half (56.5%) of the cases were male. The main groups of agents responsible for poisoning admissions are shown in Table 1. Paraffin (38.6%) and rodenticides (14%) accounted for most admissions. The mortality rate for natural toxins was significantly greater than that of pesticides and household chemicals (Table 1). All deaths in the natural toxins group resulted from traditional medicines, with a class mortality rate of 16 deaths per 100 admissions (95% CI: 8.0%–27.7%). Conclusion: The fact that household chemicals, especially paraffin accounted for the highest number of hospital admissions in this study points to a need for toxicovigilance and public education programmes aimed at raising awareness of the proper storage of these chemicals. The high mortality rate associated with traditional medicines could result from their inherent toxicity or a worsening of the initial diseases. Further investigation will help identify whether interventions in this area could reduce poisoning mortality.
References: 1. Eddleston, M. Patterns and Problems of Deliberate Self-Poisoning in the Developing World. Q. J. Med. 2000, 93, 715–731. 2. Tagwireyi, D.; Ball, D.E.; Nhachi, C.F.B. Poisoning in Zimbabwe: A Survey of Eight Referral Hospitals. J. Appl. Toxicol. 2001, 21, in press.
Table 1. Poisoning Admissions and Deaths per Toxic Group (Abstract 109)
110 METHOD AND UTILIZATION OF TEST ON TRENDS IN CHILDHOOD INTOXICATIONS
Sydow A, Wagner R, Desel H
Giftinformationszentrum-Nord (GIZ-Nord Poison Control Center), Bereich Humanmedizin, Georg August-Universität Göttingen, Göttingen, Germany
Objective: More than half of all cases that have been reported to the GIZ-Nord poison control center in Göttingen during the past five years were pediatric cases. In a study on these data leading causes of intoxication were examined. In order to identify time trends and problem areas weighted analysis of data was applied to nested groups of substances and products to develop recommendations for prevention of childhood poisoning. The focus was placed on pediatric intoxications with drugs. Methods: Of 100,319 human exposures to toxic substances reported to the GIZ-Nord poison control center 54,440 pediatric cases were selected for analysis. Trend-tests according to COCHRAN/MANTEL/HAENSZEL were used in order to evaluate the nil-hypothesis that a trend of cases with symptomatic intoxication with a given substance or group of products is identical with the trend of all exposures in the respective age group within a 5-years period (1996–2000). These tests were applied to 236 drugs and groups of drugs that were related to symptomatic childhood poisoning. The WHO ATC classification was used for drug grouping. Results: Trends differed by age groups. For small children (0 to 9 years of age) no significant increase in intoxications with single drugs or drug groups was detected within the 5-years period. For this age group significant decreases in the frequency of intoxications were found for the groups of spasmolotic/anticholinergic drugs, drugs with effects on the alimentary tract and metabolism, for clenbuterol, cough and cold medications, the group of drugs with effects on the respiratory tract, and for the entire group of drugs (p<=0.02). For the adolescent age group (10–18 years of age) a significant increase in the frequency of intoxication was shown for the group of psychoanaleptic drugs. Significant decreases were shown for codeine (all ATC classes combined), cough and cold medication and the subgroup of drugs with effects on the respiratory tract, the subgroup of psycholeptic drugs, and the entire group of drugs. Conclusion: Drugs are important causes of intoxication in children and adolescents. Poison control centers are not only committed to counsel in cases of intoxication but have to analyze and report data to the public and to decision makers in order to raise awareness for sources of intoxication and develop prevention strategies. Trend-tests and weighted analysis of data (using structured grouping of substances) help in shedding light on important and dangerous substances or products. The alarming increase in the frequency of intoxications with psychoanaleptic drugs in adolescents must lead to further studies and reflections.
111 A MULTICENTER STUDY TO CHARACTERIZE GUANFACINE EXPOSURES CALLED INTO POISON CONTROL CENTERS
McFee RB, Caraccio TR, McGuigan MA
Long Island Regional Poison Control Center, Winthrop University Hospital, Mineola, New York, USA
Background: National prescribing data indicate an increase in prescribing of psychotropic medications to adolescents and children, yet it is estimated that almost 80% of these medications are inadequately studied for safety or effectiveness. Non-psychiatric drugs such as guanfacine are also increasingly being prescribed to children for off-label psychiatric indications, including ADHD. To our knowledge this is the first multicenter study to characterize the circumstances of guanfacine exposures. Objective: To characterize the toxic effects, exposure intent, and outcomes associated with guanfacine ingestions, especially as pertain to children <6 and <12 years. Methods: A retrospective case series including calls placed to nine AAPCC certified regional poison control centers involving guanfacine as a single or multiple drug event between January 1999–August 2001. Endpoints of interest included circumstances, intent, adverse reactions, extent of toxicity, management and outcome. Results: n=156, Ages 7 months–54 years, 33% female, 67% male. 63 were <=6 YR, 112<12 YR. Thirty-seven percent involved co-ingestions, primarily methylphenidate, risperidone, clonidine and depakote; >70% involving <=6 YR were single ingestions. Calls by intent: unintentional 33%, therapeutic errors 49%. Intentional ingestions 18% associated with older patients. Greater than 50% of exposures <=6 YR involved medications belonging to the patient. Forty-eight were referred to a HCF and 11 were hospitalized 1–3 days (1.6-day average). Of note, several young children were prescribed multiple psychotropic medications; the youngest were 4 YR. Toxic symptoms: 45% no toxicity-average dose 1.6 mg (0.25 25 mg), 23% mild-average dose 2.67 mg (1–8 mg), 20% moderate toxicity-average dose of 3.0 mg (.5–40 mg), <1% had severe symptoms at 10 mg. There were 4 potentially toxic (1 mg) and 8 unknown (1 mg) cases. Greater than 25% of multi-drug ingestions were associated with self-harm. Conclusion: There has been a significant increase (36%) in the number of guanfacine calls from 1999 to 2000; 2001 exposure trend continues. According to the International Psychopharmacology Algorithm Project, efficacy and safety data about guanfacine is considered category C-based upon clinical opinion, case reports, or open uncontrolled trials. Despite this lack of clear scientific evidence, guanfacine continues to be widely prescribed to young children and adolescents. In our study, a significant percent of children experienced therapeutic errors with their own medication. Of concern, polypharmacy is being practiced on young children. Although all outcomes were benign, and no deaths occurred, a significant percentage of children were referred to HCF with signs of toxicity. The important public health problem of psychiatric medication use in children remains understudied.
112 INFORMATION SOURCES REFERRAL IN A POISON CENTER: A PROSPECTIVE STUDY
Locatelli C, Butera R, Bernareggi G, Bove A, Agazzi A, Fasola D, Carli M, Manzo L
Poison Control Center, IRCCS Maugeri Foundation and University of Pavia, Italy
Background: Information is essential for poison center (PC) activities. However, the role of information sources (e.g. databases) has been over-emphasized. Objective: To evaluate information sources (IS) referral for clinical management of poisoning cases in a PC. Methods: A prospective study was conducted during a 2-months period in a PC mainly operating for health services. The study was performed at daytime (from 8.30 a.m. to 8.30 p.m.), in randomly identified time periods representative of the whole day. Calls related to expert advice requests (EARs) for new cases of poisoning were included. Animal poisoning cases, follow-up calls and non-emergency related inquiries were excluded. Data analysis included (i) IS referral, (ii) type of information searched for, (iii) clinical problem complexity and (iv) answer quality. Information searched in IS was divided into technical (e.g. commercial products composition, toxicokinetics data) and clinical (e.g. toxic effects, diagnostic tests, treatment). Clinical problem complexity was assessed using a previously validated score system (range 8–20). Answer quality was evaluated by a referee. Data were registered by an independent observer. The way of working of PC physicians experienced more or less than 5 years was compared. Student t-test and χ2 analysis were used for statistical analysis. Results: A total of 205 EARs were studied. Sixty-three percent of EARs (129/205) were answered on the sole basis of personal professional experience. In 37% (76/205) of cases IS referral was considered, mainly to get technical information (technical information 43%, 33/76; clinical information 26%, 20/76; both 30%, 23/76); in 8% (6/76) of cases IS were lacking; in these cases the PC physician was able to provide information on specific diagnosis and therapy, and the referee commended the advice provided. Quality evaluation did not show any error, mistake or incompleteness in answers given without IS referral. Complexity level of EARs answered by PC physicians experienced more (n=122) or less (n=83) than 5 years was comparable (12.4±1.8 vs. 12.4±1.9, t-test p=0.95). More experienced physicians referred to IS in 24% (29/122) of cases, compared to 57% (47/83) for less experienced ones (test χ2 p<0.01). The type of information searched was mainly technical for more experienced physicians and both technical and clinical for less experienced physicians (clinical information/technical information ratio 0.58 and 0.90 respectively). Conclusion: The study shows that toxicologic advice is not merely based on IS referral. This act is progressively reduced as the result of growing professional experience. When attempting to organize the treatment of a poisoned patient there is no substitute for the knowledge base and experience obtained from years of handling poisoning cases.
113 ACCIDENTAL STAGGERED PARACETAMOL OVERDOSES IN THE UNITED KINGDOM: EPIDEMIOLOGY AND OUTCOME
Dargan PI, Jones AL
National Poisons Information Service (London), Guy's and St Thomas' NHS Trust, London, United Kingdom
Objective: Accidental overdoses with paracetamol do occur and are often assumed to be rare events but there is little data about them in the UK, where paracetamol overdoses represent 48% of all poisoning episodes. We report a case series of 19 accidental paracetamol overdoses collected by a prospective survey of all calls about paracetamol poisoning made to one NPIS centre. Methods: We conducted a prospective survey of all calls regarding paracetamol poisoning to NPIS (L) over a fourteen-week period (during May–August 2000) relating to paracetamol poisoning; a follow up call was made the next day to obtain data on patient outcome. Results: 280 calls were received concerning paracetamol poisoning over the 14-week period and follow up data was available for 100% of these. 19 (6.8%) were accidental and 261 (93.2%) were intentional. The accidental group had a median age of 51 years (range 35–65) and a male: female ratio of 3:1; compared to median age 28 years (range 0–70 years) and male: female ratio 1: 1.9 in the non-accidental group). The mean dose of paracetamol ingested was 17.7 g (260 mg/kg); range 8–32 g (112–464 mg/kg). All of the accidental overdoses were staggered and the mean time from first ingestion to presentation was 32 hours (range 14–78 hours) and the mean time from the last dose of paracetamol to presentation was 6.5 hours (range 1–26 hours). Concomitant ingestants were ibuprofen in 2 cases, codeine in one, diclofenac in one and MST in another case. 3 patients were in “high risk” groups (2 had chronic excessive alcohol consumption, 1 was on regular anticonvulsant medication). 17 patients received treatment with N-acetylcysteine. 5 patients developed hepatotoxicity—of these 4 had biochemical (ALT>1000 iu/L) and INR evidence of hepatotoxicity at presentation. 2 patients had a rise in INR during the first 24 hours after presentation (only one of these being in an “at risk” group). All ALT and INR abnormalities resolved with 24–48 hours of presentation and no patients went on to develop acute liver failure. Conclusion: Telephone follow up calls are an effective method of data collection in such cases. The commonest group taking deliberate overdoses in the UK are young women, but in contrast in this series, the group accidentally ingesting overdoses tended to be older men. None of the 19 accidental paracetamol overdoses developed serious sequelae, though 17 required treatment with N-acetylcysteine.
114 BUPROPION SR IN OVERDOSE: SUBSIDIZED POISONING
Falkland M,1 McMorrow J,2 McKeown R,1 Cooper GM,1 Buckley NA1
The Canberra Hospital, Canberra, Australian Capital Territory, Australia1, Royal Perth Hospital, Perth, Western Australia2
Background: Bupropion (Zyban SR™), also known as amfebutamone, was listed on the Pharmaceutical Benefits Scheme (PBS) in Australia in Feb. 2001 as a 150 mg, 120 tablet pack for use within a comprehensive treatment program, as an aid in smoking cessation. Bupropion was not previously marketed in Australia as an antidepressant, as was the case in the U.S. and Europe. Extensive media exposure combined with low cost as a result of the PBS listing resulted in a very high uptake in the community. 239,195 PBS prescriptions for bupropion were dispensed from Feb–May 2001. This implies exposure of approximately 1.2% of Australia's population to bupropion in just four months. Case Series: Many patients with overdoses of bupropion have been admitted to hospitals around the country. We describe a series of patients whose symptoms included nausea, vomiting, confusion, hallucinations, coma and seizures. In one case, a seizure did not occur until 19 hours after ingestion, most likely due to the slow release nature of the product, and the presence of active metabolites with even longer half-lives. Warnings on the use of this product have been issued by The Australian Adverse Drug Reactions Advisory Committee after receiving large numbers of reports, particularly of neurological and hypersensitivity reactions. To the end of November 2001 the committee had received 1237 reports in connection with the use of Zyban SR. These have included reports of seizures (83) and angioedema (46). Conclusion: Experience in Australia shows that consumer driven prescribing may expose patients to significant risk. Measures to encourage more balanced media reporting of medicines are needed. Appropriate patient selection and follow up counselling is important as the whole seven week course is supplied at the start of therapy. Consideration should be given to supply in smaller quantities. The slow release nature of Zyban SR™ raises questions about management of overdose, and in particular, the length of observation time.
115 CASES STUDIES OF AN INTERNET POISONS INFORMATION SYSTEM FOR PHARMACO- AND TOXICOVIGILANCE—ANTIPSYCHOTICS AND GAMMA HYDROXYBUTYRATE
Good AM, Strachan FE, Oliver JJ, Kelly CA, Bateman DN
National Poisons Information Service, Edinburgh Centre, Royal Infirmary of Edinburgh, Scotland, United Kingdom
Objectives: To examine the potential of an Internet poisons information system for pharmaco- and toxicovigilance. Background: TOXBASE provides on-line information on poisoning to registered medical professionals in the UK and Ireland. Among the system's uses are (A) in pharmacovigilance—to investigate the effects of licence changes on database access; (B) in toxicovigilance—on-line case reporting. Methods: (A) Hits on the website were analysed for accesses to antipsychotic agents for the eight months before and after the UK Medicines Control Agency's restriction on the use of thioridazine in 12/2000, following reports of rare but serious cardiotoxicity1. (B) Case report feedback forms were analysed for gammahydroxybutyrate (GHB) by age, sex, amount ingested, co-ingestants, features and management. Results: (A) The mean number of accesses for antipsychotics per month was 666 (range 596–749) for April–July 2000 and 1012 (range 866–1080) for May to August 2001. Expressed as a percentage of antipsychotic accesses for April–July 2000 thioridazine had a mean of 37.9% (range 34.9–41.8%). Accesses declined following the licence change to a mean of 7.6% (range 5.8–8.4%) of antipsychotic use for May–August 2000. In contrast accesses for chlorpromazine increased from a mean of 16.5% (range 15.5–19.0%) to 28.5% (range 27.5–30.1%). Novel antipsychotic accesses were either increasing before the license change (olanzapine, risperidone) or were unaffected (clozapine, quetiapine, sulpiride). In contrast access to other older antipsychotics increased similarly to chlorpromazine. (B) Between January and March 2001 we requested information on GHB. 24 reports were received (6.9% of TOXBASE GHB accesses). The age range of those who took GHB was 15–39 (average 22.8 years; 7 female, 17 male). Doses were seldom known. Five had possibly also taken ecstasy and 16 alcohol. Features (frequency>1) were coma GCS3 (8), respiratory depression/arrest (8), drowsiness (6), bradycardia (5), hypotension (5), dilated pupils (3), hypothermia (3), coma (unknown GCS) (2), agitation (2), confusion (2). No fatal cases were reported. Management was generally supportive with oxygen, ventilatory support and fluids. Two patients were reported to have been managed in ITU. In 9 cases naloxone was given, where quoted, doses were low (400–800 mcg), with improvement in GCS in 1 case and no improvement in 3, no further information offered in the remainder. Conclusions: TOXBASE can provide information on epidemiology and on clinical features of poisoning. Changes in the license for thioridazine has lead to an increase in enquiries for predominantly older antipsychotics.
References: 1. MCA/CSM. Curr. Probl. 2001, 27.
116 HYDROXYCHLOROQUINE POISONING: A PROSPECTIVE SERIES OF 6 CASES
Isbister GK,1 Dawson AH,1,2 Whyte IM1,2
1Discipline of Clinical Pharmacology, University of Newcastle, Australia; 2Department of Clinical Toxicology, Newcastle Mater Hospital, Newcastle, Australia
Objective: Hydroxychloroquine is rare, but important poisoning, due to its significant toxicity. There are only case reports of poisonings in the literature and so little information on the spectrum of toxicity. We describe a prospective consecutive case series of hydroxychloroquine poisoning. Methods: Consecutive cases of hydroxychloroquine poisoning to a regional toxicology service were identified by searching a database of all presentations between January 1987 and June 2001. Data included: age, sex, indication for hydroxychloroquine, dose ingested, co-ingestants, clinical effects, adverse outcomes and clinical severity. Results: There were 7245 deliberate self-poisoning that presented to the Hunter Area Toxicology Service (HATS) in the study period. Of these there were only 6 cases of hydroxychloroquine poisoning in the 13 year period. There was one death (14 g) and one severe life-threatening poisoning (4 g), the latter requiring significant treatment in intensive care for 6 days. In both cases QRS>140 msec and systolic BP<90 mmHg, and the patients were both comatose on presentation (<2 hrs). The patient who died presented in cardiac arrest with a QRS>200 msec and initially responded to bicarbonate, diazepam and inotropes, but died from re-occurrence of a wide complex arrhythmia and persistent hypotension 36 hours later. There were 3 cases with mild effects, and in all of these the patients had a normal GCS, blood pressure and QRS width on presentation (4–5 hrs). Conclusion: This series confirms how rare hydroxychloroquine poisoning is and that it causes severe and life-threatening poisoning. 4 g can cause severe toxicity, and the onset of clinical effects is rapid and within 2 hours in this series. Although a small series, it suggests that the absence of coma, hypotension and a widened QRS, 4 hours after the overdose, is consistent with minimal complications.
117 PARAPHENYLENE-DIAMINE POISONING. A DESCRIPTIVE STUDY OF 20 CASES
Aliane F, Iddir W, Choukri AB, Abtroun R, Alamir B, Reggabi M
Centre Anti-Poisons—Laboratoire de Toxicologie, Centre Hospitalo-Universitaire de Bab-El-Oued, Alger, Algeria
Objectives: In North Africa, women use, as a traditional hair dye substance, “Hadira Souda” which is derived from a nontoxic plant. Since this is now becoming very rare, it is substituted by a chemical product; the highly toxic paraphenylene-diamine (PPD). The aim of the study was to evaluate the clinical profile and the outcome of systemic PPD poisoning. Methods: PPD poisonings collected by the poison centre over a 9-year period were analyzed. Results: 20 cases of poisoning by various amounts of PPD were found. The age ranged from 18 months to 57 years (mean: 20.4) and sex ratio (m/f) was 0.42. Poisoning occurred after ingestion in 95% and after topical use in 5%. Circumstances were intentional (75%), accidental (20%) and criminal (5%). The respiratory failure due to an upper airway obstruction was the most prominent clinical feature in 90% of the cases. Other symptoms included myoglobinuria with black urine (50%), myalgia (20%), vomiting (10%) and macroglossia (10%). 65% died between one half hour and 20 days of ingestion despite supportive therapy. Postmortem examination showed nonspecific multi-organ hemorrhages. In a severely poisoned woman 7 months pregnant, a caesarian section was performed but the fetus was dead with a grayish tegument appearance. Discussion: The respiratory failure related to a contact reaction (angioneurotic edema with an acute upper airway obstruction) which appears even after ingestion of very small amounts of PPD. The other symptoms, especially rhabdomyolysis complicated by acute renal failure, are related to a systemic effect of PPD. The mechanism of PPD systemic toxicity remains uncertain. Conclusion: In this series PPD ingestion resulted in severe poisoning. No antidote therapy is available, and supportive care failed to prevent a high mortality rate.
118 VALPROATE POISONING: A 10 YEAR SINGLE CENTRE CONSECUTIVE SERIES
Isbister GK,1 Balit C,2 Whyte IM,1 Dawson AH1
1Discipline of Clinical Pharmacology, University of Newcastle, Newcastle. 2New South Wales Poisons Information Centre, Sydney. 3Department of Clinical Toxicology, Newcastle Mater Hospital, New South Wales, Australia
Objective: Valproate is often regarded as having significant toxicity based on case reports of massive overdoses. The study aimed to describe the changing epidemiology of valproate poisoning and its severity in a consecutive single centre series. Methods: Consecutive cases of valproate poisoning to a single toxicology treatment centre were identified by searching a database of all presentations between January 1991 and February 2001. Data included age, sex, indication for valproate, dose ingested, co-ingestants, clinical effects, adverse outcomes and clinical severity. Results: There were 92 presentations of valproate overdoses in 10 years, 17 cases of valproate alone. There was an increase in valproate poisoning over time: 1991–93: 0–1; 1994–98: 5–10; 1999: 13; 2000: 41. Valproate was the patient's own in 48 cases: 15 for epilepsy, 32 as a mood stabiliser, 1 as an analgesic adjunct. In the 17 valproate alone cases, 9 were asymptomatic, 7 had mild effects and 1 had moderate toxicity (hypotension). There were no deaths or major adverse effects except hypotension. Drowsiness, nausea and vomiting were the main toxic effects. In all 92 patients, there was 1 death (pulmonary embolus), 1 patient with acidosis, mild hepatotoxicity and thrombocytopenia, and 1 patient with mild hepatotoxicity. In all others, moderate to severe effects were consistent with the co-ingestant. Conclusion: This study demonstrates a significant increase in valproate poisoning over the last 5 years. It confirms that valproate causes mild toxicity in the majority of cases. It doesn't confirm the impression of serious toxicity created by numerous case reports of massive overdoses.
119 JUST HOW TOXIC IS CITALOPRAM?
Laing WJ, Good AM, Gordon LD, Bateman DN
National Poisons Information Service, Edinburgh Centre, Royal Infirmary of Edinburgh, Scotland, United Kingdom
Introduction: Amongst the SSRIs, citalopram may have a more toxic profile in overdose1. We have reviewed 5 years (1997–2001) of telephone enquiries to a poisons information service to compare the relative toxicity of citalopram with the other SSRIs available in the UK (fluoxetine, fluvoxamine, paroxetine, and sertraline) using the features at time of the initial call to the unit. Venlafaxine was excluded because of its different action (it is an SNRI). Only one of the seven patients who ingested fluvoxamine had confirmed toxicity; this patient had also ingested another drug (diphenhydramine). Method: We retrospectively analysed our call database from 1997–2001 for calls involving these drugs. Using the scoring assigned to the call, based on apparent features or predicted toxicity, we compared the toxicity (at time of call) between the various drugs. Where only an SSRI had been ingested, we have assumed that all features are attributable to the SSRI; in other cases we compared clinical features with those expected for each drug ingested, and ascribed a likely toxic agent. Discussion: Toxicity associated with the ingestion of SSRIs alone appears to be of similar magnitude for all four drugs where we have a significant number of cases (Table 1). Coma was reported with sertraline, paroxetine and citalopram ingested alone. In all cases where other drugs have also been taken, serious symptoms seem to be more readily attributable to the co-ingested drug than to the SSRI. Conclusion: These results suggest no difference in the toxicity of citalopram as compared to other SSRI drugs available in Scotland, as reported by telephone enquirers. Reference: 1. Personne, M.; Sjöberg, G.; Persson, H. Citalopram Overdose—Review of Cases Treated in Swedish Hospitals. Clin. Toxicol. 1997, 35, 237–240.
Table 1. Comparison of Toxicity of 5 SSRIs (Abstract 119)
120 A PROSPECTIVE STUDY ON INTOXICATIONS WITH ANTIPSYCHOTICS
Zoelen GA van,1 Vries I de,1,2 Meulenbelt J1,2
1National Poisons Control Centre, National Institute for Public Health and the Environment, Bilthoven; 2Department of Intensive Care & Clinical Toxicology, University Medical Centre Utrecht, Utrecht, The Netherlands
Introduction: Antipsychotic overdoses are considered potentially life threatening, but the toxicological data of most members of this pharmacologically and structurally diverse group of drugs (phenothiazines, thioxanthenes, diphenylbutylamines, butyrophenones, benzamides and atypical antipsychotics) are limited. Moreover, about the effect of co-ingested drugs on the toxicity of antipsychotics little is known. To gain more information on the frequency and severity of clinical effects, a prospective study was conducted from August 1, 1996, until August 1, 1997. The objective of the study was to examine the effects of acute overdose after intoxications with antipsychotics alone or in combination with other drugs. Methods: All cases of possible overdose with antipsychotics alone or in combination with other substances in which the Poisons Control Centre was consulted in the study period were taken into consideration. The consulting physicians were asked to complete a standard enquiry form. Questions concerned the patient (age, relevant medical history), the circumstances of exposure (estimated dose, products involved, coingestion of substances), symptoms, additional investigations (laboratory analysis), and treatment. Results: 756 enquiry forms were sent, 328 of which were returned. Of the returned enquiries only the fully completed ones were studied (72%; N=235). In 67% (N=158) of the cases other products were co-ingested besides antipsychotics. Clinical effects were reported in 70% of the mono-intoxications (N=77) and in 88% of the mixed-intoxications (N=158). In the mono-intoxications drowsiness (N=32), lethargy (N=12), tachycardia (N=11) and nausea (N=10) were the most frequently reported symptoms. In the mixed-intoxications (N=158), benzodiazepines (34%), antidepressants (23%), alcohol (8%), antihistaminics (7%), parasympatholytics (6%), and other products (22%) were co-ingested. In these cases the most observed symptoms were drowsiness (N=79), sopor (N=23), confusion (N=20), tachycardia (N=31), bradycardia (N=11), hypotension (N=15), hypertension (N=13) and nausea (N=15). Cardiovascular symptoms were reported in 19 mono-intoxications, mostly after ingestion of atypical antipsychotics (37%) or phenothiazines (21%). In the mixed-intoxications, cardiovascular symptoms were reported in 52 cases, most frequently after ingestion of phenothiazines (40%), thioxanthenes (25%) and butyrophenones (23%). In one case, after ingestion of pipamperone (2 mg/l), and probably fluoxetine, torsades des pointes was reported. In 40% of the mixed-intoxications, and in 22% of the mono-intoxications, patients received intensive care observation. Conclusions: In our case-series severe cardiovascular symptoms were rarely observed, consequently at present no firm statement can be made regarding the cardiotoxicity caused by antipsychotics in overdoses in combination with other ingested drugs.
Table 1. Agents Involved in QTc and QRS Changes (Abstract 120)
121 SUICIDE WITH MIRTAZAPINE—HARDLY POSSIBLE
Schaper A, Färber E, Ebbecke M, Desel H
GIZ-Nord (Poison Control Centre for the Federal States of Bremen, Hamburg, Lower Saxony and Schleswig-Holstein), University of Göttingen, Göttingen, Germany
Objective: Mirtazapine is a new tetracyclic antidepressant drug with effects on central presynaptic alpha-2-receptors and several 5-HT receptor subtypes. Compared to tricyclic antidepressant substances like amitriptyline, mirtazapine is regarded less dangerous concerning cardiotoxicity and its potential to provoke central nervous effects like seizures. It was introduced to the German market in 1996 and its use has increased ever since. Our data and experience with mirtazapine overdose were analyzed in order to achieve a therapy algorithm for the treatment of mono-intoxications caused by mirtazapine. Methods: In a retrospective study we analyzed all mono-intoxications of mirtazapine, in that GIZ-Nord poison centre was consulted. The analysis included the following parameters: 1. numerical development of mirtazapine intoxications from 1996 until 2001; 2. severity of these intoxications with allocation to the levels “severe”, “moderate”, “minor” and “no symptoms” (according to Poison Severity Score=PSS); 3. amount of drug intake and its relationship to PSS level. Results: Altogether our poison centre was involved in 73 cases with mirtazapine overdose. With the exception of 6 cases in all other patients the modus was suicidal. The development over the past years was as follows: 4 cases in 1996, 8 cases in 1997, 11 cases in 1998, 9 cases in 1999, 15 cases in 2000 and 26 cases in 2001 (until october). Concerning severity we stated the following levels. No symptoms: 31 cases (max. 900 mg), minor: 32 cases (max. 2250 mg), moderate: 3 cases (max. 900 mg) and severe: 1 case (600 mg). In brackets is added the maximum dosage of mirtazapine that caused the respective level of severity. We had no fatalities in mirtazapine mono-intoxication. In 6 of our cases a PSS classification was not possible due to incomplete documentation. In one patient with a severe intoxication cardiac arrythmia was detected. Seizures were not observed and even following an intake of 2250 mg only a minor intoxication resulted. The symptomatology of the patients included dizziness, sleepiness and gastrointestinal discomfort. Conclusion: Our data show, that indeed mirtazapine mono-intoxication seems to be less dangerous compared to poisonings with classic tricyclic antidepressant substances, where fatalities are well documented and published. We did not define a single fatality and found only minor symptoms of intoxication. Thus we draw the final conclusion, that mirtazapine mono-intoxications can be compared to those caused by benzodiazepines. In our opinion gastrointestinal detoxification is inappropriate in these patients and ECG monitoring is rather optional.
122 THE FATAL TOXICITY OF ANXIOLYTIC AND SEDATIVE DRUGS IN THE UNITED KINGDOM (1983–99)
Buckley NA,1 McManus PR2
1Dept of Clinical Pharmacology & Toxicology, The Canberra Hospital, Australian Capital Territory, Australia, 2Drug Utilisation Sub-Committee, Department of Health & Aged Care, Australian Capital Territory, Australia
Objective: Sedative and anxiolytic drugs are among the most common drugs leading to fatal poisoning and large variations between these drugs have been noted. Two previous studies have examined the fatal toxicity of some of these drugs in the UK. (King & Moffat, 1983; Serfaty & Masterton, 1993). Neither examined toxicity due to a number of non-benzodiazepine non-barbiturate drugs or longitudinal changes in fatal toxicity indices. The aim of this paper is to establish the frequency with which anxiolytic and sedative drugs resulted in fatal poisoning, and secondly, to examine for pharmacological/toxicological correlations with fatal toxicity, thirdly to look for longitudinal changes in the FTI. Methods: We obtained data on the number of fatal poisonings between 1983 and 1999 in England, Wales and Scotland due to a single antidepressant drug from the Departments of Health in the UK. This was divided by the number of prescriptions in England and Scotland for these drugs over this time to derive a FTI of deaths per million prescriptions. We examined correlations between the FTI and the LD50 in animals, potency (benzodiazepines only) and lipid solubility. Results: Chloral hydrate, chlormethiazole, barbiturates, and related sedatives had much higher FTIs than benzodiazepines, buspirone, zopiclone and zolpidem. Using Poisson regression, there was no positive relationship between the FTI of anxiolytic and sedative drugs and their lipid solubility. There was a weak but significant correlation between the FTI and the LD50 in rodents. Conclusion: The continuing availability of some sedative/anxiolytic drugs with high FTIs should be reconsidered as a public health measure. Longitudinal changes in sedative drug poisoning support the effectiveness of this approach. The ratio of the therapeutic dose and the dose that cause acute lethal toxicity in animals is the best potential pre-clinical indicator of subsequent toxicity in overdose.
References: King, L.A.; Moffat, A.C. Med. Sci. Law 1983, 23, 193–198. Serfaty, M.; Masterton, G. Br. J. Psych. 1993, 163, 386–393.
123 CORONERS' INQUESTS INTO DRUG-RELATED DEATHS IN NEW ZEALAND: IDENTIFYING COMMON THREADS
Smith NA
Department of Pharmacology and Toxicology, University of Otago, Dunedin, New Zealand
Objectives: To describe the circumstances surrounding drug-related overdoses in New Zealand and identify important details that should be collected routinely from a prevention perspective. Methods: The New Zealand coroners' inquests into drug-related deaths undertaken between January/February 1999–January/February 2000 were reviewed. Results: Of the 128 deaths (73 male, 55 female; mean age 37 years, range 3–80 years), most involved more than one agent and in 40% of cases these did not belong to the victim: opiates (n=50), non-opioid analgesics (n=16), benzodiazepines (n=41), antidepressants (n=37), antipsychotics (n=15), cardiovascular agents (n=9), solvents and pesticides (n=13), alcohol/methanol as the main agent (n=22; additionally, alcohol was co-ingested in 47% of the remaining overdoses), cannabis (n=10) and miscellaneous (n=27). In only 8 cases did the records state that the victim's medications had been supplied under “close control” to prevent overdose. Almost 60% of the overdoses were intentional; the majority of unintentional overdoses involved illicit drug use. In one-third of the cases, one or more of the following featured: active or history of substance abuse, depression and previous suicide attempts. Psychosis under treatment and an emotional disturbance precipitated the overdose in 15% of cases. Most victims were socially isolated; 69% unemployed and 77% not in an active relationship. How the medical and medication history may have affected the outcome of the overdose remained unknown in 60% of cases as these details were not recorded. For toxicovigilance purposes Coronial guidelines on the data to be collected need to be developed.
124 ACUTE POISONINGS WITH PESTICIDES IN POLAND
Czerczak S, Rogaczewska A
National Poison Information Centre, Nofer Institute of Occupational Medicine, Lodz, Poland
Objective: Pesticides constitute a large group of commonly used chemicals with diverse chemical structure and toxicity. Pesticide poisonings may be due to suicidal attempts or accidental ingestion associated with improper storage in unlabelled containers. Methods: 1990, 1994–1995 and 1997–1998 supplied by Poland's regional toxicological centres. Results: Table 1 shows cases of acute poisoning with pesticides recorded by the individual toxicological centres during the periods: 1989–1990, 1994–1995 and 1997–1998. The data show that the number of pesticide-poisoned patients treated in the centres in 1997–1998 was over 50% lower than in 1989–1990. In the pesticide group, the most of poisonings were due to organophosphorous compounds (39.2% in 1989–1990; 45.1% in 1994–1995 and 28.1% in 1997–1998). The highest mortality rate was associated with dypyridyl derivative poisonings. In 1994–1995 66% of pesticide poisoned patients treated at the toxicological centres there were suicidal attempts, 31.5%—there were accidental poisonings, 2.5% were classified as “other”. In 1997–1998 self-poisonings with pesticides were lower—54%, accidental poisonings—34%, and “other”—18% (mainly occupational poisonings and poisonings due chemical accidents). Conclusion: The data show that the number of patients treated for pesticide poisoning at Poland's toxicological centres has been reduced. Self-poisonings were the most frequent category of pesticide poisonings, but accidental poisoning accounted for about 1/3 pesticide poisonings reported in 1994–1995 and 1997–1998. Therefore, more effective methods of prevention should be implemented, and pesticides should be stored in the original packages in order to reduce the risk of their accidental intake.
Table 1. (Abstract 124)
125 PESTICIDE POISONING: AN EPIDEMIOLOGICAL EVALUATION IN A POISON CONTROL CENTRE
Martínez-Arrieta R, Ramón MF, Ballesteros S, Cabrera J
Servicio de Información Toxicológica de Madrid, Spanish Poison Control Centre, Instituto Nacional de Toxicología, Spain
Background: Reduction of pesticide poisoning is a desired objective of Poison Control Centres. In order to do this, more epidemiological information is needed. Consults about poisoning with pesticides intended for agricultural use are a common source of work in our PCC. The purpose of this study is to describe the epidemiological factors of those exposures in order to propose adequate prevention measures. Methods: From January 1995 to December 2000 all exposures to agricultural chemicals collected in our Service were analyzed. The following data were included: age, gender, route of exposure, etiology, prognosis score and the level of health care intervention used. Results: A total of 4,847 consults involving agricultural chemical exposures were made during the study period. 77.6% came from health care professionals (general practitioners or hospitals) and the rest from the public (patients, family or friends of the victim). In the latter case, our Service recommended the patient be transferred to a hospital or to another health care facility (47.2%); the rest were managed at home. Mild or no symptoms were described in 52.5%; 21.2% were self-referral calls and 64.2% were managed at home. Moderate or severe poisonings represented 47.5% of the total number of calls; 84.6% came from health care professionals. Their aetiology was occupational (acute and chronic exposures) 46.6%, accidental 29%, intentional 20.8%, the rest were undetermined. 12.6% were moderate and severe cases were seen in children less than 14 years old, and 44.6% were less than 2 years. Pediatric pesticide poisoning was due to cholinesterase inhibitors 27.5%, rodenticides 26.1%, fungicides 8.4% and herbicides 5.6%. Adult poisonings were due to occupational exposure in 53.6%, intentional in 23.8% and accidental in 19.2%. The main substances implicated were cholinesterase inhibitors 31.1% and herbicides 17.5%. Conclusions: Although adults are the main population exposed to agricultural chemicals, children less than 2 years old were also affected with severe clinical prognosis in some occasions. Childhood poisoning could be reduced by proper educational campaigns among their families. Additional activities should be done in order to implement preventive measures in pesticide applications. PCCs play an important role in detection and proposal of prevention measures both to workers and the general population.
126 INCREASING FREQUENCY OF SERIOUS OR FATAL POISONINGS IN DOGS CAUSED BY ORGANIC FERTILIZERS DURING THE SUMMER OF 2001 IN GERMANY
Ebbecke M, Hünefeld D, Schaper A, Desel H
GIZ-Nord (Poison Control Center for the Federal States of Bremen, Hamburg, Lower Saxony and Schleswig-Holstein), University of Göttingen, Göttingen, Germany
Objectives: Organic fertilizers contain plant and animal compounds, mainly by-products from farming and slaughterhouses. In the years before 2001 these products have never played a major role in poisonings, monitored by our poison control center. Between March and July 2001 we have seen a series of seven fatal and eleven serious intoxications of dogs caused by the uptake of organic fertilizers. All the ingested products contained coarse meal from castor-oil plants (Ricinus communis), a by-product of the castor-oil industry. Ricinus communis contains ricin, a highly toxic protein that complexes rRNA and inhibits protein synthesis. Usually this coarse meal has to be heat-denatured to inactivate active ricin before it comes to the market. We suspected an incorrect heating process at the producing companies and informed the federal ministries of agriculture prior to analysis of the fertilizers. Case Series: During summer 2001 we observed an increasing frequency of dogs poisoned by castor-oil plant containing fertilizers. Seven dogs died, twelve were seriously intoxicated. 89% of them suffered from vomiting, half of these bloodily, 68% showed haemorrhagic diarrhea, 5% suffered from macroscopic haematuria and another 5% from cyanosis. The analysis of the fertilizer samples showed that they contained up to 10μg/g active ricin, which confirmed our assumptions. As a result, all castor-oil plant containing fertilizers were temporarily taken off the market. No more poisonings with these products have been observed after July 2001. Conclusion: Organic fertilizers are not per se safe products. Untreated coarse meal from castor-oil plants, which is a frequently used component of these fertilizers, can contain high concentrations of the extremely toxic protein ricin. Our cases and another case series published 20 years ago show that the heat-inactivation of ricin is not always ensured. Suspected intoxications should be reported to the responsible authority.
127 IRON AND COPPER VARIATION CONTENTS IN HUMAN LIVER SAMPLES: IMPLICATION IN CHARACTERIZATION OF HEMOCHROMATOSIS AND WILSON'S DISEASE
Soares ME,1 Miranda H,2 Bastos ML1
1CEQUP, Laboratório de Toxicologia, Faculdade de Farmácia do Porto; 2Serviço de Medicina 1, Hospital de Santo António, Porto, Portugal
Objectives: Quantification of iron and copper in the liver is very important for the diagnosis of hereditary hemochromatosis and Wilson's disease, respectively. Although, the hepatic copper content may present regional differences (1). The aim of this work was to evaluate the variation of both metal contents in 12 biopsies specimens. Methods: Biopsy specimens from explanted livers of patients submitted to liver transplantation were collected under decontaminated conditions, sectioned in several subfragments depending on the sample size (between 10 and 17 each), dried and submitted to acid digestion. Validation of the method and quantification of Cu and Fe was performed by Electrothermal Atomization Atomic Absorption Spectrometry as previously published (2). Results: Among the 12 analysed biopsies specimens 7 were positive and 5 negative both for Wilson's disease and for Hemochromatosis. For all the specimens analysed it was found oscillation in contents of Cu and Fe as it is summarized in Table 1. Conclusion: There is a variation of both metal contents in the subfragments of liver biopsies specimens. For contents near the threshold values, depending on the site of collection of the specimens, the results can be positive or negative as it was found for samples 9 and 12 for Cu and 8, 9 and 12 for Fe. Thus, caution in diagnosis must be taken especially for low positive values.
References: 1. Faa, G.; Diaz, G.; Farci, G.; et al. Variability of Copper Levels in Biopsy Tissue from a Cirrhotic Liver. J. Trace Elem. Electrolytes Health Dis. 1990, 4, 49–50. 2. Soares, M.E.; Areias, J.; Pedroto, I.; Pinho, C.; Bastos, M.L. Doseamento de Cobre e Ferro em Fragmentos de Biópsia Hepática Humanos por Espectrofotometria de Absorção Atómica com Atomização Electrotérmica. J. Português Gastrenterol. 1998, 5, 28–33.
Table 1. Contents (Mean Values) of Cu and Fe in Biopsy Liver Specimens and Respective Oscillation (Maximum and Minimum Values)
128 THE EFFECT OF HERBAL REMEDIES AND DIETING IN PARACETAMOL POISONING: TWO CASES
Strachan FE, Kelly CA, Oliver JJ, Bateman DN
National Poisons Information Service, Edinburgh Centre, Royal Infirmary of Edinburgh, Scotland, United Kingdom
Objective: The paracetamol treatment nomogram is widely used in the management of paracetamol poisoning and is a reliable predictor of the need to treat with the antidote N-acetylcysteine (NAC). A number of factors, including concomitant therapy with enzyme inducing drugs such as carbamazepine and St John's Wort; nutritional deficiency and chronic alcohol misuse, indicate a higher risk of hepatotoxicity. We report 2 cases of patients admitted to our unit who developed signs of hepatotoxicity despite paracetamol levels below the normal treatment line (4 h post ingestion). Case Report 1: a 28 year old female presented 3 h following a deliberate overdose with paracetamol (16 g). She gave no history of alcohol or drug misuse or concomitant medication, and was within normal weight range for her height. On admission her vital signs and LFTs were within normal range. Her paracetamol level was 178 mg/l (below normal treatment line), NAC was not administered. Fifteen hours later she developed nausea, vomiting and generalised abdominal pain: INR 1.6 and ALT 628 U/L. On repeat examination she revealed that she had recently been on a detox programme for heroin and benzodiazepine use, was on a low carbohydrate diet and was taking herbal remedies to detox and to reduce anxiety. Case Report 2: a 31 year old male presented 3 h following a deliberate overdose of paracetamol (35 g). He gave no history of drug or alcohol misuse or concomitant medication, and was obese. On admission: heart rate 102/min; respiration rate 22/min; ALT 90 iu/l; all other LFTs within normal range, salicylate not detected. His paracetamol level was 174 mg/l, therefore he was not treated with NAC. Twelve hours later he developed nausea, vomiting and severe abdominal pain: INR 1.4 and ALT 2045 U/L. On repeat examination he revealed that he was on a reducing diet and taking the herbal supplement Xenadrine to aid weight loss. Both patients were discharged 3 days later following extended treatment with NAC [standard regime plus 500 ml NAC (×2, case1; ×4, case2) 50 mg/kg over 8 hours]. Summary: We have presented 2 cases where patients developed significant liver toxicity following paracetamol overdose. Neither patient indicated evidence of high risk factors. However, both patients were taking herbal supplements and, despite being of average (case 1) and above average (case 2) weight, both patients may have been glutathione deficient due to poor nutritional state. Conclusion: The effect of herbal supplements and dietary change without apparent weight loss on the hepatotoxicity of paracetamol in overdose requires further assessment.
129 ETHYLENE DICHLORIDE POISONING BY INGESTION: ABOUT TWO CASES
Payen B,1 Bastin C,2 Nackers P,2 Laterre PF,1 Wittebole X,1 Haufroid V,3 Lefebvre C,4 Geubel A,4 Hantson Ph.1
1Department of Intensive Care Medicine, Cliniques St-Luc, Université catholique de Louvain, Brussels, Belgium. 2Department of Emergency and Intensive Care, Hopital St-Joseph, Gilly, Belgium. 3Laboratory of Toxicology, 4Department of Internal Medicine, Cliniques St-Luc, Université catholique de Louvain, Brussels, Belgium
Background: Intoxication by accidental ingestion of ethylene dichloride is rare and is usually severe.1 We report two recent cases with favorable outcome. Case Series: A 18-year-old man (68 kg, 178 cm) ingested for recreational purpose 15 ml of ethylene dichloride after ingestion of ethanol. Onset of symptoms was within 6 hours, including severe headache and vomiting, but the patient was admitted to the hospital only after 36 hours. Consciousness was preserved. Oliguria was noted initially and persisted. Liver function tests were slightly abnormal and steadily worsened. Hypoprothrobinemia occurred on the third day (INR 6.26). Other biological data were: ASAT 1603 IU/l (<43), ALAT 3014 IU/l (<36), bilirubin 171 μmol/l (<17.1), ammonia 23.5 μmol/l (<57.8), creatinine 326.2 μmol/l (<106). Electrocardiogram and echocardiography were normal. His 28-year-old friend (70 kg, 170 cm), who was also chronically abusing ethanol, ingested 50 ml of ethylene dichloride. Ten hours after ingestion, he complained of nausea, black vomiting and abdominal pain. He was admitted 36 hours after intoxication. He was perfectly conscious and presented with the following biological data: ASAT 37136 IU/l, ALAT 17083 IU/l, INR 4.43, bilirubin 75.2 μmol/l, ammonia 47 μmol/l, creatinine 219.2 μmol/l. Abnormal left ventricle contractility was noted at echocardiography. Both patients received supportive therapy and also intravenous N-acetylcysteine and alprostadil. The second patient experienced, after discharge from the Intensive Care Unit, two episodes of cardiogenic pulmonary edema. The outcome was favorable for the two patients, with a slow recovery of renal function. Hemodialysis was not required. Cardiac follow-up could not be obtained in the second patient. Discussion: Ethylene dichloride is used as a solvent for fats, glues… Death has been observed after ingestion of as little as 15 ml and is usually due to circulatory or respiratory collapse. Significant ingestion may result, as in the present series, in rapidly progressive hepatorenal failure. Hypoprothrobinemia may be observed as a result of midzonal liver necrosis. Encephalopathy was absent in our patients. There is no specific therapy; N-acetylcysteine has been suggested as a potential antidote, but its efficacy is not documented. As with other halogenated hydrocarbons, a complete recovery of hepatic and renal injury is possible. Reference: 1. Yodaiken, R.E.; Babcock J.R., Jr. 1,2-Dichloroethane Poisoning. Arch. Environ. Health 1973, 26, 281–284.
130 HEPATOTOXICITY AND EXOTIC PLANTS: A NEW CHALLENGE IN BOTH THE DEVELOPING AND THE DEVELOPED COUNTRIES
Résière D, Mégarbane B
Cornwall Regional Hospital, Montego Bay, Jamaica and Réanimation Médicale et Toxicologique, Hôpital Lariboisière, Paris, France
Objectives: Acute exotic plant poisonings are the cause of serious health problems in both developed and developing countries and are associated with several social and economic consequences. The American Association of Poison Control Centers reported 122,578 plant exposures in 1998. European data indicate an annual incidence of 0.2–3.0/100 inhabitants. 5–10% of all calls to poison control centers concern plant ingestions, with 83% involving children. Incidence of plant ingestion is increasing because of the development of travel and the revival of traditional medicine and phytotherapy. Exotic plant intoxication may effect multiple organ systems, including the nervous, cardiovascular, GI, hepatic, renal and hematologic systems. Liver is one of the main targets of toxicity. Methods: A systematic Medline review of published data concerning the risk factors, the pathophysiology and the clinical features for exotic plants which may induce hepatic injuries (hepatitis, jaundice, cholestasis, steatosis, portal hypertension, cirrhosis, hepatic thrombosis and failure). Results: Several syndromes of hepatic diseases may be attributed to exotic plant ingestion. Ackee fruit (Blighia sapida) intoxication is responsible for hypoglycemia due to blockage of hepatic gluconeogenesis by the hypoglycine A present in unripe fruits. Hepatic micro or macrovesicular steatosis was also reported as a consequence of mitochondrial damage. Pyrrolizidine alkaloids include many herbal products, as Heliotropium senecio (gordolobo), Crotalaria, Symphytum officinalis (Comfrey) that are used in Jamaica to make bush tea. They are metabolized to alkylating responsible for veno-occlusive disease (Budd-Chiari syndrome) and cirrhosis. Germander are present in some slimming agents in France and may cause centrilobular necrosis. Chaparral may produce periportal injury, cholangitis and cholestasis. In South Eastern Asia, kombucha tea (a mixture of yeast and bacteria), Jin Bu Huan (present in varying preparations) and Syo-Saiko (a mixture of 7 herbs) have been associated with hepatic injuries. These poisonings are generally unintentional with exotic fruit ingestion or herbal medicine use. However, the causality link with hepatotoxicity is sometimes difficult to establish. Factors facilitating hepatic toxicity are an inadequate selection of the ingested part of the plant, the inappropriate stocking conditions and the possible contamination of plants by various chemical agents, heavy metals and microorganisms. Conclusion: Acute exotic plant poisonings represent an increasing health problem. The real incidence of these intoxications is difficult to estimate not only in the developing countries but also in developed ones. Further coordinated studies and effective strategies to understand and prevent plant toxin-induced liver injury should be conducted.
131 ARE THE PACK SIZE RESTRICTIONS ON PARACETAMOL SALES ADHERED TO IN THE UNITED KINGDOM?
Norman E, Dhairiwan R, Dargan PI, Jones AL
National Poisons Information Service (London), Guy's and St Thomas' NHS Trust, London, United Kingdom
Objective: Paracetamol is the commonest drug taken in overdose in the UK accounting for almost half of all admissions with self-poisoning and an estimated 100 to 200 deaths per year. In September 1998 in an attempt to decrease the number and severity of paracetamol overdoses, legislation was introduced in the UK to limit sales of paracetamol to a maximum of 32 tablets of 500 mg in pharmacies and 16 tablets of 500 mg in supermarkets and other non-pharmacy outlets. The aim of this study was to investigate whether these pack size restrictions are being adhered to. Method: During 2000, the authors posed as a patient with knee pain and attempted to purchase in excess of the restricted amount of paracetamol in both pharmacies and non-pharmacy outlets in South London. Results: Table 1 summarises the number of paracetamol tablets purchased in the study. In all of the thirteen outlets we were able to purchase more than the restricted amount of paracetamol and on two occasions we purchased four times the amount allowed by the legislation. Conclusions: We had no difficulty in purchasing a hepatotoxic dose of paracetamol much in excess of the UK restricted amounts in both pharmacy and non-pharmacy outlets in South London. Any studies that are examining the impact of the pack size reductions on paracetamol overdoses should be interpreted in the light of the knowledge that there is limited compliance with the legislation.
Table 1. Number of Paracetamol Tablets Purchased at Different Outlets in South London (Abstract 131)
132 SOTALOL OVERDOSE: HOW DANGEROUS IS IT?
Hermanns-Clausen M,1 Desel H2
1Poisons Information Centre VIZ-Freiburg, Freiburg, 2Poisons Information Centre GIZ-Nord, Göttingen, Germany
Objectives: Sotalol is a non cardioselective β-blocking agent with additional class III antiarrhythmic properties. It is used for treatment of ventricular arrhythmia and of supraventricular tachycardia. Like other drugs that prolong repolarisation sotalol has arrhythmogenic properties. These effects are dose-dependent: the incidence of Torsade des Pointes tachycardia in adult patients treated with sotalol has been reported to 2.4%. In contrast to this well known side effects only few data are available about dose-dependent toxicity of sotalol overdoses. Case Series: We have analysed retrospectively all sufficiently documented sotalol overdoses reported to the poisons information centres GIZ-Nord (between 1/1996 and 10/2001) and VIZ-Freiburg (between 5/2000 and 10/2001). Results: CHILDREN: 1 baby, a toddler, daily dose. Only the toddler who had ingested the highest dose of sotalol (13 and a 7 yo girl ingested a therapeutic dose of sotalol, 2 toddlers had accidentally eaten more than 8 mg/kg, the maximum mg/kg) showed ECG-changes (without any hemodynamic complication). The other children were asymptomatic. A 12 yo girl who tried to commit suicide with 1.92 g sotalol developed severe bradycardia. Atropine and glucagon were successfully applied. ADULTS: 32 adult patients were included in this study after ingestion of 480 mg to 16,000 mg sotalol. Patients with severe intoxications typically developed Torsade de Pointes tachycardia combined with bradycardia and hypotension. Severe toxicity was observed after ingestion of 1.6 g sotalol or more. Occasionally, overdoses up to 4.8 g were tolerated without major hemodynamic complications. Circulatory failure was observed after ingestion of 2.4 g, death occurred after 4.8 g. Two other patients who died had ingested an unknown amount of sotalol. Minor bradycardia or QT-prolongation were seen after 640 mg. Conclusions: CHILDREN: 3 children in this case series who accidentally had taken a single sotalol dose less than 8 mg/kg (the maximum therapeutic daily dose) were asymptomatic-as expected. Only Children who have ingested sotalol overdoses should be monitored. Further case reports and case series are needed. ADULTS: In this study only adult patients with sotalol ingestions above 1 g developed severe hemodynamic complications. Because QT-prolongation have been reported in a patient taking a single doses of 640 mg sotalol every patient who has ingested more than 480 mg (maximum daily dose) should be monitored. Gastric lavage should only be considered after ingestion of a life-threatening dose of sotalol (>2.4 g). The removal of sotalol through hemodialysis is recommendable only when large doses have been taken or the patient is renally insufficient. Treatment of Torsade de Pointes tachycardia includes magnesium sulfate, in case of pronounced bradycardia and QT-prolongation treatment with atrial overdrive pacing is indicated. Hypotension was successfully treated with dopamine or norepinephrine with glucagon.
133 GLUCAGON TREATMENT OF COMBINED BETA-BLOCKER AND CALCIUM CHANNEL BLOCKER TOXICITY
Goldberg J, Wadhwa A, Shrestha M
Medical College of Pennsylvania and the Mercy Hospital of Philadelphia, Philadelphia, Pennsylvania, USA
Background: Beta-blockers and calcium channel blockers are among the most prescribed medications in the world. They have a narrow therapeutic index and are, by the nature of the ailments being treated, frequently given to elderly patients with cardiovascular and cerebrovascular disease. Glucagon has been reported to be beneficial in both beta-blocker and calcium channel blocker toxicity. We report the case of patient who presented with symptomatic poor cerebral perfusion taking these medications, who responded dramatically to bolus treatment with intravenous glucagon. Case Presentation: S.W., a 73 year-old gentleman with a history of diabetes mellitus, hypertension and coronary artery disease, presented to the emergency department (ED) with lethargy, confusion, near syncope, mild shortness of breath, and chest burning. He had been taking propranolol 60 mg BID, verapamil SR 240 mg QD, glyburide 10 mg QD, and lisinopril 10 mg QD. His blood pressure on arrival was 88/46 mm Hg, with a heart rate of 42 beats/minute. Respiratory rate was 22 breaths/minute; he was afebrile and had a pulse oximetry of 96% saturation on room air. Lung exam revealed only mild rales at the bases. Chest radiograph revealed moderate cardiomegaly with only mild volume overload. ECG revealed a junctional bradycardia at a rate of 38 beats/minute, a bifascicular block, and flipped T waves in III, F, and V3–5. Attempts were made to insert a temporary transvenous pacemaker without success. Glucagon 1 mg IV was then given every 5 minutes for 3 doses. Shortly after the 3rd dose the cardiac rhythm reverted to a normal sinus rhythm at 70 beats per minute. The blood pressure rose to 120/70, and he was markedly more alert, oriented, and less dizzy. The flipped T waves in the III and F resolved. He ruled out for a myocardial infarction. He remained alert with normal heart rate and blood pressure till discharge the next day. Discussion: Our patient with symptomatic propranolol and verapamil toxicity responded dramatically to a very simple treatment, averting the need for a transvenous pacemaker, which was actually attempted and unsuccessful. The relatively low dose of glucagon, with no subsequent intravenous infusion requirement despite its short half-life, may have been effective because this was not an “overdose” situation. The lack of the need for an intravenous infusion also dramatically decreased the cost of glucagon treatment. Conclusion: Non-overdose cardiac patients on beta-blockers and calcium channel blockers with symptomatic bradycardia and mild hypotension should be first given a trial of a few doses of intravenous glucagon before attempts are made at more invasive treatments.
134 QT PROLONGATION ASSOCIATED WITH LEVO-ALPHA ACETYL METHADOL
Traub SJ,1 Wani S,2 Hoffman RS,1 Nelson LS1
1New York City Poison Control Center, New York, New York, USA. 2Lincoln Hospital and Medical Center, Bronx, New York, USA
Objective: Levo-alpha acetyl methadol (LAAM) is a very long-acting opioid agonist used as an alternative to methadone maintenance therapy for opioid addiction because of its superior dosing profile. LAAM is rarely associated with arrhythmias due to QTc interval prolongation. We report a case of LAAM-associated syncope resulting from QTc prolongation. Case Report: A 45 year old female presented to the Emergency Department after suffering 5 episodes of syncope in one day. She had two similar episodes one month previously. She denied any nausea, sweating or chest pain with these episodes. There was no bowel or bladder incontinence. Her only medication was Orlaam® (Levo-alpha acetyl methadol); she took 135 mg every Monday and Wednesday and 175 mg on Friday for over a year. Vital signs were: pulse, 60/minute and regular; blood pressure, 170/100 mm Hg; respiratory rate, 12/minute; temperature, afebrile. There was no orthostasis. Her heart and lung examinations were normal. She had ecchymoses on the forehead and fresh needle track marks on her upper extremities. There were no neurologic deficits. The ECG demonstrated normal sinus rhythm with a corrected QT (QTc) interval of 740 msec. Serum electrolytes, including magnesium, were within normal limits. LAAM was discontinued, and she was observed in a monitored setting for three days with no further episodes of syncope or documented cardiac arrhythmias. At the time of discharge, her QTc at discharge had fallen to 650 msec. Conclusion: In this patient's case, LAAM appeared to be associated with QTc prolongation, which may have precipitated ventricular arrhythmias and syncope. Physicians should be aware that LAAM may prolong the QT interval, and that the drug is contraindicated in patients with known baseline QT prolongation.
135 PROLONGED HYPOTENSION IN ACUTE GUANFACINE OVERDOSE
Muller AA, Ishimine P, Henretig FM, Lin E
The Poison Control Center, Philadelphia, Pennsylvania, USA. University of California—San Francisco, San Francisco, California, USA
Objective: Guanfacine is an alpha-2 agonist used in the treatment of hypertension and attention deficit disorder. Hypotension associated with overdose of this drug typically lasts for 24–36 hours. We present a patient with prolonged hypotension despite low guanfacine levels. Case Report: An 18 year-old male with history of acute lymphoblastic leukemia, hydromorphone dependency, intermittent cocaine abuse and depression took six 1 mg Watson guanfacine tablets, assuming they were a controlled substance. His usual medications include paroxetine, methadone, clonazepam, TMP/SMZ, fluconazole, and gabapentin. He completed a course of cytarabine, etoposide, vincristine, and methotrexate 1 week prior to presentation. On day 2, he presented to an ER with weakness, lightheadedness, and urinary retention, but was discharged home after a period of observation. On day 3, he presented to our hospital's oncology clinic. PE revealed fatigue; initial VS: BP 63/32 mmHg, HR 63, RR 18, and T 36.2°C; ECG: sinus bradycardia; echocardiogram: normal. The patient's HR and BP remained low despite intravenous crystalloid and dopamine infusion at 5 mcg/kg/min. He remained bradycardic and hypotensive, dopamine was titrated up to 20 mcg/kg/min and was required for 7 days before successful weaning. Guanfacine levels drawn at 2, 5, and 8 days post-ingestion were as follows (ng/mL): 0.934, 0.142, and ND, respectively. These levels were sub-therapeutic despite the patient's serious medical course. Conclusion: This patient had prolonged hypotension despite low guanfacine levels, possibly reflecting synergy with prescribed medications.
136 SODIUM NITRITE INTOXICATION IN A NEWBORN
Pelclová D,1 Kredba V,2 Pokorná P,2 Srnský P,2 Vobruba V,2 Černá O,2 Sádlo M,2 Hoza J,2 Rakovcová H,1 Švarcová M,3 Špičáková D3
Department of 1Occupational Medicine with Poisons Information Centre and 2Department of Pediatrics and Adolescent Medicine of the Charles University and General Teaching Hospital, Prague, 3Department of Pediatrics and Newborns, Kladno, Czech Republic
Objective: Intoxication due to sodium nitrite occurs very rarely. Usually, these poisonings are life threatening. To our knowledge, intranatal poisoning of a baby in utero after intoxication of the mother has not been described. The baby survived, unfortunately the mother died. Case Report: A 22-year-old pregnant woman ingested sodium nitrite by mistake 30 minutes before a planned caesarean section. The dose was several times greater than the lethal dose. Because of the rapid onset of cardiopulmonary failure the caesarean section was performed urgently, and a male baby weighing 3650 g was delivered. His Apgar Score was 1, 3, 9 and he was cyanotic, but after 10 minutes was breathing spontaneously. After the methemoglobin level was found to be 80%, ascorbic acid 300 mg (Hoechst-Biotika) was immediately given i.v. and, after administration of the first dose of tolonium chloride (Toluidinblau, Köhler) 2 hours postpartum, he was transferred to a specialist department. Further doses of tolonium chloride were given, totalling 16.4 mg/kg. The methemoglobin level after tolonium chloride treatment dropped to 0.3%. On the 3rd day a severe haemolytic anaemia with Heinz bodies in erythrocytes developed, with increasing bilirubinaemia, reaching 945 μmol/l (total bilirubin), therefore an exchange transfusion was performed. Over the next few days sepsis developed, most probably enterogenous (Klebsiella pneumoniae was found in the blood). After complex treatment with antibiotics, immunoglobulins, blood derivatives, thrombocytes, ventilation and circulatory support and phototherapy, the condition of the new-born slowly improved. Signs of renal tubular damage (beta2 microglobulin, alfa1 microglobulin, albumin, hyaline casts in urine) also disappeared. After 4 weeks haematological and biochemical findings in blood and urine normalised, except for mild aminotransferase elevation. Neurological examination revealed no severe abnormalities, the EEG was evaluated as borderline to slightly abnormal with higher frequencies and rhythmicity. Auditory evoked potentials were normal. The patient was discharged on the 32nd day postpartum. Conclusion: We describe an unusual case of intranatal intoxication of a baby with sodium nitrite. This patient developed a very severe intoxication with 80% methemoglobinaemia, haemolysis, hyperbilirubinaemia, renal failure and sepsis. He recovered following antidotal treatment with tolonium chloride, exchange transfusion and very complex management. Acknowledgement: The case report was supported by MSM J13/98 111100002 and 111100005.
137 ACCIDENTAL NITROGLYCERIN POISONINGS IN CHILDREN
Taalikka P, Nyman T, Pohjalainen T, Hoppu K
Poison Information Centre, Helsinki, Finland
Objective: There is limited published information on overdoses of nitroglycerin in children when considering its availability for accidental poisonings. Due to the high first-past metabolism of nitroglycerin, an ingestion of a few sublingual tablets is usually considered to be harmless. On the other hand, it could be expected that many small children just retain the tablet in their mouth or chew it, and may thus be exposed to better bioavailability. We initiated a study in order to get a better picture about the possible toxic dose and symptoms. We focused on short acting sublingual tablets that are ingested, chewed or retained in mouth. Case Series: A prospective study of calls placed to the poison centre involving nitroglycerin exposures in children under the age of 6 was performed for the 12 month period of Feb 1999–Feb 2000. At the time of the first call, a standard data collection form was filled and the parents were asked to give permission for the study. A follow-up call and a semi-structured interview were made a few days after the incident. We received 35 inquiries about nitroglycerin in this age group and they were all included in the study. There were 21 (60%) males involved. Patients' age ranged from 1 to 4 years with a mean age of 2.5 years. The estimated amount of tablets involved was 3 tablets (each containing 0.5 mg of nitroglycerin) or less in all cases. The parents estimated that the children had ingested the tablets in 29 cases, chewed the tablets in 2 cases and retained them in mouth in 4 cases. Symptoms were reported in 8 children. However, four of these cases included symptoms that were not considered to be related to the nitroglycerin incident. For example, one boy vomited nine hours after the incident while drinking his evening milk. Details on the 4 cases with symptoms possibly related to nitroglycerin are presented in Table 1. Three of the children were observed at home, one admitted to a hospital for observation. The symptoms lasted for 1.5–3 hours. None of the patients had any symptoms that could be classified as moderate or severe. Conclusion: Even if the amount involved is often unclear, severe nitroglycerin poisonings in children are rare. In addition, due to the low bioavailability of nitroglycerin, the risk of getting symptoms is quite small.
Table 1. Symptomatic Patients (Abstract 137)
138 DELAYED CLINICAL EFFECTS IN MODIFIED-RELEASE AMITRIPTYLINE POISONING: A CASE REPORT WITH TOXICOKINETIC DATA
Greene SL,1 Dargan PI,1 Antoniades H,2 Wyncoll D,2 Jones AL
1National Poisons Information Service (London) and Intensive Care Unit, 2Guy's and St Thomas' NHS Trust, London, United Kingdom
Objective: There are no published reports of delayed clinical sequelae occurring in modified release tricylcic antidepressant poisoning. We report a case in which significant clinical effects were seen for up to 4-days after ingestion of modified-release amitriptyline. Case Report: A 40-year-old female with post-partum depression presented approximately 16-hours after ingestion of one hundred 50 mg modified-release amitriptyline (lentizol®), with thirteen 50 mg clomipramine and ten 20 mg fluoxetine tablets. On arrival in the emergency department she had a GCS 7/15, HR 105 bpm, BP 125/65 mmHg, twelve-lead ECG showed QRS prolongation of 119 ms. Soon after arrival she had a convulsion treated with 4 mg IV lorazepam and 50 mL IV 8.4% sodium bicarbonate. She then had runs of VT treated with 100 mL IV 8.4% sodium bicarbonate, and was intubated and ventilated and transferred to ICU. Over the following 6-hours she required a further 250 mL of IV 8.4% sodium bicarbonate to maintain a pH>7.45 and she had no further convulsions or arrhythmias. Her acid–base and QRS duration together with serum amitriptyline concentrations (HPLC) are shown in Table 1. She subsequently developed further features of tricylcic toxicity (metabolic acidosis requiring up to 100 mL/hour of 8.4% sodium bicarbonate and QRS prolongation as shown in Table 1) and so a decision was made to give activated charcoal at 18, 26 and 32-hours post admission. As shown in Table 1 her amitriptyline concentration continued to rise and peaked at 42-hours post-ingestion (26-hours post admission). She was extubated on day 4, discharged from ICU on day 5 and discharged from hospital on day 9 after review by the psychiatrists (her antidepressant was changed to venlafaxine). Conclusions: We report a case of severe poisoning secondary to ingestion of modified-release amitriptyline in which delayed absorption (concentrations peaking at 42-hours post-ingestion) resulted in a prolonged clinical course. Clinicians treating patients with modified-release tricyclic antidepressant poisoning should be aware of the potential for delayed and prolonged clinical effects and consider the use of gastric decontamination methods such as repeat-dose activated charcoal or whole bowel irrigation up to 36-hours post-ingestion.
Table 1. Toxicokinetic–Toxicodynamic Data in a Patient with Modified-Release Amitriptyline Poisoning (Abstract 138)
139 HYDROFLUORIC ACID INGESTION RESULTING IN RECURRENT VENTRICULAR FIBRILLATION: DOCUMENTED FLUORIDE LEVELS
Rivera W, Velez LI, Carrasco M, Sprueil R, Delaney KA
Division of Emergency Medicine, The University of Texas Southwestern Medical Center and The North Texas Poison Center, Dallas, Texas, USA
Objective: Hydrofluoric (HF) acid is one of the strongest inorganic acids and is used mainly for industrial purposes in glass etching, metal cleaning and electronics manufacturing. Some household products like rust removers, aluminum brighteners and heavy-duty cleansers also contain HF. Exposure usually is accidental and often is due to inadequate use of personal protective measures. HF acid is readily absorbed from the skin and GI tract and has been reported to cause gastric burns, inflammation, necrosis, hemorrhage and perforation. Most ingestions reported in the literature have resulted in death. Case Report: An 82-year-old woman was brought to the Emergency Department (ED). She had ingested approximately 8 oz of rust stain remover 6 hours before, and then called a neighbor. Prior to arrival the patient had abdominal pain and one episode of emesis. Upon arrival to the ED vital signs were: BP 129/82, P 108, RR 24 and T 95.8 F. On physical exam there were no burns in the oropharynx. The rest of the exam is unremarkable. While attempting to insert a urine catheter, the patient went into ventricular fibrillation (VF). Defibrillation was performed three times, followed by intubation and 10 cc of 10% CaCl2. The VF recurred multiple times, requiring a total of 20 shocks and a total of 50 cc of 10% CaCl2 and 3 grams of MgSO4. Propofol was initiated for sedation, and the patient was admitted to the ICU. Initial laboratories (drawn during and after resuscitation) were as follows: WBC 15×103/uL, Hgb 15 g/dL, Hct 44%, PLT 275×103/uL, Na 142 mmol/L, K 4.2 mmol/L, Cl 110 mmol/L, HCO3 19 mmol/L, BUN 20 mg/dL, Cr 0.9 mg/dL, Ca 11.4 mg/dL, Mg1.7 mEq/L. ABGs showed respiratory alkalosis. The aspirin, acetaminophen, ethanol, and urine toxicologic screen for common drugs of abuse were negative. The serum fluoride was reported later at 2 mg/L (normal less than 0.2 mg/l), and the urine fluoride was 72.46 mg/L (normal 0.2–3.2 mg/L). The Calcium level ranged from 8.1 up to 19.5 throughout the hospital stay. A pre-treatment calcium level was not drawn. The patient developed hemorrhagic gastritis on the second day in ICU, but was successfully extubated and discharged four days after admission. Conclusions: This case report illustrates a significant ingestion of HF that resulted in recurrent VF. Although there is not a pre-arrhythmia calcium level, we can speculate that the VF was probably precipitated by a long QT interval due to hypocalcemia. The arrhythmia was aggressively treated with calcium salts. We also document elevated serum and urine fluoride levels.
140 MANAGEMENT OF FLUORHYDRIC ACID POISONINGS
Humbert P,1 Raspiller MF,2 Roger F,1 Léonard C,1 Jaeger A,3 et les Centres Anti-poison Français.
1SAMU 972, CHU La Meynard, Fort de France, France; 2Centre Antipoison, CHU, Hôpital Central, Nancy, France; 3Réanimation Médicale, CHU, Hôpital de Hautepierre, Strasbourg, France
Objectives: In Martinique, the tropical climate contributes to a large use of anti-rusts for linen cleaning. Poisonings by anti-rusts are still frequent in this country: 8 voluntary poisonings with 2 deaths were reported in 2000. The aim of this study was to update the management protocol which had been used by the SAMU 972 since 1995. Methods: Review of the literature regarding fluorhydric acid (FH) and ammonium bifluorure (NH4F2) poisonings and inquiry to French Poison Centres (FPC) regarding their management protocols. Results: Since 1988, the French regulation imposes the substitution of HF by NH4F2 in fluoride-containing household products and since 1994 the maximal concentration of NH4F2 is limited to 10%. Review of the literature shows a wide range of fluoride plasma concentrations in lethal cases (factor of 1 to 20), an important mortality (50%) during the first hours related to severe dysrhythmias and differences in management protocols. The inquiry among the FPC also showed important differences in treatment. Several treatments are considered as useless or even dangerous: vomiting, ingestion of milk, gastric lavage if the HF concentration is higher than 10%, intravenous administration of calcium and/or magnesium salts without clinical or electrocardiographic disturbances. According to the literature and the inquiry of FPC inquiries, an updated protocol is proposed to the SAMU 972 for the management of HF-poisoned patients. The protocol includes: gastric suction by a nasogastric tube, neutralization by oral calcium gluconate or chloride, IV administration of calcium gluconate or chloride and/or magnesium sulfate for dysrhythmias, mechanical ventilation in case of coma and/or convulsions. In case of gastointestinal bleeding or peritoneal syndrome, a gastric fibroscopy is indicated. In severe poisonings, hemodialysis may be considered. Conclusion: The management of HF ingestions is not clearly established. According to the literature and the protocols used by FPC, an updated protocol is proposed. This protocol will be evaluated by the SAMU 972 in Martinique where poisonings by anti-rust products containing HF and NH4F2 are still common.
141 PULMONARY INJURIES AFTER ACUTE IRRITATIVE INHALATORY INTOXICATION
Naumovski J, Pilovski G, Bozinovska C, Kovkarova E, Petkovska L
Clinic of Toxicology and Urgent Internal Medicine, Skopje, Macedonia
Objectives: Household cleaners contain various concentrations of sodium peroxide, perborate or hypochlorite in water. When used these may produce chlorine or ammonia gas, which if inhaled may cause respiratory tract irritation. This study aimed to identify clinical criteria associated with a need for observation or hospital treatment. Methods: A prospective study of patients acutely poisoned with irritant fumes of household bleaches, detergents or other sanitizing agents. Data collected included: subjective complaints, objective clinical features present at admission, radiological and laboratory findings, the need of hospital or ambulance treatment and complications grouped by the type of substance being used. Results: Most patients admitted had used their own mixture of Hydrochloric acid and bleaches containing Sodium hypochlorite (N=45; 56.2%). The rest were exposed to commercial sanitizing agents (N=12;15%), Hydrochloric acid (N=7; 8.75%) and Sodium hypochlorite (N=16; 20%). 70 pts (87.5%) were accidentally exposed while cleaning at home in poorly ventilated spaces with no protection device, and only 10 (12.5%), were exposed while cleaning at their working place. Clinical features are given in Table 1.
Statistical analysis showed predominance of symptoms and need of hospitalization in the group of patients that used their own mixture (p=0.0043, Chi2=0.57). Conclusion: Acute inhalatory poisoning with irritant fumes is a frequent problem in our clinical practice. Mixing household cleaners of various types is a common practice among housewives unaware of the danger from inhalation of the fumes of their own cocktails, especially in poorly ventilated spaces. The inhaled gases caused respiratory tract irritation with cough, labored breathing and sometimes pulmonary edema. Headache, vertigo, cyanosis and hypotension are common and additional criteria for hospitalization. Symptomatic patients with such inhalational exposure require clinical observation for acute and late pulmonary toxicity.
Table 1. Notified Subjective Complaints and Physical Findings Grouped by Agent (Abstract 141)
142 LACK OF SIGNIFICANT TOXICITY FROM ACUTE INHALATION OF DRY PAINT CONTAINING TRIGLYCIDYLISOCYANURATE (TGIC)
Rubinstein B, Shrestha M, Greenberg M
The Medical College of Pennsylvania and the Philadelphia Poison Center, Philadelphia, Pennsylvania, USA
Background: Dry paint or “Dry-Lac” is particle paint devoid of the solvent required for liquid paint. Triglycidylisocyanurate (TGIC) is a triepoxy compound used as a cross-linker agent for the polyester resins. It has been previously reported to cause skin and lung sensitization, but there are no reports of acute inhalation. Some of the dry paint powder is fine enough to get into the lower respiratory tract. Case Presentation: A parcel handler who was moving boxes in the back of a truck lifted a box containing powdered dry-paint and set it down on the floor of the truck. A green fog emanated from the defects of the box and occupied the space in front of his face. He inhaled a few breaths of the material and sought advice from his supervisor. He initially was asymptomatic, and then in the course of minutes developed some eye and nose irritation, a dry cough, and a burning chest discomfort. He was taken to a local emergency department (ED) where he was found to be in mild distress with normal vital signs and pulse oximetry. There was no wheezing. The chest radiograph and the ECG were normal. The eyes were irrigated using a Morgan Lense™, and 3 treatments of nebulized albuterol (2.5 mg or 0.5 cc diluted in 2.5 cc of saline) were administered, resulting in symptomatic improvement of the eye and chest irritation. He was watched overnight and discharged the next day in good condition. One of the patient's coworkers went into the parcel truck after the powder had leaked out and had similar, but less severe symptoms, and was discharged from the ED. Conclusion: From an acute inhalation of dry-paint containing TGIC we found only toxicity consisting of subjective symptoms. Symptoms improved over hours to days, and the one-time exposure to this date has not resulted in any persistant effects.
143 SEVERE METHYLSALICYLATE POISONING WITH DELAYED UPPER AIRWAY OEDEMA IN A CHILD
Levy S, Dargan PI, Jones AL
National Poisons Information Service (London), Guy's and St Thomas' NHS Trust, London, United Kingdom
Objective: Oil of Wintergreen (methylsalicylate) is a constituent in a number of different liniments and ointments used for the relief of musculoskeletal pain. 1 mL of 98% methylsalicylate is equivalent to 1400 mg of aspirin. We report a case in which ingestion of a small volume of methylsalicylate by a child resulted in severe salicylate toxicity and later respiratory tract irritation with upper airway oedema and stridor. Case Report: An 18 month old girl (11 kg) was found at home with an open bottle of 98% methylsalicylate. The bottle was noted to be nearly full, so the carer took no action until four hours later when the child developed vomiting and was taken to her local hospital. On arrival she was alert, had a heart rate of 128 bpm, BP 110/60 mmHg and a respiratory rate of 40 per minute. Her initial plasma salicylate concentration (6 hours after ingestion) was 653 mg/L (4.7 mmol/L); arterial blood gases showed: pH 7.33, PaCO2 2.45 kPa, PaO2 11.96 kPa, BE -12, HCO3 9.9 mmol/L; renal function remained normal throughout her hospital stay. She was given an inadequate sodium bicarbonate infusion for 8 hours (0.1 ml/kg 8.4% sodium bicarbonate) and did not achieve adequate urinary alkalinisation (her urine pH was 5.4). Over the next eight hours she continued to vomit, and developed drowsiness (GCS 9/15) and mild stridor. Her salicylate concentration at 14 hours after ingestion was 622 mg/L (4.5 mmol/L). She was intubated and ventilated and transferred to PICU, and the poisons centre were contacted for further management advice. She was commenced on adequate alkalinisation (a bolus dose of 1 mL/kg of 8.4% sodium bicarbonate to correct her metabolic acidosis, followed by an infusion of 0.2–0.3 mL/kg/hour of 8.4% sodium bicarbonate to alkalinise her urine to pH 8.5). After a further 24 hours her salicylate concentration was 236 mg/L (1.7 mmol/L), her metabolic acidosis had settled and she was extubated. Immediately after extubation, she developed upper airway obstruction with stridor and severe respiratory distress. There was no response to two 5 mL nebulisers of 1 in 1000 epinephrine given over 5 minutes. She was therefore re-intubated and treated with dexamethasone (0.25 mg/kg/day). She was successfully extubated after 5 days and discharged from hospital the following day. At follow up she has no residual respiratory problems. Conclusions: This case demonstrates the potential for severe salicylate poisoning with small ingestions of methylsalicylate in children. Clinicians should also be aware of the potential for unusual complications such as delayed upper airway oedema after ingestion of concentrated methylsalicylate solutions.
144 REFRIGERANT GAS R404A EXPOSURE WITH DELAYED SEQUELAE
Brown R,1 Jones AL,2 Dargan PI2
1Accident and Emergency Department, King's College Hospital and 2National Poisons Information Service (London), Guy's and St Thomas' NHS Trust, London, United Kingdom
Objective: Refrigerant gases are toxic when inhaled in high concentrations or for extended periods. This case is unique as exposure took place over several hours and the patient developed severe, late onset pulmonary oedema. Case Report: A previously fit and asymptomatic 55 year old male presented after waking at in the early hours of the morning with breathlessness. He had a history of increasing dysponea for one week, with some nocturnal dyspnoea and decreased exercise tolerance but no chest pain, palpitations or associated symptoms. He smoked 40 cigarettes per day. He worked as a driver of a refrigerated vehicle and a week prior to the onset of symptoms, whilst driving, he became aware of an odd smell and located it to the refrigeration unit. Despite the onset of a headache, he continued to be exposed to the fumes for 3–4 hours; he did not describe palpitations or dyspnoea at the time of the exposure. The coolant leak was identified as 404A (which contains a blend of fluoranes 125, 134A and 143A) and repaired. On examination he was tachycardic (120 bpm) with oxygen saturations on air of 86%. He had bilateral basal coarse crepitations, a JVP of +4 cm and he was given intravenous furosemide, diamorphine and high flow oxygen. Nasal CPAP was required to maintain oxygen saturations, and he was treated with a GTN infusion and an ACE inhibitor. His ECG showed sinus tachycardia and T wave inversion in the inferior leads (II, III and aVF). CXR showed bilateral pulmonary oedema. Cardiac enzymes, cholesterol and triglycerides were normal. An echocardiogram showed an ejection fraction of <30% with localized akinesia on the postero-lateral wall and reduced anterior wall movement and coronary angiogram showed triple vessel disease. Conclusion: It is possible that pre-existing coronary artery disease increased susceptibility to the cardiotoxicity of the refrigerant gas in this patient and that fluorocarbons unmasked previously asymptomatic disease, although we cannot exclude a fluorocarbon effect alone or ischaemia alone. Successful management of the cardiac failure with CPAP is described.
145 THE PREVALENCE OF ASYMPTOMATIC LEAD POISONING IN PATIENTS WITH ANAEMIA
Dargan PI,1 Jones AL,1 Sculley M,2 House IM,1 Wood D,1 Woodcock S2
1National Poisons Information Service (London), and 2Department of Haematology, Guy's and St Thomas' NHS Trust, London, United Kingdom
Objective: Lead poisoning can cause anaemia at blood lead concentrations greater than 400–500 μg/L. However, many patients with blood lead concentrations in this range are asymptomatic or have only vague, non-specific symptoms and may not seek medical attention. The aim of this study was to assess the prevalence of lead poisoning in patients with anaemia in South-East London, a multi-cultural area with social deprivation in which studies 20-years ago demonstrated a high prevalence of lead poisoning. Methods: All blood samples received by the haematology laboratory of one hospital in South-East London for full blood count analysis, in which the haemoglobin was less than 11.5 g/dL in adult males and 11 g/dL in adult females and children (under 12-years), were assessed. Macrocytic samples (MCV>100fL) and samples in which there was an obvious cause for anaemia such as a haematological malignancy or acute blood loss were excluded from further analysis. The remaining samples were analysed for blood lead concentration using atomic absorption spectroscopy. Results: A total of 988 samples were analysed: 290 (29.3%) were male, 698 (70.7%) were female; median age 49-years, 106 samples (10.7%) in children under 12-years. The median haemoglobin was 10.3 g/dL (range 4.2–10.9) in females, 10.65 g/dL (5.2–11.4) in males and 10.7 g/dL (6.7–10.9) in the children. The median blood lead was 26.3 μg/L (range 30–240 μg/L; 95th centile 75.4 μg/L); there were 15 samples (1.5%) with a blood lead concentration greater than 100 μg/L, 5 (1%) greater than 150 μg/L and 1 (0.1%) greater than 200 μg/L. In the 106 children, the median blood lead was 23.4 μg/L (range 5–145 μg/L; 95th centile 61.2 μg/L). There was only one child (145 μg/L) with a blood lead concentration greater than 100 μg/L. There was a very poor correlation between haemoglobin and blood lead concentration (R2=0.008). Conclusions: This study shows that the population of South-East London have a similar median blood lead concentration to that reported in eight regions of England in 1996.1 It is reassuring that in this, former high exposure area, there is a low prevalence of significant lead poisoning, even in an anaemic population. In comparison to previous UK studies,1 we found a smaller proportion of people with blood lead concentrations above 100 μg/L, particularly in children. There was a poor correlation between blood lead concentration and haemoglobin in this population of asymptomatic individuals with anaemia. Thus routine screening of such samples for lead poisoning cannot be justified.
References: 1. Delves, H.T.; Diaper, S.J.; Oppert, S.; et al. Blood Lead Concentrations in UK Have Fallen Substantially Since 1984. BMJ 1996, 313, 883–884.
146 ACUTE HEMOLYSIS INDUCED BY PERCUTANEOUS TRICHLORFON EXPOSURE
Deng JF, Wu ML, Tsai WJ, Ger J
Division of Clinical Toxicology, Department of Medicine, Taipei Veterans General Hospital, Taiwan
Introduction: Trichlorfon (Dimethyl-2,2,2-trichloro-hydroxyethylphosphonate), an organophosphate, could be absorbed by inhalation, transdermal, transconjunctival, and gastrointestinal exposure. Organophosphate poisoning is well known for it's characteristic symptoms and signs, but acute hemolysis caused by trichlorfon is not reported. We report a case who developed acute hemolysis and renal function impairment after percutaneous trichlorfon exposure. Case Report: A 54-year-old man used trichlorfon to bath his dog for killing the dog's parasites. He used a high concentration for getting better effect and did not wear protective gloves during the procedure. Half an hour later, the dog died and he had abdominal cramping pain. Then, severe nausea and vomiting developed 4 hours later and chillness, high fever, and cold sweating occurred 8 hours later. Initial management included atropine and pralidoxime infusion. On the next day, marked leucocytosis (WBC 301900/cumm), elevated blood urea nitrogen (35 mg/dL), creatinine (2.3 mg/dL) and total bilirubin (5.0 mg/dl) were noted. A full evaluation including plasma hemoglobin, haptoglobin, urine hemosiderin confirmed the presence of acute hemolysis. The erythrocyte-cholinesterase and plasma-cholinesterase activities were normal. He eventually recovered and was discharged on the eleventh day. Conclusion: Although hemolysis in dogs after trichlorphos poisoning has been reported in 1979, it is a rare clinical feature of trichlorfon or other organophosphate intoxication.
147 DELAYED RECOGNITION OF CORNEAL ABRASION FROM CONCENTRATED HYDROGEN SELENIDE (H2SE) GAS EXPOSURE
Shrestha M, Peltz H, Baniukewitz A, Bryant S, Greenberg M
MCP/Hahnemann University, Frankford Bucks and Mercy Hospitals, Philadelphia, Pennsylvania, USA
Background: Hydrogen Selenide (H2Se) gas is extremely irritating to mucous membranes and can be destructive at high concentrations. H2Se is highly soluble in water and can combine with membrane moisture, depositing selenium (Se) or Se containing compounds. Case Report: A 53 year-old-male worker was exposed to a cloud of 100% H2Se gas in an accidental workplace release. He complained of immediate burning of the nose, throat, eyes, and chest, with severe dyspnea, cough and congestion. On arrival to the hospital he was in respiratory distress. After emergency department (ED) stabilization, he was admitted to the intensive care unit, where he was noted to have severe photophobia and cojunctival injection. Ocular pain increased over time, and within 24 hours eye pain and photophobia were severe. Visual acuity was decreased. Fluorescein staining of both eyes revealed horizontally oriented, elliptically shaped corneal abrasions. Topical antibiotics and midriatics were administered and the ocular symptoms improved over the course of 48 hours. Two other similarly exposed workers also developed corneal abrasions. Conclusions: The location and shape of the abrasions were consistent with a noxious gas contacting eyes held partially closed by squinting. Ocular exposure to water-soluble irritant gases may result in corneal damage that goes initially unrecognized. In any case involving exposure to water-soluble gases, early ocular irrigation must not be overlooked and may help to reduce corneal damage.
148 IATROGENIC HYPERMAGNESEMIA IN A PRE-ECLAMPTIC PATIENT RECEIVING MAGNESIUM SULFATE
Dincq AS,1 Laterre PF,2 Wittebole X,2 Hantson Ph.2
1Department of Anesthesiology, 2Department of Intensive Care Medicine, Cliniques St-Luc, Université Catholique de Louvain, Brussels, Belgium
Objective: To present a case of poorly symptomatic hypermagnesemia consecutive to iatrogenic intravenous magnesium overdose in a pre-eclamptic patient. Case Report: A 29-year-old patient was delivered by Cesarean section under general anesthesia after 32 weeks of twin pregnancy. The reason for premature delivery was the progression of the clinical signs of pre-eclampsia. Her neurological examination did not reveal any abnormality. Magnesium sulfate infusion had been started 8 hours prior to Cesarean section at a rate of 1.6 g/hr in order to maintain magnesium serum concentration between 1.5 and 2 mmol/l. The admission biological data were calcium 3.4 mmol/l (4.3–5), phosphorus 2.5 mmol/l (1.2–2.35), magnesium 1.8 mmol/l (0.70–0.95), urea 15.3 mmol/l (5.3–17.8), creatinine 106 μmol/l (53–123.8). Creatinine clearance was calculated at 107 ml/min. It was decided to wean the patient from the mechanical ventilation and she was extubated 5 hours after admission. The patient was perfectly conscious; deep tendon reflexes were weak, but not abolished. Respiratory rate was 15/min; heart rate, 100/min; arterial blood pressure 175/87 mmHg. Immediately after extubation, the nurse realized that the magnesium sulfate infusion was empty, and that the patient had inadvertently received 30 grams magnesium sulfate over the 4 hours preceding extubation. Magnesium serum concentration was urgently determined and was 5.05 mmol/l (calcium was 3.3 mmol/l and phosphorus 2.2 mmol/l). The physician administered 1.375 grams of calcium gluconate by intravenous route. The patient did not present any sign of magnesium overdose and, in particular, no respiratory depression. The electrocardiogram was not modified and no cardiovascular adverse events were noted. No specific treatment was required. The magnesium serum concentration decreased only slowly despite the fact that renal function was not impaired. Magnesium serum level was still 1.35 mmol/l six days after magnesium overdose. Discussion: Signs of hypermagnesemia may occur at serum concentrations in excess of 2 mmol/l. The major life-threatening clinical manifestations are lethargy or coma, respiratory depression, cardiac conduction delays or asystole. Magnesium serum levels greater than 7.4 mmol/l are usually fatal.1 Patients with impaired renal function are more likely to experience severe hypermagnesemia as magnesium is effectively eliminated via renal route; hemodialysis can remove significant quantities of magnesium in severely intoxicated patients. Calcium salts will somewhat antagonize respiratory depression induced by hypermagnesemia, presumably by displacing magnesium from cell membranes. Conclusion: Iatrogenic magnesium sulfate overdose resulted in a marked hypermagnesemia in a pre-eclamptic woman with normal renal function. No significant adverse events were noted and the treatment was supportive. Reference: 1. Vissers, R.J.; Purssell, R. Iatrogenic Magnesium Overdose: Two Case Reports. J. Emerg. Med. 1996, 14, 187–191.
149 IATROGENIC LIDOCAINE INTOXICATION WHILE PERFORMING A ‘ROUTINE’ PROCEDURE
Van der Pant KAMI,1 Hammoen K,2 Leeuwen HJ van,1 Meulenbelt J1
1Department of Intensive Care and Clinical Toxicology and 2Department of Surgery, University Medical Centre Utrecht, Utrecht, The Netherlands
Objectives: Lidocaine is an aminoacyl amide, widely used as a local anesthetic and parenterally administered antiarrhytmic. Toxicity has been reported via various routes of administration. A lidocaine overdose may result in drowsiness, seizures, respiratory depression, loss of consciousness, areflexia, hypotension, ventricular arrhythmia and asystole. Studies suggest that lidocaine toxicity becomes increasingly likely when the plasma concentration exceeds 6 mg/L. Intoxication with lidocaine is relatively common, can be life-threatening, requires immediate evaluation and intensive care observation. Case Report: A 63-year-old, 65 kg, woman underwent a Belsey-operation because of refractory gastroesophageal reflux disease. The postoperative course was complicated by a left sided pleural empyema and progressively bilateral pleural effusion. It was decided to perform bilateral pleural space drainage. This procedure took place at the emergency room, where vials of 30 cc lidocaine 2% (with rubber top for multiple use) are available for local anesthesia. The vials were prepared under good manufacturing practice by the hospital pharmacy. On the right side the local anesthetic procedure with circa 20 cc lidocaine 2% elapsed uncomplicated. Shortly after administration of circa 20 cc lidocaine for the chest tube insertion on the left side the patient had a generalized seizure. At that moment a total dose of circa 800 mg lidocaine was administered. She was treated with 1 mg clonazepam IV and because of respiratory failure and central nervous system depression she was intubated and mechanically ventilated. On admission to the intensive care unit the patient had a blood pressure 78/45 mmHg; pulse rate 75/min; temperature 37°C. Laboratory results (before mechanical ventilation) included an arterial blood gas analysis with pH 7.38; pCO2 45 mmHg; pO2 73 mmHg; HCO3 25 mmol/L, potassium 3.0 mmol/L, sodium 136 mmol/L, glucose 9.2 mmol/L, calcium 1.45 mmol/L (normal in relation to albumin concentration), magnesium 0.61 mmol/L. Electrocardiogram showed STT segment changes, but no signs of myocardial infarction. Computerized tomography of the brain showed no abnormalities. The clinical symptoms directly after the lidocaine administration were very suggestive of a lidocaine overdose. Lidocaine plasma concentration confirmed this (See Figure 1). Analysis of the used vials showed a lidocaine concentration of 2% as indicated on the label. Patient received supportive treatment with IV fluid resuscitation, dopamine, and mechanical ventilation. After a few hours the patient recovered completely without neurological symptoms and mechanical ventilation was discontinued. Conclusion: Physicians using lidocaine as a local anesthetic should be aware of the potential toxicity. Unexplained hypotension, respiratory failure, and CNS symptoms after lidocaine administration should direct the physician to the diagnosis. In this case it wasn't realized that a potential toxic dose (12.3 mg/kg) was administered. The variation in concentration in available vials may contribute to the risk for an overdose. In order to prevent the iatrogenic intoxications with lidocaine a protocol should be followed in which the maximum dose per kg body weight and the lidocaine concentration to be used for the particular procedures is described.
150 MILD TOXICITY AFTER TENFOLD IATROGENIC OVERDOSE OF DOXAPRAM IN A PRETERM INFANT
Seidel C,1 Wintgens J,2 Lentze MJ1
1Poison Control Center Bonn, Germany. 2Elisabeth-Hospital Rheydt, Germany
Objective: Doxapram is used as an analeptic in preterm infants. Intravenous loading doses range from 0.5 to 2.5 mg/kg, the intravenous maintenance dose is 1 mg/kg/h. The data on the onset of therapeutic effects range from 1–8 hours. Overdose experience in the literature is limited. Severe cases may have nausea, vomiting, tachycardia, hypertension, hyperpnea, seizures, hemolysis, coma and respiratory failure. We report a case of an iatrogenic overdose which is to our knowledge the highest continuous infusion rate. Case Report: A preterm twin infant (born at 28 weeks gestation), 37 days old, 1.730 g, had been intubated and ventilated for about 10 days because of severe apnoea and bradycardia with superimposed pneumonia. After extubation the patient was first treated with therapeutic doses of doxapram followed by oral coffein, phenobarbital and clarithromycin in therapeutic doses. For prevention of apnea the patient was treated for 6.5 hours with Doxapram 0.95 mg/kg/h (1.62 mg/h) intravenously. While moving the child in the ICU, the infusion rate was accidently changed to 16.2 mg/h (9.5 mg/kg/h). This ten-fold dose continued for approximately 4.5 hours (±15 min). The error was noted when the infusion was finished. The overall dose for 4.5 hours was 72.9 mg. There were no cardiovascular or neurologic effects during the infusion or afterwards. Blood pressure and heart rate remained stable. Seizures might have been prevented by the phenobarbital. The only symptoms noted were nausea and increased gastric residuals. Some episodes of apnea occurred several hours later but could be explained by the prematurity of the infant. Conclusion: The only toxic effects of doxapram seen in a premature infant after the intravenous administration of 9.5 mg/kg/h for 4.5 hours were gastrointestinal disturbances. The margin of safety of doxapram might be wider than it has been thought. Since the infant did not respond well to therapeutic doses, there might also be an individual sensibility towards doxapram. Further case reports are needed to assess the toxicity of this substance.
151 ACCIDENTAL INTRAVENOUS INFUSION OF GOLYTELY® IN A 4-YEAR OLD FEMALE
Guzman DD, Teoh D, Velez LI,1 Rivera W,1 Shepherd JG1
Children's Medical Center of Dallas, 1The University of Texas Southwestern Medical Center and the North Texas Poison Center, Dallas, Texas, USA
Objective: GOLYTELY® is an isosmotic solution of high molecular weight polyethylene glycol and electrolytes that is administered orally or via nasogastric tube. It is used in toxicology to cleanse the GI tract after some ingestions by inducing diarrhea. Currently there are few published reports of accidental IV GOLYTELY® infusions. In 1959, a case series described the IV administration of polyethylene glycol 300 (PEG) to 9 patients. Seven patients developed proximal tubular necrosis and 2 patients died. The osmotic activity of PEG and the electrolyte concentration should not produce fluid shifts. Because GOLYTELY® is composed mainly of PEG, it is important to direct therapeutic interventions to PEG toxicity. We report an accidental IV infusion of GOLYTELY® in a pediatric patient that did not result in systemic toxicity. Case Report: A 4-year old female presented to the Emergency Department (ED) after ingestion of approximately 24–50 mg (80 mg/kg) tablets of 6-mercaptopurine (6-MP) two hours prior to arrival. She had vomited twice after receiving syrup of ipecac at home. At the ED, her vital signs were: T 36.2 F, HR 102, RR 42 and BP 115/67. On physical examination, she was alert and in no distress. The remainder of the examination was normal. The patient received 1 gm/kg of activated charcoal and was started on GOLYTELY® via NGT. After one hour it was noted that the GOLYTELY® was being administered IV (391 ml infused) and the infusion was immediately stopped. Electrolytes, BUN, creatinine, serum glucose, lactic acid, venous blood gas and glycol levels were sent. There was no evidence of acidosis (HCO3 28 mmHg, pH 7.35) or renal failure (BUN 8 mg/dL, Creatinine 0.3 mg/dL). Glycol levels were undetectable. The patient was admitted for observation and antibiotic therapy. She was discharged free of complications 36 hours after admission. Discussion: There have been many reports of accidental infusion of GOLYTELY® into the lungs through a misplaced NG tube, leading to pulmonary edema and pneumonitis. However, accidental IV infusion is rare. Toxicity from GOLYTELY® is mainly related to the molecular weight of the PEG in the formulation. Low MW (200 to 400) PEG has produced systemic toxicity, while higher MW (3000 or greater) are less toxic. Because GOLYTELY® contains PEG, it is very important to monitor acid–base status, renal function and pulmonary function in symptomatic patients. Fortunately, this patient did not suffer any systemic toxicity from the IV infusion of GOLYTELY®.
152 TRAMADOL OVERDOSE—FIVE YEARS EXPERIENCE IN SWEDEN
Carlvik B, Persson H, Sjöberg G, Westberg U
Swedish Poisons Information Centre, Stockholm, Sweden
Objective: Tramadol, a synthetic opioid agonist, was introduced on the Swedish market at the end of 1996 as an alternative in the treatment of moderate to severe pain. In order to assess the acute toxicity of tramadol in human overdose, a retrospective review of the inquiries and case reports received by the Swedish Poisons Information Centre between 1997 to 2001 was carried out. Case Series: During this 5-year period the Swedish Poisons Information Centre received a total number of 883 calls concerning tramadol overdose (90% adults, 9% children <10 years and 1% unknown), with an increasing number each year. During the period 104 cases of tramadol poisoning could be investigated in detail through the study of hospital case records (95% adults and 5% children <10 years). In this patient material the sex distribution was 72% females and 28% males. The poisonings were graded according to the Poisoning Severity Score (PSS) as shown in Table 1. Common symptoms were dizziness, CNS depression, seizures, miosis, agitation, dizziness and gastrointestinal symptoms. Respiratory depression was uncommon and only observed after ingestion of high doses (in general >2 g). Also significant cardiovascular disturbances were rare. Apart from one fatal case, that because of insufficient data was not possible to grade, there were no deaths in this material. Seventeen patients received naloxon, mainly for prophylactic reasons. Conclusion: The use of tramadol is steadily increasing in Sweden. The clinical features of tramadol intoxications are usually mild to moderate and seem to be less severe than for other opioides, in particular dextropropoxyphen. Inter-individual variations make it difficult to establish a precise dose–response relation. In this material a dose of <600 mg produced only mild intoxications (PSS 0–1), whereas doses of >2 g in general produced moderate or severe intoxications (PSS 2–3).
Table 1. (Abstract 152)
153 INTOXICATION WITH NEUROLEPTIC DRUGS REPORTED TO THE SLOVAK TOXIGOLOGICAL INFORMATION CENTRE IN THE YEARS 1996–2000
Caganova B, Plackova S, Kresanek J, Batora I
Toxicological Information Centre, Department of Industrial Medicine and Toxicology, Derer Hospital, Bratislava, Slovakia
Objective: The Toxicological Information Centre (TIC) in Bratislava has frequently been consulted for advice on neuroleptic agent exposures in recent years. To obtain more information about neuroleptic overdoses in Slovakia, we performed a retrospective analysis of all telephone calls to our Centre. Methods: All telephone inquiries involving neuroleptic exposures were extracted from our databases for the period 1996–2000. The following data were analysed: age, sex, intent of exposure (accidental or suicidal), substances ingested and clinical severity. Results: During the 5-year period 2887 drug intoxications were reported to the Slovak TIC, of which 324 (11.2%) involved neuroleptic drugs. Neuroleptic exposures in female (56.6%) were more prevalent than those involving males (43.3%). Suicidal poisonings (54.7%) were more common than accidental poisonings. The majority of cases (63.8%) were adults, the most frequently involved age groups were those aged 19–30 years and those younger than 5 years of age. Phenothiazines were responsible for 53.3% of all cases. More than 22% of exposures involved atypical antipsychotics. The majority of patients overdosed with neuroleptics were asymptomatic or developed only mild toxicity (63.4%). Moderate or severe symptoms occured in 36.6% of all exposures, 5 cases (1.5%) resulted in death. All these deaths occurred in adults, 3 resulted from clozapine and 2 were associated with a co-ingestant. Conclusion: Intoxications involving both conventional and atypical neuroleptics are among the more common enquiries to the Slovakia TIC. Clinical symptoms occurred early and were usually of mild to moderate severity. In our experience children appeared at particular risk from antipsychotic poisoning.
154 TRENDS IN SEVERE MIXED DRUG POISONING AMONG OPIOID-ADDICTED DRUG USERS WITH THE ADVENT OF HIGH DOSE BUPRENORPHINE SUBSTITUTION
Gueye PN, Mégarbane B, Borron SW, Adnet F, Galliot-Guilley M, Ricordell I, Tourneau J, Goldgran-Tolédano D, Baud F
INSERM U26, Hôpital Fernand Widal and Réanimation Médicale et Toxicologique, Hôpital Lariboisière, Paris, France
Objectives: Since 1996, high dose buprenorphine has been approved in France as substitution treatment of heroin addiction. As soon as 1998, forensic series reported several deaths in addicts using buprenorphine, frequently in association with benzodiazepines. To date, other opiates and opioids, including methadone and morphine, are also used as substitution treatment in heroin addicts. Our objective was to assess the trend in incidence and mortality in severe opiate/opioid poisonings, since buprenorphine obtained administrative authorizations for heroin substitution. Methods: We conducted a retrospective study using data from 1996 to 1999, with collection of: 1) pre-hospital data in a northeast suburb of Paris with a high prevalence of heroin addiction (SAMU 93); 2) in-hospital data from our toxicological intensive care unit in northeast Paris; and 3) post-mortem data for 1998 and 1999. Results: From 1996 to 1999, the detection of buprenorphine among opiate/opioid poisoned patients has steadily increased while opiates have diminished. Increased buprenorphine detection correlated directly with increasing sales over this time period. In spite of the increased use of buprenorphine, the mortality associated with opiate/opioid poisonings has diminished both in pre-hospital and in-hospital environments. Our data suggest that the availability of high dose buprenorphine has not been associated with an inordinate incidence of severe poisonings. Our study detected a high frequency of multiple opiate/opioid use in severe poisonings, as well as the frequent association of other psychotropic drugs including ethanol. While the relevance of mixed drug poisonings involving opiates or opioids with psychotropic drugs including ethanol. While the relevance of mixed drug poisonings involving opiates or opioids with benzodiazepines has been previously outlined, the risk of the combination of opiates with opioids or of two or more opioids in these poisonings may be underestimated. Conclusion: Our data demonstrate the recent tendency of multiple drug consumption in the addict population in Paris area. They emphasize the need to further clarify interactions between opiates, opioids and other sedative drugs. The risk/benefit ratio of specific antidotes such as naloxone and flumazenil in these complex poisonings has yet to be defined.,
155 THE NATURE OF SHORT-TERM REPEAT ADMISSIONS FOR SELF-HARM
Good AM, Chalmers CD, Kelly CA, Oliver JJ, Bateman DN
National Poisons Information Service, Edinburgh Centre, Scottish Poisons Information Bureau, Royal Infirmary of Edinburgh, United Kingdom
Objectives: To evaluate the nature of repeat attenders at a poisoning treatment centre. Methods: All repeat admissions to ward 1A at the Royal Infirmary of Edinburgh (RIE) for the year 1 October 2000 to 30 September 2001 were analysed retrospectively for age, sex, type of admission, and agent(s) taken. This was compared with the same data for single admissions. Background: The RIE has, as part of the Acute Admissions Unit, a 9 bedded ward dedicated to the care of self-harm and poisoned patients, aged over 12 years. This includes poisonings, alcohol and drug abuse and other forms of self-harm e.g. hangings, wrist slashing. As well as medical care they have access to social and psychiatric services and counselling from liaison alcohol and psychiatric nurses. Despite this a number of patients are repeat attenders. Results: In the year studied there were 3050 admissions. 360 patients were admitted on more than one occasion during the year (see Table 1) and were responsible for 36% of admissions; the average number of admissions/patient was 3 (range 2–15); 165 (46%) males and 195 (54%) females.
2% of re-admissions were on the same day, 25% within 1 day to 1 week, 30% within 1 week to 1 month and 42% more than 1 month. The peak age group for both males and females was 30–39 years. 1967 patients were admitted only once in the year; 967 males (49%) and 1000 females (51%). Peak age groups were 20–29 for males. Almost identical numbers were in 20–29 and 30–39 for females. Of agents taken the top ten for repeat attenders were paracetamol (409), ethanol (238), diazepam (136), dihydrocodeine (74), dextropropoxyphene (59), codeine (52), ibuprofen (50), amitriptyline (49), chlorpromazine (48), paroxetine (38). There were also 83 admissions for deliberate self-harm. For single admissions the top ten were paracetamol (650), ethanol (376), diazepam (167), ibuprofen (141), dextropropoxyphene (100), codeine (93), dihydrocodeine (87), amitriptyline (69), heroin (69), caffeine (69). Repeaters were more likely to have taken chlorpromazine (4.4% against 1.8%; odds ratio 2.5). Conclusions: Approximately 15% of patients were admitted more than once in the year and were responsible for 36% of admissions. One quarter of re-admissions were within 1 week and more than 50% within 1 month of the previous overdose. Patients with multiple admissions were more than twice as likely to have taken chlorpromazine than single attenders.
Table 1. (Abstract 155)
156 ADMISSIONS TO CARDIFF POISONS TREATMENT UNIT INVOLVING DRUGS OF ABUSE, BETWEEN 1989 TO 2000
Spears RA, Thompson JP, Routledge PA, Hutchings A
National Poisons Information Service, Cardiff Centre. Therapeutics and Toxicology Centre, UWCM, Cardiff, United Kingdom
Objectives: The NPIS (Cardiff Centre) collects data on patients admitted. This study identifies trends in admissions involving drugs of abuse between 1989 and 2000. Method: Patients admitted to the poisons unit were interviewed by staff and the substances taken recorded. The admission database was searched for details of patients admitting to taking drugs of abuse (solvents, amphetamine, butane, cannabis, cocaine, ecstasy, gammahydroxybutyric acid (GHB), glue, heroin, khat, liquid lighter fuel, lysergic acid diethylamide (LSD), and Psilocybe semilancelata (magic mushrooms)). Results: Total admissions to the unit have increased form 894 in 1989 to 1791 in 2000. Drugs of abuse were involved in 17 cases in 1989 and 198 cases in 2000, an increase from 1.9% to 11% of total admissions (see Table 1).
Of the drugs studied, amphetamine was most commonly involved; increasing from 2 admissions in 1989 to 90 in 1999 and 48 in 2000. Ecstasy admissions increased from four cases before 1992, to 21 cases in 1994 and 68 cases in 2000. Third commonest was cannabis, increasing from 1 case in 1989 to a peak of 30 in 1999 with 25 in 2000. Fourth commonest was heroin and fifth LSD, with a total of 143 and 65 cases respectively. Fifty two percent of patients had also taken alcohol, 16.7% benzodiazepines, 9.1% paracetamol and 5.4% antidepressants. Conclusion: Admissions to the Cardiff Poisons Treatment Unit have doubled over the last 12 years, whilst those admitting to taking drugs of abuse has risen sixfold. Most of the latter are young (one third under 20 years) or male. Generally, more females are admitted, and typical patients are older (17.2% under 20 years). The reason patients admitted to the unit having taken drugs of abuse has increased to such a great extent is unknown. It could reflect a growing drug problem in the area. Alternatively patients may now be referred to the unit who would not have been referred in the past.
Table 1. (Abstract 156)
157 CRITICAL CARE INTERVENTIONS REQUIRED FOLLOWING DRUG OVERDOSE
Kelly CA,1 Din S,2 Bateman DN1
1National Poisons Information Service, Edinburgh Centre, Royal Infirmary of Edinburgh, Scotland, United Kingdom. 2Medical School, University of Dundee, Scotland, United Kingdom
Objectives: Drug overdose remains a significant cause of morbidity and mortality. Our objective was to determine the complications arising in drug overdose patients requiring admission to a critical care facility and the therapeutic interventions undertaken. Methods: A retrospective review of case records was undertaken for all patients admitted to the intensive care or high dependency unit of a large urban teaching hospital, with the Scottish Liver Transplantation Unit on site. Complications were defined as decreased conscious level (Glasgow coma score ≤8), hypotension (systolic BP <100 mm Hg), ECG changes (QRS ≥0.12 ms or QTc ≥0.45 ms), seizures or chest X-ray (CXR) abnormality (infiltration or collapse). All therapeutic interventions were recorded. Data were analysed using Chi square test were appropriate. Results are expressed as mean±SEM. Results: Data were analysed on 81 male and 89 female consecutive patients admitted over a 24 month period from January 1999 to December 2000. Mean length of stay in a critical care area was 2.76±0.22 days. The commonest drugs ingested were tricyclic antidepressants (32.4%), paracetamol (20%), benzodiazepines (17.6%) and opiates (13.5%). Decreased conscious level was seen in 153 patients (90%), hypotension in 33 patients (19.4%), ECG changes in 25 patients (14.7%), seizures in 22 patients (12.9%) and CXR abnormalities in 53 patients (31.2%). The most frequent interventions required were intubation and/or ventilatory support (62.9%), antibiotics (28.8%) and inotropic support (18.8%). In hospital mortality was 11.2%. Mortality was significantly greater following paracetamol ingestion (p<0.0001), but many of these patients had fulminant hepatic failure and had been transferred from other units for assessment for liver transplantation. Conclusions: The majority of patients admitted had depressed conscious level and required intubation and ventilatory support. A GCS ≤8 or abnormal CXR findings on admission may be useful triage tools for assessing potential need for critical care intervention.
158 INCIDENCE OF SEVERE ADVERSE EFFECTS MANAGED IN A SLOVENIAN INTENSIVE CARE UNIT
Fortuna M,1 Perharic L2
1Centre for Intensive Internal Medicine, University Medical Centre, Ljubljana; 2Institute of Public Health, Ljubljana, Slovenia
Objective: Occurrence of adverse effects related to medications may be precarious secondary to various factors including individual differences in drug absorption, metabolism and elimination, altered pharmacodynamics and pharmokinetics due to co-existing diseases, and use of multiple drugs. The aim of this study was to establish the frequency and type of severe adverse effects managed in our Centre for Intensive Internal Medicine (CIIM). Methods: A retrospective analysis of patients' notes with a diagnosis of severe and life threatening side effects has been performed. We included those patients who required admission to CIIM secondary to the severity of adverse effects and those who developed adverse effects in CIIM. A diagnosis of an adverse effect was established clinically and when possible confirmed analytically. Results: Among 20318 patients managed in CIIM between 1985 and 2000, we identified 186 (0.9%) patients who fulfilled the criteria stated in the methods. The average patient age was 68 years (range: 39 to 94 years). There were 99 men and 87 women. In patients aged 65 and above, the incidence of adverse effects was significantly higher than in younger patients (p<0.001). The distribution between the sexes was not significantly different. In majority of the patients a previous history of cardiac disease had been established. The most frequently implicated medications were cardiovascular agents which were identified as a cause of side effects in 93 (50%) patients: verapamil in 40, digoxin in 19, amiodarone in 14, beta blockers in 13, other antiarrhythmic agents in 4, and ionotropic agents in 3. Adverse effects related to other medications featured as follows: thrombolytics and anticoagulants in 32 patients (17.2%), multiple medications in 24 (12.9%), insulin and oral hypoglycaemic agents in 11 (5.9%), and other medications in 26 (14%). The most frequently occurring serious adverse effect was hypotension with cardiogenic shock in 56 patients (30.1%), followed by bradycardia and asystole in 54 (29%), haemorrhage in 32 (17.2%) and other adverse effects in 44 (23.7%) patients. Temporary cardiac pacing was required in 27 patients. Despite intensive therapy 18 (11.3%) patients died. Conclusion: The results of our analysis suggest that development of serious adverse effects is more likely in the elderly treated with cardiovascular medications. We recommend a close follow up of these patients in order to reduce the occurrence of severe adverse effects. The patients treated with verapamil merit particular attention. In view of their possible severity and unfavourable outcome prompt recognition and treatment of adverse reactions associated with cardiovascular agents is essential.
159 FATAL ANAPHYLACTOID REACTION TO N-ACETYLCYSTEINE: CAUTION IN ASTHMATICS
Appelboam AV,1 Dargan PI,2 Jones AL,2 Knighton J1
1Queen Alexandra Hospital, Portsmouth and 2National Poisons Information Service (London) Guy's and St Thomas' NHS Trust, London, United Kingdom
Objective: Paracetamol overdose remains a common cause of admission for self-poisoning in Europe and intravenous N-acetylcysteine (NAC) is the treatment of choice for patients with significant poisoning. We report a case of an asthmatic patient who died following the administration of NAC after a paracetamol overdose. Case Report: A 40-year-old female presented after taking an intentional, staggered, paracetamol overdose of 15 g over the preceding 48-hours. She had a history of severe, steroid dependent asthma. She also had a history of depression, treated with fluoxetine and a previous, untreated, paracetamol overdose three years earlier. She was not in a “high risk” group for paracetamol poisoning and had no known drug allergies. On arrival, she was alert, with no respiratory distress or cyanosis and examination of her chest revealed clear, bilateral breath sounds with no wheeze. She was obese with a weight of 101 kg and so the dose of paracetamol ingested was 74 mg/kg/24 hours. This mg/kg dose was, however, calculated on total rather than lean body mass and there was some doubt about the dose ingested and so she was treated with IV NAC at the standard UK initial dose of 150 mg/kg over 15-minutes. After 5-minutes she developed dyspnoea, there was no rash, tongue swelling, or hypotension, but chest auscultation revealed widespread wheeze. The NAC was stopped and 5 mg nebulised salbutamol, 1 mg IM epinephrine, 100 mg iv hydrocortisone and 10 mg iv chlorpheniramine administered. Despite these measures, followed by 1 mg IV epinephrine she continued to deteriorate and had a respiratory and secondary cardiac arrest. She was intubated and ventilated and her output returned only when her bronchospasm settled, after nine minutes of resuscitation. She was transferred to ICU for further management. Her ventilation improved rapidly, but she had severe hypoxic brain damage and died one week later without regaining consciousness. Liver and renal function tests and INR remained normal throughout. Conclusion: Anaphylactoid reactions to NAC occur in approximately 3–5% of cases, but these are usually mild and respond to stopping the NAC and symptomatic treatment with antihistamines. There have been deaths reported after excessive doses of NAC, but this is the first report of a fatality associated with a therapeutic dose of NAC. Asthmatic patients are more likely to develop adverse reactions to NAC1 and the treatment of asthmatic patients with NAC requires caution, however asthma should not be seen as a contraindication for NAC.
References: Schmidt, L.E.; Dalhoff, K. Risk Factors in the Development of Adverse Reactions to N-Acetylcysteine in Patients with Paracetamol Poisoning. Br. J. Clin. Pharmacol. 2001, 51, 87–91.
160 CHARACTERISTICS OF THE POISONS INFORMATION SERVICE IN RUSSIA
Ostapenko YuN, Khonelidze RS, Kovalenko LA
Toxicology Information and Advisory Center at the Russian Federation Ministry of Health, Sukharevskaya Ploschad, Moscow, Russia
Objectives: To describe some institutional features and the prospects for the development of poisons information services in Russia. Methods: Analysis of data from poisons treatment centers, the Russian Toxicology Information and Advisory Center (RTIAC), and the Russian Federation Ministry of Health. Results: Data were collected from 43 poisons treatment centers. Of their patients 94% had been given treatment by ambulance teams prior to their arrival at the center. In 2000 these centers treated 29.3% of all patients hospitalized with poisoning. There were 4441 inquiries to the RTIAC. Of these 83% were from physicians of various specialties, including 47% from ambulance stations, 21% from resuscitation physicians and 2.0% from toxicologists. Inquiries concerned the diagnosis (18.7%), appropriate management (21%), and need for hospitalization (29.2%) of poisoned patients. Assistance in obtaining toxicological laboratory analyses accounted for 13.9% of inquiries, poisons information 4.1%, the need for an ambulance 4% and other inquiries 9.1%. The majority of inquiries (98%) were from the Moscow region. Conclusion: Inquiries to RTIAC were predominantly from medical professionals. Cooperation with toxicologists from the Moscow City Center for Poisoning Treatment facilitated a high standard of telephone consultations, and production of information and reference materials and software systems. The nature of the inquiries indicated the need to include experienced toxicologists in the staff of poisons information and advisory centers. RTIAC provided consultancy primarily to the Moscow region. This was largely because of the vast size of the Russian Territory and the high costs of long-distance phone calls, limiting access by financially constrained Russian medical institutions. In addition the network of poisons treatment centers provided consultancy at the local level. There is no unified system for the provision of poisons information and advice in Russia today, making it practically impossible to carry out epidemiological studies and poisons prevention activities. RTIAC has prepared a proposal for developing poisons information services in Russia oriented primarily at the territories having no poison treatment centers, taking into account local features and WHO/IPCS recommendations.
161 A MODEL DETERMINING MINIMUM STAFFING LEVELS FOR POISONS INFORMATION CENTRES—THE IMPACT OF THE EUROPEAN UNION WORKING HOURS DIRECTIVE
Campbell A
National Poisons Information Service, Guy's and St Thomas' Trust, London, United Kingdom
Objective: To assess the impact of implementation of the Working Hours Directive (WHD) on the staffing levels needed to provide a 24-hour poisons information service (PIS). Method: The WHD outlines criteria for good practice for shift-workers providing 24-hour cover. These include: a) A break if working day exceeds six hours; b) Minimum rest periods of 11 hours per 24 hours and 35 hours per week; c) A maximum work period for night workers of 8 hours per 24 hours. A model was developed for determining the minimum staffing requirements, ensuring these WHD criteria are met, and that there is: 1) Flexibility for full holiday entitlements and special leave (sickness, maternity etc) to be taken without disruption of normal service; 2) Dedicated time for essential non-telephone work (outreach, continuing education etc.); 3) A contingency arrangement guaranteeing continuity of service during periods of staff shortage, recruitment and training. The findings are compared with the staffing levels of the National Poisons Information Service London (NPISL) for selected years from 1993–2001. Results: A minimum staffing requirement (n) can be calculated using the equation n=365 (d+x)/c−(h+52 g) where d=number of shifts needed per day (determined by workload), x=number of sessions desired per day for contingency cover, c=contracted number of shifts per worker per annum, h=contracted number of days holiday per annum and g=desired number of sessions per week for non-phone work. As a worked example the NPISL employs Clinical Scientists as Poisons Information Specialists for whom c=234.4 and h=38.5 days inclusive of statutory days on average. Using these values I have calculated values for n (rounded up) for various levels of daily cover, contingency cover and non-phone work (see Tables 1 and 2). Conclusions: Comparison of the calculated results with past NPISL staffing indicates the model provides a realistic estimate of staffing needs for a single centre operation, although not all the WHD criteria are yet met by NPISL. The model also indicates the NPISL has historically operated with little capacity to withstand staffing crises. This year staffing reduced to 79% for several months, due to maternity and high staff-turnover, severely limiting non-phone activities and the ability to train new staff effectively. For many PISs balancing the WHD requirements against financial pressures, whilst ensuring staffing rosters are flexible and attractive will be difficult. This model could be adapted for multi-centre operations sharing resources out-of-hours, as is the case in some European countries.,
Table 1. (Abstract 161)
Table 2. Staffing at NPISL 1993–2001 (Abstract 161)
162 DO SCOTLAND'S HOSPITALS HAVE SUFFICIENT ANTIDOTES READILY AVAILABLE FOR AN EMERGENCY?
Good AM,1 Bhati R,2 Bateman DN1
1NPIS, Edinburgh Centre, Royal Infirmary, Edinburgh, United Kingdom, 2Warrington Hospital, United Kingdom
Objectives: To determine the timely availability of antidotes to Scottish accident and emergency departments (A&E) and minor injuries units (MIU), in light of the current climate of terrorism. Methods: A questionnaire was sent to the principal pharmacist for Scottish hospitals with A&E or MIU requesting information on 27 specific antidotes recommended by WHO1 to be held in major hospitals and currently relevant to the UK, 9 special antidotes (to be held in regional centres) and 29 obsolete antidotes. Antidote availability and timeliness were compared with WHO recommendations and amounts of antidotes compared with previous publications. Results: 31 of 33 questionnaires (94%) from A&E departments and 14 of 21 (67%) of MIUs were returned. All hospitals surveyed had atropine, benzylpenicillin, glucagon, naloxone and vitamin K. Only 2 hospitals (both with A&E departments) had access to all 27 specific antidotes. Of the 28 hospital A&Es who reported availability times 19 (68%) had 20 or more of the specific antidotes available in appropriate times, average 21(range 12–27). For the 13 MIUs who reported availability times only 1 had more than 20 antidotes, average 13 (range 8–21). Specific antidotes—Organophosphate poisoning—in the UK atropine and pralidoxime mesylate (P2S). All hospitals surveyed held atropine. P2S is provided by the Department of Health to designated centres only. Cyanide poisoning—in the UK dicobalt edetate or sodium nitrite/sodium thiosulphate. 22 hospitals (49%) had at least 1 antidote available within 30 minutes. 18 (40%) still hold amyl nitrite (no longer recommended). None had solutions A & B. 26 (58%) hospitals hold hydroxocobalamin (not licensed in the UK for cyanide poisoning) within 30 minutes, but in insufficient quantity for treating cyanide poisoning. Heavy metal poisoning—36(80%) hold desferrioxamine. 31 (69%) have at least 1other chelating agent. 1 centre holds DMPS/DMSA; 2 Prussian Blue; 24 (53%) sodium calcium edetate. 8 MIU and 1 A&E held no heavy metal antidotes. Special antidotes—digoxin specific antibodies 14(31%) A&E (only 3 had 20 or more vials), fomepizole 1 A&E, pyridoxine 16 (36%) within 30 minutes but only 1 A&E had 5 g, sufficient for 1 patient. 22 (49%) hold Zagreb antivenom (adder). Obsolete antidotes—5 (11%) hold fructose (ethanol), 11(24%) still hold ipecac, 20 (44%) have fuller's earth (paraquat), 21 (47%) methionine (paracetamol). Conclusions: The availability of antidotes is variable. Fewer than 50% of hospitals had a cyanide antidote available within 30 minutes. Almost all A&Es and >half MIUs held at least one heavy metal antidote. Reference: 1. Pronczuk de Garbino, J.; Haines, J.A.; Jacobsen, D; et al. Evaluation of Antidotes: Activities of the International Programme on Chemical Safety. Clin. Toxicol. 1997, 35, 333–343.
163 QUESTIONNAIRE BEFORE AND AFTER ELABORATING GUIDELINES FOR THE USE AND STOCKING OF ANTIDOTES
Maia P, Pinho C, Martins H, Reis E, Vale L, Aragão I, Manuel A, Silva F, Barrosa MT, Lopes M
Hospital Geral de Santo António, Porto, Portugal
Objectives: The efficacy of antidotes may vary considerably and although their use may only be an adjunct to supportive care, their lack may trigger catastrophic consequences for the poisoned patient1,2. In order to evaluate on-site availability of selected antidotes (deferoxamine mesylate, digoxin immune Fab, European vipers antivenom, ethanol, hydroxocobalamin, naloxone hydrochloride, obidoxime and pyridoxine, to treat 1 seriously poisoned 70 kg patient) in all North Portugal hospitals that provide emergency department care, we performed a questionnaire before and after producing guidelines for the use and stocking of antidotes. Methods: A questionnaire was sent (1999) to the Pharmacy Department of these 31 hospitals, which afterwards received the handbook “Manual de Antídotos”, (elaborated by this Group of Toxicology). The same questionnaire was mailed one year after (2000) and both results were compared. Results: All questionnaires were completed and returned. Results are presented in Table 1. Conclusions: Potentially lifesaving antidotes to treat 1 seriously poisoned 70 kg patient were insufficiently stocked in all but 1 hospital. The stock of the selected antidotes increased from the first to second questionnaire, except for digoxin immune Fab and hydroxocobalamin. An improved and integrated management may solve this problem of insufficient stocking, specially in a group of selected hospitals.
References: 1. Dart, RC; et al. Insufficient Stocking of Poisoning Antidotes in Hospital Pharmacies. JAMA 1996, 276, 1508–1510. 2. Woolf, A.D.; Chrisantus, K. On-site Availability of Selected Antidotes: Results of a Survey of Massachusetts Hospitals. Am. J. Emerg. Med. 1997, 15, 62–66.
Table 1. Availability of Antidotes Sufficient to Treat 1 Seriously Poisoned 70 kg Patient (Abstract 163)
164 ANTIDOTE AVAILABILITY IN NORWAY
Solheim L,1 Andrew E,1 Jacobsen D1,2
1National Poisons Information Centre, Oslo, Norway. 2Medical Division, Ullevaal University Hospital, Oslo, Norway
Objective: Antidotes are therapeutic substances that are used primarily to counteract the toxic actions of poisonous agents1. They play an important role in the treatment of poisonings. Antidote availability is crucial for the poisoned patient, and it is important that hospitals keep antidotes in stock to treat these patients. In Norway there is no national antidote programme. The aim of the present study was to investigate the antidote availability in Norwegian hospitals. Methods: In October 2000 the National Poisons Information Centre (NPIC) sent questionnaires to all Norwegian hospitals treating poisoned patients asking which antidotes were kept in stock. Within 8 weeks 42 hospitals had replied. After a follow-up letter 63 (95%) hospitals replied. Hospitals were classified as local (42), central (19) and university (5) hospitals. Results: The results showed that few hospitals had what NPIC would consider an appropriate antidote supply (Table 1). There also seemed to be some inconsequence within hospitals as to which antidotes were kept in stock and which were not. Only 20% of university hospitals had all the necessary antidotes. 17% of the central hospitals and 37% of the local hospitals had the recommended antidotes. N-acetylcystein was the only antidote present in all hospitals. Ethanol was present in all university hospitals, 94% of central hospitals and in 92% of local hospitals. Flumazenil and naloxone were not present in all hospitals. Conclusions: This study demonstrated that the antidote supply in Norwegian hospitals (all public) were in part insufficient and lacked consistency. This was also recognized by those responsible for the antidote supplies in the different hospitals, who asked for national guidelines. Such tentative guidelines were made by the NPIC2. Geography, nearby industry and cooperation among nearby hospitals where taken into account in making these guidelines—especially regarding expensive antidotes that are rarely used (Table 2). Focus was also put on the relative efficacy of antidotes (Fig. 1). As such, this study hopefully will result in a better supply of antidotes in Norwegian hospitals based on the guidelines from the NPIC. Follow-up studies are planned.
References: 1. Haines, J.A.; Jacobsen, D.; Meredith, T.; Pronczuk de Garbino, J. International Programme on Chemical Safety—Antidotes Project. Clin. Toxicol. 1997, 35, 125–126. 2. Solheim, L.; Andrew, E.; Jacobsen, D. Tidsskr. Nor. Laegeforen. 2001, in press.
165 SURVEY ON THE AVAILABILITY OF CYANIDE ANTIDOTES IN INDUSTRIAL SETTINGS
Smet H, Daeleman W, Mostin M
Poisons Centre, Brussels, Belgium
Objective: The aim of this study was 1) to assess the nature of safety equipment and antidotes available in industrial settings using cyanides; 2) to identify those hospitals where poisoned patients will be transferred and their current antidotal treatment. Methods: A structured questionnaire was mailed to 101 factories notified to the Federal Ministry of Labour and Employment for the use of cyanides and to 42 reference hospitals. Results: 72 (71%) factories returned the questionnaire, 11 of which specified that they no longer use cyanides; the remaining 61 factories were further studied. This survey revealed 52 (85%) factories having first aid equipment available and 47 (77%) keeping 1 or more antidotes; 14 (22.9%) factories keep no antidote at all. The methemoglobinemia inducing agents are frequently chosen with a marked preference for 4-DMAP (44.2%); chelation on cobalt is represented with hydroxocobalamin (18%) and dicobalt edetate (8%). Amyl nitrite (29.5%) and sodium thiosulphate (47.5%) are widely available while sodium nitrite (1.64%) has lost its place in therapy. Furthermore 42 (100%) reference hospitals returned their questionnaire and that revealed 78.6% hospitals keep one or more antidotes while 21.4% of them keep no specific treatment at all. Both methemoglobinemia inducers with 4-DMAP (33%) and sodium nitrite (14%) and chelation on cobalt with hydroxocobalamin (38%) and dicobalt edetate (11.9%) are present. It is important to note that the availability of hydroxocobalamin has increased from 1.5% in 1990 to 38%. Conclusion: From this survey it appears that 1) the number of industrial settings using cyanides has decreased by >50% over the last decade; 2) in order to set out optimum guidelines for the therapeutic approach one should promote the collaboration between the occupational physicians and the emergency department; 3) the participation for first aid training sessions in industry should be encouraged; 4) there is a need for information on recent trends in treatment protocols and 5) pharmacists report difficulties obtaining antidotes that must be purchased outside the country.
166 MERCURY POLLUTION IN DENTIST'S WORK-PLACES
Schach-Boos V,1,2 Jahanbakht S,1,2 Livardjani F,1 Flesch F,1 Jaeger A,3 Haïkel Y4
1Centre Antipoison, Hôpital Civil, Strasbourg, 2AD Scientifique, Strasbourg, 3Réanimation Médicale, Hôpital de Hautepierre, Strasbourg, 4Faculté de Chirurgie Dentaire, HUS, Strasbourg, France
Objectives: Dentists and their assistants may be occupationally exposed to mercury by using mercury amalgams. Exposure may occur during dental work with mercury amalgams but also continuously by inhalation of mercury contaminated air. The aim of the study was to evaluate the mercury air pollution in dentist's work-place and to identify the potential sources of mercury release. Methods: Mercury concentrations in the air were measured directly with a portable device using the cold vapor atomic absorption spectrophotometry technique. Analyses were performed at the dental faculty in a work-place including 40 dentist's working stations. Results: Mercury concentrations in the air increased progressively from 10 to 18 μg/m3 during the week. At different working stations three critical sources of potential continuous mercury vapor release were identified: the mercury concentrations ranged from 0.10 to 110.10 μg/m3 around the separators, used 8 months, from 0.80 to 2925.10 μg/m3 in the suction tubes and from 92.5 to 1204.3 μg/m3 in the waste containers. Measures performed from June to September 2001 showed an increase of these concentrations, especially in the suction tubes, but high mercury concentrations were also measured around the separators. Conclusion: In France, salvage of mercury wastes in dentists' work-places is mandatory since April 2001 in order to decrease the release of mercury in the environment (water). Separators are used for this purpose. However, our study shows that the separators used at the present time are not completely tight to mercury vapors which may be responsible for a potential increase of the occupational exposure of dentists to mercury.
167 ACCIDENTAL REPEATED HUMAN EXPOSURE TO ORGANOCHLORINE COMPOUNDS: CORRELATION OF BIOLOGICAL INDICATORS WITH CLINICAL SURVEILLANCE
Santos APM,1 Lopes MF,2 Batoréu MC2
1Toxicology Laboratory, Faculty of Pharmacy of Lisbon, Lisboa, Portugal. 2Centro de Saúde de Sintra, Sintra, Portugal
Introduction and Objectives: The neurotoxic effects due to an acute intoxication by organochlorine compounds (OCC) such as OC pesticides, is very well known. However, it is quite difficult to recognize the clinical effects of repeated exposure to low or medium concentrations of those compounds. The repeated exposure to those persistent compounds, may interfere specially with the nervous, endocrine and reproductive system and may decrease the blood cell count. The aim of this study is to correlate the changes in biomarkers of exposure and effect with the clinical dysfunctions in workers accidentally exposed to OC pesticides. Case Report: We studied a group of persons (14) working in a administrative service in an old building, where some years ago the floor was treated with OC pesticides with subsequent application of other pesticides in the conservation of the documents archived. Most of the workers have developed delayed toxic symptoms such as chronic fatigue, weight loss, dermal reactions, and one pregnant worker has possible fetus toxicity. In this study, we investigated the correlation between the OCC exposure and the clinical symptoms observed, determining the white and red blood cells, the platelets, thyroid hormones and the concentrations of some OC pesticides in serum. Conclusions: The preliminary results indicate that, in all the workers studied, there was a significant increase of OC pesticides concentration in serum, when compared with the control values. Concerning the OC pesticides identified and quantified in serum, we observed the following distribution: 85% of the cases for DDE, 57% for DDT and 50% for lindane. In 79% of the workers studied the hematological (Hb, RBC, leucocits and platelets) and/or endocrine parameters (free T3 and T4) were altered. In a first approach, it is possible to correlate the decreasing of Hb, leucocytes, platelets, free T3 and T4 thyroid hormones with higher concentrations of OC pesticides in serum. Finally, we may suggest these biological indicators as a complementary tool for the diagnosis of low repeated exposure to OCC.
References: 1. Keith, L.H. Environmental Endocrine Disruptors; Wiley: New York, 1997. 2. Environ. Health Perspect. 1999, 107 (Suppl. 4), 605–611. 3. Annu. Rev. Public Health 1997, 18, 211–244. 4. J. Chromatogr. B 1998, 716, 147–152.
168 IS HEART DONATION POSSIBLE AFTER FATAL METHANOL POISONING?
Hantson Ph,1 Moniotte S,2 Moulin P,3 Ruyffelaere P,4 Gillard L,4 Wittebole X1
1Department of Intensive Care Medicine, 2Laboratory of Pharmacotherapy, 3Department of Pathology, Cliniques St-Luc, Université Catholique de Louvain, Brussels, Belgium. 4CH de la Basse Sambre, Auvelais, Belgium
Background: A significant experience exists with kidney transplantation with grafts obtained from methanol poisoned donors, but data concerning other organs are more limited.1 The possibility of heart donation after evidence of brain death following methanol poisoning is a matter of concern. Case Studies: In the two last consecutive patients admitted with severe methanol poisoning leading to fatality, we considered kidney and liver donation. As for the heart, we decided not to transplant the organ but to investigate postmortem pathological changes. The first donor (D1), a 46-year-old man with a history of chronic ethanol abuse, was admitted comatose to the hospital, probably more than 12 hours after the ingestion of an unknown amount of methanol. The toxicological analysis revealed an initial serum level of 320 mg/dl. A profound metabolic acidosis was observed, with an arterial blood pH of 6.79, and total bicarbonate (CO2) 5 mmol/l. Hemodynamic conditions were stable. The admission electrocardiogram (ECG) did not reveal any abnormality. By the following day, the absence of brainstem reflexes was noted and the patient was declared brain dead. Metabolic acidosis was corrected at this time. The diuresis was maintained and serum creatinine level was 53 μmol/l. The kidneys were transplanted with success. The liver was not considered (macrovacuolar steatosis). The second donor (D2), a 41-year-old man, had presented a cardiocirculatory arrest (10 minutes duration) soon after admission. The abnormal ECG findings were related to hyperkalemia. The left ventricle contractility was diffusely depressed at echocardiography and catecholamines were prescribed. A mild elevation of cardiac enzymes was noted. The methanol serum level was 95 mg/dl, with arterial pH 7.0 and total CO2 8 mmol/l. Brain death was diagnosed 24 hours later. Only the kidneys were successfully used for transplantation. In the two cases, fresh frozen sections performed on a myocardial sample did not display any significant lesion. In a third observation of moderate methanol poisoning, with favorable outcome, abnormal ECG and echocardiography findings consistent with anterior wall myocardial infarction were noted; coronary angiography did not reveal any lesion and the relationship with methanol poisoning was doubtful. Discussion: The heart is not a target organ in methanol poisoning. The experience of other transplant teams suggests that the heart may be used without unacceptable risks. Important inclusion criteria should be: absence of cardiac arrest, normal ECG and echocardiography, no need for inotropic support. Reference: 1. Hantson, Ph.; Vanormelingen, P.; Squifflet, J.P.; Lerut, J.; Mahieu, P. Methanol Poisoning and Organ Transplantation. Transplantation 1999, 68, 165–166.
169 TRANSPLANTATION AFTER ACUTE POISONING: RE-USE OF A LIVER TRANSPLANTED TO A POISONED PATIENT FROM A POISONED DONOR
Hantson Ph,1 Vanormelingen P,2 Laterre PF,1 Wittebole X,1 Lerut J3
1Department of Intensive Care Medicine, 2Transplant Coordination, 3Liver Transplant Program, Cliniques St-Luc, Université Catholique de Louvain, Brussels, Belgium
Background: The re-use of a graft after acute failure (unrelated to graft function) in the recipient is poorly documented. A successful case of early liver graft re-use 48 hours after the first transplantation is reported, with the particularity that the poisoned recipient had received the liver from a poisoned donor. Case Report: A 47-year-old man was admitted for unexplained coma. The relevant biological data were: bilirubin 169.3 μmol/l (<17.1), ASAT 4565 IU/l (<37), ALAT 4398 IU/l (<41), INR 6.9 (0.9–1.3), creatinine 258.1 μmol/l (53–123.8). Toxicological analysis revealed that acetaminophen serum concentration on admission was 18 μg/ml. The exact amount ingested and the time of poisoning remained unknown. Encephalopathy grade IV with mydriasis was noted, but with well structured grade III evoked potentials. Urgent liver transplantation was considered. A liver originating from a 15-year-old boy who died following suicidal insulin intoxication was used. Early postoperative period was uneventful but, unfortunately, the recipient was declared brain dead 29 hours later. This was the consequence of the pre-operative encephalopathy with brain edema. Considering the evolution of the liver tests and the results of echocardiography and coronary angiography, he was found eligible for liver and heart donation. Due to the progression of acute renal failure with anuria (creatinine 415.5 μmol/l), kidney donation was not considered. The heart was successfully transplanted in Germany. The liver was re-used in our center. The second liver recipient was a 53-year-old male suffering from postviral C cirrhosis complicated by a hepatocellular carcinoma. The re-used liver was again implanted using the cavo-caval technique. Post-operative recovery was also uneventful, and patient was discharged from the ICU on day 1. Outcome at 6 months is excellent for the heart and liver recipients. Discussion: There is no contraindication to organ procurement after insulin overdose with irreversible brain damage. We have a personal experience in kidney and liver donation, and other centers have also successfully used heart grafts. Also with acetaminophen, kidney, lung or heart donation has been previously reported.1 In the present case, the progression of acute renal failure was clearly a contraindication to kidney donation. Conclusion: To our knowledge, this is the first description of a transplantation with a liver graft coming from a poisoned donor that was implanted in an intoxicated patient and thereafter successfully re-used for transplantation. Reference: 1. Hantson, Ph.; Vekemans, M.C.; Laterre, P.F.; Vanormelingen, P.; Mahieu, P.; Koerner, M.M. Heart Donation After Fatal Acetaminophen Poisoning. J. Toxicol. Clin. Toxicol. 1997, 35, 325–326.
170 SUCCESSFUL MULTIORGAN TRANSPLANT AFTER DONORS DEATH CAUSED BY SUICIDAL METHANOL INGESTION
Radam M,1 Hamza A,2 Landgraf B,2 Fornara P2
1Poisons Information Centre Erfurt, Erfurt, Germany; 2Department of Urology, University Hospital at the Martin-Luther-University Halle-Wittenberg, Halle, Germany
Objectives: “Brain death” after accidental or suicidal intoxication is not so rare. Different toxins have specific organ toxicity. After a potential donor's death caused by poisoning, it is possible to remove and transplant organs without a higher risk of rejection. The specific toxicity of methanol is primarily due to central nervous effects and metabolic disorders. If the metabolic acidosis can be treated successfully, resulting organ damages are rare and the prognosis for an organ function without complications after transplant is good. Case Report: Procuring hospital: Cliniques Universitaires St. Luc, Bruxelles Belgium; Donor: female, 39-year-old, brain death after suicidal methanol intoxication; initial serum methanol concentration 0.44 mg/dL; treatment of the methanol intoxication with hemodialysis and ethanol therapy; initial blood gas analysis: pH 6.6; base deficit 36.4 mmol/L. After substitution of 600 mL sodium bicarbonate, the acid–base disorder was corrected and function of liver and kidneys was preserved. Recipients: Left kidney: successful transplant at Transplantation Centre of Martin-Luther-University Halle-Wittenberg, Germany; male, 40-year-old; hemodialysis since 6 years due to chronic glomerulonephritis, lost organ function 5 years after first kidney transplant because of chronic rejection. Result: good function to present. Right Kidney: successful transplant at Cliniques Universitaires St. Luc, Bruxelles Belgium; no detailed information about the recipient available; good function to present (telephone information from Eurotransplant). Liver: successful transplant at Hospitalier Universitaire Liege, Belgium; no detailed information about the recipient available; good function without complications until present (telephone information from Eurotransplant). Heart: provided for transplant of valves (no report). Conclusion: Death involving toxins does not seem to be a contraindication for donation of liver and kidney for transplant. Regional Poisons Control Centers can assist clinicians and organ procurement organizations in the appropriateness of toxic patients as organ donors. Organ donation from fatal methanol poisoning victims has been reported to be successful, with no long-term sequelae as a consequence of methanol poisoning. Organs reported for transplant included lungs, kidneys, liver and heart (1,2).
References: 1. Evrard, P.; Hantson, P.; Ferrant, E.; et al. Successful Double Lung Transplantation with a Graft Obtained from a Methanol-Poisoned Donor. Chest 1999, 115, 1458–1459. 2. Toll, L.L., Hurlbut, K.M., Eds. POISINDEX® System; MICROMEDEX, Inc.: Greenwood Village, CO (Vol. 110 expires 12/2001).
171 RECREATIONAL USE OF JIMSON WEED (DATURA STRAMONIUM): TEN YEARS EXPERIENCE OF THE “DRUG DEPENDENCE EVALUATION AND INFORMATION CENTRE” (DDEIC) OF MARSEILLES
Arditti J, Spadari M, De Haro L, Hayek-Lanthois M, Valli M
Drug Dependence Evaluation and Information Centre—Poison Centre of Marseilles, Hôpital Salvator, Marseilles, France
Objective: Jimson weed is an anticholinergic alkaloid-containing plant which can be used recreationally for hallucinations and delirium. In order to evaluate the importance of such voluntary poisonings, cases involving recreational use of Datura stramonium were observed in the Drug Dependence Evaluation and Information Centre of Marseilles (DDEIC—depending of the French Health Products Safety Agency and located in the Poison Centre) for 10 years (1992–2001). Case Series: 55 cases were collected, 84% men and 16% women. The average age was 22 years (13 years–53 years). Different parts of the plant were used: leaf infusions (25 cases), ingestion of seeds (14 cases), ingestion of crude leaves (5 cases), ingestion of flowers (3 cases), smoked leaves (1 case), smoked seeds (1 case), and smoked blending of several parts (5 cases). The Jimson weed was used alone for 47 cases, and with other products for 8 cases (cannabis, alcohol, ecstasy, etc.). The authors note that there was increased use of Datura during rave parties. 19 cases were collective poisonings. 5 of the 19 collective cases happened in agronomists colleges where the students had just learned the different species of toxic plants. The most frequent symptoms were hallucinations, delirium, mydriasis, tachycardia, agitation, and dry mouth. Two patients died. A 21-year-old female drowned after drinking leaf infusion as she thought she was able to walk on the sea “like Jesus.” A 30-year-old male killed himself with a gun after behaviour disturbances induced by a leaf infusion. For the first lethal mentioned case, anticholinergic alkaloids were found in blood samples with gas chromatography/mass spectrometry (GC/MS) techniques. All other patients recovered with symptomatic treatments. Conclusion: Jimson weed is a rather common plant in southern France. As some teenagers or young adults discover that they can experience hallucinations with this plant, they may try it. This study raises doubts about the public education on easily available products such as Jimson weed.
172 FEW SYMPTOMS REPORTED FOLLOWING PLANT EXPOSURE IN IRELAND
Donohoe E, Tracey JA,
National Poisons Information Centre, Beaumont Hospital, Dublin, Ireland
Objective: To examine the severity of symptoms reported to the Poisons Information Centre by patients who are exposed to plants. Methods: We retrospectively examined all plant enquiries received by the Poisons Centre in Dublin between January 1997 and December 2000. For the purpose of this study we eliminated all animal or “information only” enquiries. We identified the plants using callers knowledge, descriptions of plant parts, and location of the plant. We graded the patients' symptoms at the time of the call into minor, moderate or severe using the IPCS/EC/EAPCCT severity score. Results: 564 enquiries were received involving 502 patients. The majority (74%) of these were children aged 10 years or younger. 154 different plant species were involved. The most common agents were (1) unknown berries, (2) Giant Hogweed, (3) Arum maculatum, (4) Holly berries, (5) Cotoneaster. Exposure to unknown berries is also common in American Poison Control Centres and possibly reflects the difficulty in identification by verbal description2. There were no fatalities in our study and 78% of patients were asymptomatic. 20% of patients developed minor symptoms. 7 patients developed moderate symptoms and required admission to hospital for observation. One patient developed severe symptoms. Case Study: A 48 year old male developed vomiting, generalized seizures and coma after eating what he thought were chervil roots. He received 50 g AC in casualty and was admitted to ICU where he was self-ventilating. He remained quite agitated for 12 hours and was given iv diazepam. He had no further seizures. His ECG showed sinus rhythm with occasional atrial ectopics. His CK level rose from 998 to 40,960. His creatinine rose from 132 to 280 but he maintained a urine output. His AST and ALT peaked at 549 and 147 respectively on day 2. The plant was identified as hemlock (Conium maculatum) based on description and symptoms, but no plant material was available for accurate identification. Hemlock is a toxic plant containing the alkaloid coniine. There is no known antidote. The patient required supportive care in ICU for 48 hours. He made a full clinical recovery and took his own discharge against medical advice. Conclusion: The Poisons Centre in Dublin received 564 plant related enquiries between 1997 and 2000. 78% of patients remained asymptomatic after exposure. 8 patients required admission to hospital and 1 of these patients was treated in ICU for Hemlock toxicity.
References: 1. Krenzelok, E.P.; Jacobsen, T.D.; Aronis, J. Those Pesky Berries…Are They a Source of Concern? Vet. Hum. Toxicol. 1998, 40, 101–103. 2. Lawrence, R.A. Poison Centers and Plants: More Pollyanna Data? J. Toxicol. Clin. Toxicol. 1998, 36, 225–226.
173 ANGEL TRUMPET (BRUGMANSIA SPP.) POISONING: CLINICAL EFFECTS AND EPIDEMIOLOGY
Isbister GK,1 Dawson AH,1,2 Whyte IM1,2
1Discipline of Clinical Pharmacology, University of Newcastle, Australia; 2Department of Clinical Toxicology, Newcastle Mater Hospital, Australia
Objective: Anticholinergic plant poisoning is an uncommon poisoning and can be difficult to diagnose and treat. Our objective was to determine the epidemiology and clinical features of anticholinergic plant poisoning, to characterise the time course and pattern of clinical features and to identify important diagnostic features and severity measures. Methods: Consecutive cases of anticholinergic poisoning to the Hunter Area Toxicology Service (HATS) were identified by searching a database of all presentations between July 1990 and June 2000. Patient demographics, details of poisoning, diagnostic clinical features, adverse effects including seizures, arrhythmias, hypotension, accidental injuries, and treatments were recorded. Results: 33 patients ingested Brugmansia spp. (Angel trumpet); median age 18 years (I.Q. range 16 to 20); 27 of 33 were males (82%). 31 ingested a brewed tea or parts of the plant and 31 used it recreationally. Five patients were brought in by police for wandering and causing a disturbance. Cases were clustered, with no increase in incidence over the 10 year period. Common clinical features were: mydriasis (100%), with a mean duration of 29 hr (SD 13) and delirium (88%) with a mean duration of 18 hrs (SD 12). Tachycardia was uncommon (33%) at presentation, but in those with tachycardia an exponential decay of heart rate occurred over 4–6 hrs. Other clinical features were typical of anticholinergic effects, including dry mouth, flushed skin, reduced bowel sounds and hallucinations. There were no deaths, seizures or arrhythmias in the study. One patient had hypotension and two sustained accidental traumatic injuries. 19 patients required sedation, mainly with benzodiazepines. Physostigmine was used diagnostically in 7 cases. Mean length of hospital stay was 21 hours (SD 12 hrs). Conclusions: Anticholinergic plant abuse is sporadic in nature. Most cases were moderate in severity, requiring sedation only, and severe toxicity was rare. Diagnostic clinical findings were mydriasis and delirium, the later having important implications for management.
174 DIAGNOSIS WANDERING IN A CASE OF ACCIDENTAL POISONING BY COLCHICUM AUTUMNALE
Flesch F, Krencker E, Mootien Y, Kintz P, Ihadadène N, Jaeger A
Centre Anti-Poison, Hôpitaux Universitaires, Strasbourg, France
Objectives: Colchicine poisonings are mostly due to the ingestion of colchicine drugs. Poisonings by colchicine-containing plants are reported. We report a case of Colchicum autumnale poisoning which, without the obstinacy of the poison centre, would not have been diagnosed. Case Report: A 68-year-old women developed vomiting and diarrhea 5 hours after ingestion of a tart containing a wild plant, supposedly Allium ursium, which she had cooked according to a recipe recently demonstrated on a TV show. The next day, due to worsening of symptoms, her doctor contacted the poison centre who suggested a possible colchicine poisoning. The patient was admitted 35 hours after the ingestion to an emergency department but was transferred to a surgical unit for probable intestinal ischemia which was not confirmed. Because of biological abnormalities (thrombopenia, increased hepatic enzymes) the patient was transferred to a medical unit. Because of the suspicion of colchicine poisoning, the poison centre contacted the family who finally brought in the plant which was identified as Colchicum autumnale. The patient was transferred to the ICU on day 4. On admission, the patient had vomiting, diarrhea, hypotension and fever. Biological abnormalities were consistent with colchicine poisoning: thrombopenia (8,000/mm3 on day 5), leucopenia (1270/mm3 on day 6), hepatic cytolysis (ASAT 284 IU/L, ALAT 53 IU/L), CPK 1058 UI/L. Other abnormalities included hypokalemia, hyperglycemia, T wave inversion on ECG and β hemolytic streptococcus septicemia (day 5). On day 4, blood colchicine was 2.2 ug/L. Treatment included rehydration, correction of hypokalemia, hypoglycemic agent and antibiotics. On day 5 the patient developed alopecia. Diarrhea lasted for 15 days, alopecia for 15 days and hyperglycemia for 2 months. Total recovery was observed after 2 months. Conclusion: Accidental poisonings by colchicine containing plants (Colchicum autumnale, Gloriosa superba) are due to mistakenly eating wild plants such as Allium ursium. This case shows the role of the poison centre in making a correct diagnosis where symptoms are nonspecific.
175 COLCHICINE POISONING: INGESTION OF COLCHICUM AUTUMNALE FLOWERS
Zoelen GA van,1 Vries I de,1,2 Meulenbelt J1,2
1National Poisons Control Centre, National Institute for Public Health and the Environment, Bilthoven; 2Department of Intensive Care and Clinical Toxicology, University Medical Center, Utrecht, The Netherlands
Background: Poisonings with the plant Autumn Crocus (Colchicum autumnale) are rarely reported in literature. All parts of Colchicum autumnale contain the alkaloid colchicine. Flowers contain approximately 0.1–0.6% colchicine. The fatal ingestion of Colchicum autumnale flowers has to our knowledge only once been described. In that case, death was attributed to the ingestion of 12 flowers, whereas ingestion of 40 flowers has been survived.1,2 Colchicine is used as medication against gout. It has an antimitotic effect, which can cause the arrest of cell division in the metaphase stage. Most relevant symptoms observed after colchicine exposure in the first stage are severe gastric-intestinal symptoms. The second stage, 24–72 hours post ingestion, is characterised by multi-organ failure. If a patient survives this stage recovery can be accompanied by leukocytosis, alopecia and fever. The onset of symptoms is usually within 2–12 hours. Gastric lavage may only be useful within one hour post ingestion. Multiple dose activated charcoal may be considered because colchicine is believed to undergo an enterohepatic recirculation. Colchicine specific Fab fragments have been experimentally used in the treatment of colchicine poisoning but are not commercially available in the Netherlands.3 Therefore, treatment is mainly symptomatic (e.g. monitoring of vital signs, blood, electrolytes, fluid, and renal and liver function). Case Report: A 21-year-old male ingested 10 flowers of Colchicum autumnale to experience whether these plant parts would produce illegal drug-like effects. The patient developed severe gastric-intestinal symptoms (severe vomiting and stomach pain). After two days, the patient confessed to a physician that he had ingested 10 Colchicum autumnale flowers. About 52 hours after ingestion the Poisons Control Centre was consulted and it advised sending the patient to a hospital. On admission to a general hospital, about 54 hours after ingestion, the patient was tachypnoeic, severely dehydrated, and had a blood pressure of 80/60 mm Hg and a pulse rate of 110/minute. Intravenous fluid was given and symptomatic treatment started. The patient was transferred to an intensive care because he developed respiratory insufficiency, cyanosis, renal insufficiency and anuria. About 64 hours post ingestion the patient was referred to our hospital for further treatment. The patient died while being transported. Death was due to multi-organ failure (metabolic acidosis, renal failure, diffuse intravasal clotting, and ventricular tachycardia resulting in cardiac arrest) comparable to the effects which might be caused by severe colchicine intoxication. Unfortunately, no colchicine concentration was determined before transportation. No material was left to determine the colchicine concentration afterwards. Conclusion: Ingestion of Colchicum autumnale can result in life-threatening clinical effects. After ingestion, toxicological analysis is needed because in an early stage treatment with colchicine Fab fragments may be essential for a good prognosis.
References: 1. Ellwood, M.G.; Robb, G.H. Self-Poisoning with Colchicine. Postgrad. Med. J. 1971, 4, 245–247. 2. Danel, V.C.; Wiart, J.F.; Hardy, G.A.; Vincent, F.H.; Houdret, N.M. Self-Poisoning with Colchicum autumnale L. Flowers. J. Toxicol. Clin. Toxicol. 2001, 39, 409–411. 3. Baud, F.J.; Sabouraud, A.; Vicaut, E.; et al. Brief Report: Treatment of Severe Colchicine Overdose with Colchicine-Specific Fab Fragments. N. Engl. J. Med. 1995, 332, 642–645.
176 PLANNING POISON CONTROL CENTRE ACTIVITIES ACCORDING TO THE TYPE OF CONSULTS: SCORPION AND SCOLOPENDRA ENVENOMATIONS AS A MODEL
Ballesteros S, Ramón MF, Martínez-Arrieta R, Cabrera J
Spanish Poison Control Centre, Instituto Nacional de Toxicología, Madrid, Spain
Objective: Proper planning of a PCC includes preventive measures, campaigns and review of protocols. The purpose of this investigation was to understand health care professional and public needs through an epidemiological analysis of animal envenomations. Scorpions and scolopendras were chosen because they were frequently involved in cases in our service and they resemble each other in their habitats and envenomation clinical features. Methodology: A retrospective review of documented cases of animal contact from 1991 to 2000 was performed. Data including patient age, clinical presentation, prognosis, geographic origin, and timetable of the exposures were recorded. Results: A total of 1,700 cases of animal envenomation were registered during the study period. Scorpions and scolopendras represented 41.4%, snakes 18%, marine animals 12.3% and hymenoptera 7.5%. Therefore we chose the first group of animals to be analysed. Cases of envenomation with scorpions and scolopendras appeared consistently over the 10 years increasing in May and June with peaks in July and August. Most consults were made between 2 and 10 pm (56.6%) and Saturday had the highest number of calls. Consulting personnel were general practitioners and hospitals (44.8 and 30.7% respectively) whereas the general report of our PCC shows 60% of consults from the public. Centre and South regions of our country, which are the driest, had the highest percentage of cases. At the time of the consult minor local symptoms were present in 71.2% of cases, important local signs or local plus mild systemic symptoms in 5.8%, moderate systemic symptoms in 1.1% and life-threatening symptoms in 4 cases. The rest had no symptoms or could not be determined. Children less than 14 years composed 21.5% of the victims and 3.7% were less than 2 years old. There were 3 cases of stings due to imported scorpions. Treatment previously applied consisted of local cold, corticosteroids and antihistamines. However there were cases of erroneous or useless treatment such as tourniquet (9), incisions (4), prophylactic antibiotics (2), vinegar or ammonia (4 cases). Conclusions: Data collected by PCC might give clues to temporal increases of particular types of poisoning. Animal envenomation is highly geographic and seasonally dependent. Due to the proximity of our country to Africa, it is important to know possible migrations of animals. Call volume peaked in summer when scorpions and scolopendras are usually active and consults were mainly made by sanitary staff. This study enables PCCs to time campaigns properly, to avoid erroneous therapeutic measures and to review clinical protocols.
177 UNUSUAL LOCAL COMPLICATIONS OF VIPERA XANTHINA PALAESTINAE BITE
Bentur Y, Cahana A
Israel Poison Information Center, Rambam Medical Center, Faculty of Medicine, Technion, Haifa, Israel
Background: Vipera xanthina palaestinae (VXP) is the commonest venomous snake in Israel. The main clinical manifestations of the envenomation include fang marks, swelling, ecchymosis, bullae, gastrointestinal signs, hypotension, allergic reaction, thrombocytopenia and DIC. VXP-specific antivenin is indicated for both systemic effects and marked progressive local signs. In our experience, clinicians are often not aware of the morbidity associated with the local effect of the venom and consequently do not administer the antivenin in envenomations with local effects only. Objective: To describe unusual local complications of VXP envenomation. Case Series: 1) An 8-year old male suffered abdominal pain, vomiting and diarrhea after experiencing a sharp pain in his foot. VPX bite was suspected when swelling, ecchymosis and bullae appeared. At 48 hours a tense and tender edema involved the entire leg, groin, scrotum and penis, resulting in urine retention and necessitating urinary catheter. At this stage VXP antivenin was administered with gradual improvement. 2) A 16-year old male was bitten on his palm by VXP. Swelling, ecchymosis and bullae progressed to his arm. Antivenin was offered but the patient refused, claiming that he was immunized by several previous VXP bites. After 36 hours, tender and tense edema involved both shoulders, chest, neck and nape and he complained of dysphagia. Only at this stage was patient approval obtained for antivenin administration. 3) A male child 2 years and 3 months old was bitten by VXP on his mid-arm and was treated with antivenin. Thirty-six hours after envenomation the swelling progressed and involved the shoulder and the neck. Additional dose of VXP antivenin was given, followed by regression of the swelling. Conclusion: Inadequately treated swelling caused by VXP envenomation may involve the trunk even when the site of the bite is remote. In some cases this may compromise the function of vital structures such as the upper airways, call for unnecessary interventions and prolong hospitalization. It is recommended that VXP antivenin be administered whenever there is marked and progressive swelling even in the absence of systemic signs.
178 DEFIBRINATION SYNDROME AFTER VENOMOUS SNAKEBITE
Verhelst D,1 Hermans C,2 Dumont C,3 El Gariani A,1 Goossens E,4 Wittebole X,1 Hantson Ph.1
1Department of Intensive Care and Emergency Medicine, 2Laboratory of Hemostasis, 3Department of Internal Medicine, Cliniques St-Luc, Université Catholique de Louvain, Brussels, Belgium. 4Poisons Control Center, Brussels, Belgium
Background: In Europe, snake envenomation is infrequent, except in Mediterranean countries, and the treatment of patients coming back from Africa with a snakebite is complicated by the difficulty of snake identification and by the poor availability of specific antivenom. Case Report: A 55-year-old patient was referred for hemorrhage following a snakebite. Five days before, in Burkina Faso, he had been bitten on the left foot by a snake that could not be immediately identified. He immediately developed localized edema, and a few hours later, hematoma on the thorax and abdomen. On admission in a local hospital, biological analysis revealed a severe anemia (hemoglobin 6.6 g/dl). Abdominal echography showed major hematomas in the abdominal wall, left psoas and bladder. The day after, because of abdominal pain, he was transferred to a second hospital. He was perfectly conscious; arterial blood pressure was 80/60 mm Hg, pulse rate 112/min and temperature 38°C. A diffuse edema of the left thigh and leg was noted. Biology revealed a severe anemia with hemoglobin 3.4 g/dl and platelet count 118.000/mm3. Coagulation disorders were observed with INR>7 and fibrinogen 49 mg/dl. The patient received two units of red packed cells. On day five, he was transferred to our hospital. Multiple hematoma were observed on the thorax and abdomen and also diffuse bleeding at puncture sites. Biological data were: hemoglobin 5.3 g/dl, white blood cell count 26.570/mm3, platelet count 158.000/mm3, INR>7, fibrinogen<50 mg/dl, APTT>120 sec, D-dimers 32,000 ng/ml. The thorax and abdomen computed tomography revealed a major hematoma of the left psoas and a left pleural effusion. The patient received red packed cells, fresh frozen plasma and fibrinogen without any clinical and biological improvement. Two vials of trivalent serum against Echis-Bitis-Naja (Pasteur®) were obtained from the zoo and were administered within one hour. Tolerance was excellent and less than two hours after the end of the infusion, no more bleeding was observed with a drastic improvement in clotting disorders. The snake belonged most likely to the Echis species. Conclusion: Coagulopathy is commonly encountered in victims of African Viperidae. Blood products transfusions may be ineffective as long as the venom is circulating. As in the present case, a reliable species identification is sometimes difficult to obtain and the decision to use specific antivenom was guided by the severity of clinical symptoms and by the likelihood of an Echis snakebite. Even used with delay, the treatment was immediately effective and well tolerated.
179 PROSPECTIVE STUDY OF THE CLINICAL EFFECTS OF EXPOSURE TO THE WHITE-STEMMED GUM MOTH (CHELEPTERYX COLLESI)
Balit CR,1 Geary MJ,2 Russell RC,2,3 Isbister GK4
1New South Wales Poisons Information Centre. 2Department of Medical Entomology, ICPMR, Westmead Hospita. 3University of Sydney. 4Discipline of Clinical Pharmacology, University of Newcastle, Australia
Aims: To examine the clinical effects of injuries by the caterpillar or cocoon of the white-stemmed gum moth (Chelepteryx collesi). Methods: A prospective observational study of caterpillar, centipede and millipede exposures was conducted through the NSW Poisons Information Centre. Subjects were interviewed over the phone either at the time of the call or within 24 hours, and caterpillars and cocoons were forwarded to the investigators for identification. Subjects were followed up by phone until all clinical effects had resolved. Results: From 107 caterpillar exposures, 5 had confirmed contact with the caterpillar of C. collesi (all children aged 1–11) and 5 had confirmed contact with the cocoon of C. collesi. Another 7 subjects had caterpillar exposures consistent with C. collesi, but no caterpillar was caught. All cases occurred in the summer months. Of 10 confirmed exposures there was no difference between caterpillars and cocoons, so these 10 were considered together. The affected area was the hands (5), feet (4) or both (1), following C. collesi being picked up or trodden on. 9 had pain, none had severe pain and in 8 cases pain lasted <60 minutes. 6 subjects had more than 100 spines embedded that appeared as small black dots. In 2 cases each spine was surrounded by swelling and yellow discolouration. Despite the removal of multiple spines, they remained embedded for over 2 months in a number of cases, but caused no problems. Conclusion: C. collesi exposure is common in summer. Overall the effects were minor with mainly pain, and although spines remained in all cases, they caused no problems. Complete removal of spines is neither possible nor necessary.
180 FEATURES AND MANAGEMENT OF SCORPION (CENTRUROIDES EXILICAUDA) ENVENOMATIONS IN THE AMERICAN SOUTHWEST
Wax P
Good Samaritan Regional Medical Center, Phoenix, Arizona, USA
Introduction: Of the 40 scorpion species found in the United States, envenomation by Centruroides exilicauda (also known as the bark scorpion because of its predilection of residing in or near trees) is clearly the most medically consequential. This species is native to the American Southwest. Similar to other scorpions, Centruroides are characterized by seven sets of paired appendages (4 sets of legs, the chelicerae, the pedipalps (claws) and the pectines) and a dorsally curved tail known as the telson. It is the telson that contains paired venom glands and the stinger. Scorpions envenomate by stinging not biting. Centruroides exilicauda measures about 5 cm in length and is characterized by a subaculear tubercle near the base of the stinger. Its venom contains a variety of neurotoxins that affect fast sodium channels resulting in the uncontrolled release of a variety of neurotransmitters (including acetylcholine, norepinephrine, dopamine, glutamate and GABA), excessive neuromuscular activity, and autonomic dysfunction. Since tissue destructive toxins are not a feature of Centruroides venom, local tissue effects are minimal. In 1997 more than 8700 calls were received by the Arizona Poison Control Network regarding possible scorpion stings. Although deaths, especially among young children, were not uncommonly attributed to Centruroides envenomation during the first half of the 20th century, more recently, scorpion fatalities are rarely reported. Symptoms: Characteristic of moderate to severe Centruroides envenomation is significant neuromuscular abnormalities without cardiopulmonary or hematological effects. Local inflammation is minimal and a puncture wound is usually not detectable. A four part grading system to aid in the treatment of Centruroides envenomation has been utilized since the mid 1980s. Grade I and II envenomations are limited to pain and paresthesias only with Grade I symptoms localized to the site of sting and Grade II defined by remote and local findings. A “tap test” over the sight of the sting site may greatly exacerbate the local pain. Grade III and IV envenomations are characterized by systemic symptoms manifested by cranial nerve dysfunction (involuntary roving dysconjugate eye movements, hypersalivation, dysphagia, upper airway difficulties, slurred speech) or skeletal neuromuscular dysfunction (extreme restlessness, fasciculations, choreiform movements, opisthotonus). The inability to lie still is characteristic of these neuromuscular abnormalities and is often associated with extreme discomfort (and inconsolable crying in children). Grade III consists of cranial nerve or skeletal muscle dysfunction while Grade IV is characterized by both abnormalities. Symptom onset occurs almost immediately after the sting with maximal intensity noted at about 5 hours although Grade IV effects may be seen as early as 15–30 minutes after stings in infants. Improvement without antivenin therapy usually occurs within 9–30 hours but paresthesias may persist up to 2 weeks. Children appear to have a higher frequency of more serious envenomations. In one large study 34% of children had Grade III or IV envenomations compared to 6% in adults. Management: Patients with Grade I or II envenomations who present for medical attention should be observed for several hours in order to evaluate for systemic progression and offered oral analgesics such as ibuprofen as needed. The treatment of choice for the Grade III or IV systemic Centruroides envenomations and the utility of antivenin remain controversial. Since airway compromise is usually the most critical problem that develops in severe envenomations, attention to maintaining an unobstructed airway including intubation as needed is required. The two different approaches to the treatment of severe Centruroides envenomation include the use of specific Centruroides antivenin or sedation, ICU admission and possible intubation. Antivenin proponents argue that the use of the antivenin results in the rapid reversal of systemic symptomatology often within 30 minutes, decreasing the need for hospitalization and avoiding the risks of oversedation. Many of these patients who receive the antivenin can be sent home from the emergency department after a short period of observation. Opponents of this approach argue that the goat-derived antivenin is associated with the unnecessary risk of both anaphylactic reactions and serum sickness. In lieu of antivenin, treatment of severe Centruroides envenomations requires intravenous sedation with a benzodiazepine or barbiturate often necessitating intubation and some times paralysis. Prospective controlled clinical trials are needed to better determine optimal management strategies.
181 MUSHROOM POISONING MANAGEMENT: COLLABORATION BETWEEN THE POISON CONTROL CENTRE AND THE LABORATORY
Ballesteros S, Iturralde MJ,1 Ramón MF, Martínez-Arrieta R, Cabrera J
Servicio de Información Toxicológica. 1Sección de Biología, Instituto Nacional de Toxicología, Madrid, Spain
Objective: The incidence of mushroom poisoning is increasing due to the rising popularity of mushroom foraging. Many edible mushrooms resemble poisonous ones and are impossible to differentiate by telephone descriptions, even by experts. Our objective was to evaluate the possibilities of collaboration between a Poison Control Centre (PCC) and the Section of Biology located in the same institution. Methodology: A retrospective review of cases of mushroom ingestion reported to our PCC by telephone during 10 years. Laboratory diagnosis of suspected mushroom poisoning was studied during the same time period. Mycological identification consisted of botanical classification with macroscopic and microscopic characteristics and searching of mushroom toxins (amanitins, muscarine, psylocine and psylocibine) in suspicious samples. Results: Of 355 mushrooms exposures, 75% of patients were already symptomatic at the time of the consult to the PCC. The incidence of pathology associated to mushrooms was: gastrointestinal 31%, hepatotoxic 24.5%, cholinergic 14.4%, neurologic 4.5%, hallucinogenic 4.5% and cardiovascular 1.4%. Laboratory results showed a similar percentage of syndromes in the 38 episodes of intoxication analyzed. Identification of samples corresponded to the clinical features, which were already present at the time of analysis. Misidentification or no identification at all by telephone was seen in 13.5% of cases. On the other hand mushroom poisoning in infants and children younger than 14 years differed. There were 51 consults (mean age 5 years, range 9 months–13 years). Fifty-one percent were asymptomatic at the moment of the consult and no data were provided in 41% of occasions. Ninety-four percent ingested non-cooked mushrooms. Geographic site of exposure were gardens, school lawns and wooded areas. Only 3 children ate cooked poisonous mushrooms with their family. No children were treated on site but all were referred to health care facilities. Nine different substrates of growth were given as an identification clue corresponding to mushrooms, which produce very different syndromes. Laboratory analysis of samples eaten raw showed non-toxic species in 75% of cases, the rest produced gastrointestinal or muscarinic syndromes. Vegetative symptoms or pathology not due to mushrooms were another reason of consult and it was differentiated both by clinical and lab methods. In our experience no case of mixed mushroom ingestion was observed. Conclusions: Many mushroom poisonings can be managed after clinical features. However there are a percentage of cases, mostly children who eat raw samples that cannot be identified in this way. Although many species are non-toxic it cannot be excluded that poisonous ones are involved. Ruling out a pathology due to mushrooms was another reason for lab analysis although the clinical features were mostly definitive.
182 INCIDENCE OF MUSHROOM POISONING IN IRELAND 1997–2000
Cassidy N, Casey PB, Tracey JA
National Poisons Information Centre, Beaumont Hospital, Dublin, Ireland
Objective: The aim of this study was to analyse the epidemiology of mushroom poisoning in Ireland, from 1997 to 2000, as reported to the National Poisons Information Centre (NPIC). Methods: A retrospective review of computerised records was carried out to evaluate mushroom type, circumstances, caller identification, patient age, sex, and clinical effects. Results and case report: The NPIC received 114 enquiries relating to mushrooms/fungi out of a total of 51,589 enquiries (0.22% of enquiries). There were 62 enquiries relating to 60 cases of deliberate ingestion of psilocybe hallucinogenic mushrooms and 1 request for information only. The mean age of patients was 17.5 years, male to female ratio was 1:7 and 89% of patients were symptomatic. The predominant clinical features were hallucinations and dilated pupils. 43 enquiries concerned unknown mushrooms, comprising 41 cases of human exposure, 1 animal exposure and 4 requests for information only. Ingestion of unknown mushroom species occurred predominantly in children under 12 years (34/42 cases) and the mean age was 3.5 years. 31.7% of human cases were symptomatic primarily with gastrointestinal features. There were 9 enquiries relating to 5 cases of toxic mushroom ingestion, which occurred as a result of incorrect species identification by amateur mycologists. Ingestion of Agaricus xanthodermus (Yellow Stainer) was reported in 2 adults and clinical features included headache, dry mouth, muscle weakness, and abdominal pain. Ingestion of Boletus satanus resulted in vomiting, sinus tachycardia and abdominal pain in a 4 year old female. Her father developed diaphoresis, sinus tachycardia, and first degree heart block. We report an unusual case of renal impairment and sequelae following ingestion of amanita mushroom species. Case Report: A 65 year old male developed severe gastrointestinal symptoms, 6–12 hours post ingestion of different types of wild mushrooms, some of which were subsequently identified as Amanita phalloides (Death Cap) and Amanita virosa (Destroying Angel). The patient presented to the emergency department, approximately 60 hours post ingestion, with acute renal failure and hepatic impairment (urea 30.9 mmol/l, creatinine 472 μmol/l, INR 1.65, ALT 1014 IU/L). Haemodialysis was commenced and an infusion of silibinin (Legalon®) was administered 72–84 hours post ingestion and chemotherapy continued for 5 days. Peak liver damage occurred 5 days post ingestion (INR 3.07) but organ transplantation was not required. Hepatic function normalised but the patient was haemodialysis dependent for 5 weeks. Renal sequelae persisted and 2 years post exposure, serum creatinine levels remain elevated (>250 μmol/l). Conclusion: Ingestion of poisonous mushrooms is uncommon in Ireland and severe poisoning is rare. Renal failure with sequelae, and hepatic impairment with subsequent recovery occurred following ingestion of potentially fatal amounts of amanita mushroom species.
183 GLUCAGON TREATMENT OF MILD INTESTINAL OBSTRUCTION FROM ILLICIT DRUG BODY PACKING
Shrestha M, Roberts J
The Medical College of Pennsylvania and the Mercy Fitzgerald Hospital, Philadelphia, Pennsylvania, USA
Background: Body packers transporting illicit drugs, usually cocaine or heroine, frequently swallow large numbers of “packets”. The main medical morbidity/mortality from this practice is that of rupture, obstruction and subsequent perforation. The “packets” that we have encountered are exceedingly sturdy, made of repeatedly wrapped thin sheets of plastic that do not rupture. We report the case of body packer given propylene glycol with obstructive symptoms that resolved with treatment with intravenous glucagon. Case Report: A man in his 30 s was brought to the emergency department (ED) having admitted to airport customs officials that he had swallowed a large number of packets containing cocaine approximately 7 hours earlier in an effort to smuggle the material into the USA from Jamaica. He was symptomatic for only mild abdominal fullness without nausea or vomiting. Vital signs were normal. The abdomen was soft with normal bowel sounds, with only mild diffuse tenderness. He was started on oral propylene glycol. After 500 mL was ingested, the patient developed slightly worsened abdominal discomfort and vomited up 9 packets. Intravenous metaclopromide 20 mg was given without relief. An obstruction series showed almost all of the packets in the stomach, and a few air fluid levels. Two hours after the metaclopromide, intravenous glucagon was given. A few minutes after the second 1 mg dose, the patient felt a symptomatic relief of the abdominal discomfort. A repeat obstruction series showed many of the packets in the intestines. The patient eventually passed over a hundred packets with no further vomiting. Conclusion: Glucagon, a potent smooth muscle relaxer, may relax the pylorus sufficiently to allow multiple “packets” ingested by body packers to pass through into the intestines. This therapy should be considered if the propylene glycol, used to push the packets through the bowel, results in discomfort, or there are other signs of gastrointestinal obstruction.
184 MULTIPLE-DOSE ACTIVATED CHARCOAL USED TO TREAT VALPROIC ACID OVERDOSE
Su M, Fong J, Howland MA, Nelson LS
New York City Poison Control Center; St John's University College of Pharmacy; Maimonides Medical Center, New York, New York, USA
Objective: Valproic acid (VPA) is commonly used for the treatment of various psychiatric conditions and seizure disorder. In overdose, significant toxicity and death may occur. Treatment of overdose is generally supportive but may include hemodialysis or hemoperfusion. Although activated charcoal may also be used, multiple-dose activated charcoal (MDAC) is reported to be ineffective in enhancing elimination of VPA. We report the case of a child who ingested a large quantity of Depakote® in whom MDAC appeared to significantly enhance the elimination of VPA. He also developed a transient proximal tubular wasting syndrome similar to Fanconi syndrome, which is not previously reported with acute overdose of VPA. Case Report: A 15 year-old male with a behavioral disorder presented to the hospital after an intentional ingestion of approximately 115 tablets (250 mg each) of Depakote complaining of dizziness and vomiting. He was started on the medication two days prior to presentation and was taking no other medications at that time. His vital signs were: blood pressure 130/87 mm Hg; pulse 100 beats/minute; respirations 20/minute; and temperature 98.9°F (37.2°C). He was delirious and had pinpoint pupils but no focal neurologic findings were present. The patient was sedated with 2 mg of intravenous lorazepam because of agitation and a nasogastric tube (NGT) gastric lavage did not return pill fragments. Activated charcoal (60 g) was administered via the NGT. His initial serum VPA concentration was 915 μg/mL and the peak of 1183 μg/mL after two hours later. He received three additional doses of activated charcoal (30 g) via the NGT every 6 hours. Over the next 48 hours, serial VPA concentrations (for a total of 11) revealed a calculated serum half-life, while receiving MDAC, of 6.45 hours. After MDAC was discontinued, the apparent serum half-life was 24.75 hours. He remained hemodynamically stable at all times and his condition gradually improved. On the second hospital day, the patient developed a transient nephropathy similar to Fanconi syndrome (i.e. phosphaturia, kaliuria, proteinuria) that resolved by the third hospital day. He was discharged to a psychiatric care facility 4 days later. Conclusion: Although previous studies report minimal efficacy of MDAC on enhancing VPA elimination, this case report demonstrates that MDAC may actually be effective. Furthermore, this is the first described case of Fanconi syndrome developing from an acute overdose of VPA.
185 EFFECT OF YOGHURT ON THE ADSORPTION OF ACETAMINOPHEN (PARACETAMOL) TO ACTIVATED CHARCOAL, SIMULATED IN IN VIVO STUDIES
Hoegberg LCG,1 Friis-Hansen L,1 Angelo HR,1 Christensen HR2
Departments of 1Clinical Biochemistry and 2Clinical Pharmacology, Bispebjerg Hospital, Copenhagen, Denmark
Objectives: Gastric decontamination using activated charcoal (AC) is the primary treatment for many poisonings. However getting children to drink the recommended dose (1 g/kg) AC slurry is difficult. In these cases it would therefore be of value, if charcoal was administered as other mixtures than the recommended water slurry. A reduction in adsorption capacity of acetaminophen to AC of 13% compared to control when ice cream was mixed with AC to a palatable suspension is described (1). A AC/yoghurt mixture is often used with success in pediatric poisonings in our neighboring Nordic countries, but no investigations have been made on the AC/yoghurt mixture. The general recommendation is that AC should not be mixed with anything but water (2). The potential advantage of the AC/yoghurt mixture is the easy administration both in hospitals and at home. We therefore wanted to a) examine the adsorptive capacity of AC grains poured on top of yoghurt in simulated in vivo conditions (pH 1.2 and 7.2), and b) to evaluate the palatability of the AC/yoghurt mixture. Acetaminophen was used as test drug (1,3). Methods: AC, fluids, and acetaminophen were mixed with yoghurt (nutrition facts: protein 3.2%, fat 3% and carbohydrate 12%). The method was as described earlier (1,3). To evaluate the palatability, we asked 12 children (3–13 years) to comment the taste of a AC/yoghurt mixture. Results: The maximum adsorption capacity of AC with added yoghurt was 544 mg acetaminophen/g AC (pH 1.2), 569 mg acetaminophen/g AC (pH 7.2). The palatability study showed that the AC/yoghurt mixture is accepted as well as a AC/ice cream mixture (not published), and that the children in general will happily eat the mixture, although the AC grains make the mixture a little gritty. The AC does not take away the fruity flavor of the yoghurt. The only drawback is the grayish color of the mixture. Conclusion: In the presence of yoghurt the adsorption capacity is reduced by 15–17% (p<0.05) compared to control without yoghurt (3), and the palatability study showed that the AC/yoghurt mixture is well accepted in children.
References: 1. Hoegberg, L.C.G.; Angelo, H.R.; Christophersen, A.-B.; Christensen, H.R. Effect of Food and Ice Cream on the Adsorption of Paracetamol to Activated Charcoal (Abstract). J. Toxicol. Clin. Toxicol. 2000. 2. Alison, J.; Paul, D. Churchill's Pocketbook of Toxicology; Churchill Livingstone: London, 2001. 3. Hoegberg, L.C.G.; Angelo, H.R.; Christophersen, A.-B.; Christensen, H.R. Effect of Ethanol and pH on the Adsorption of Paracetamol to High Surface Activated Charcoal, In Vitro Studies. J. Toxicol. Clin. Toxicol. 2001.
186 THE USE OF NOVEL PYROLYSED CARBON ADSORBENTS IN POISONING: AN IN VITRO STUDY
Scorgie KA,1 Dargan PI,2 Jones AL,2 Davies JG,1 Phillips GJ,1 Lloyd AW,1 Mikhalovsky SV1
School of Pharmacy and Biomolecular Sciences,1 University of Brighton and National Poisons Information Service (London),2 Guy's and St Thomas' NHS Trust, London, United Kingdom
Objective: Current indications for charcoal haemoperfusion include severe poisoning with aspirin and carbamazepine. The haemoperfusion column most commonly used in the UK is the Gambro Adsorba 300C. Novel adsorbents are currently being developed, including mesoporous polymer based carbons, produced by the pyrolysis and steam activation of a vinylpyridine–divinylbenzene copolymer (SCN) and styrene–divinylbenzene copolymer (SUCS). The flexibility offered by these adsorbents through close control of their chemical purity, pore-size distribution and surface chemistry makes them highly biocompatible and allows the targeting of specific molecules. The aim of this preliminary in vitro study was to investigate the potential use of these novel adsorbents in clinical toxicology. Methods: Phosphate buffered saline was made up to pH 7.4 and added to bovine serum albumin at 500 mg/L; drug concentrations of 500 mg/L for the aspirin study and 80 mg/L for the carbamazepine study were constructed. 1 g of wet carbon was added to independent flasks, the two respective drug solutions were then added and the flasks then placed in a shaking incubator for 1 hour at 37°C. SUCS was used as the adsorbent for the aspirin studies and SCN for the carbamazepine studies. The Adsorba carbon was used as the control adsorbent under the same experimental conditions. All samples were analysed using UV spectrophotometry at 279.5 nm for aspirin and 286 nm for carbamazepine. Results: The results are summarised in Table 1. In summary, adsorption to the SCN and SUCS carbons is greater than adsorption to the Adsorba carbon for carbamazepine and aspirin respectively. Conclusions: These results demonstrate the ability of the mesoporous carbons SCN and SUCS to adsorb carbamazepine and aspirin in vitro. Whilst other factors will influence drug adsorption in vivo and require further evaluation, this preliminary study shows that these adsorbents have potential for the treatment of aspirin and carbamazepine poisoning. These adsorbents may also have other benefits in critically ill poisoned patients and are currently being further investigated regarding their adsorption capacity for inflammatory molecules in sepsis and uraemic toxins, including middle molecules, in acute renal failure.
Table 1. Adsorption of Carbamazepine and Aspirin to the Study (SCN and SICS) and Control (Adsorba) Carbons (Abstract 186)
187 EXTRACORPORAL ELIMINATION PROCEDURES IN A CASE OF ACUTE CUTANEOUS CHROMIC ACID POISONING
Felgenhauer N,1 Pfab R,1 Eyer F,1 Schramel P,2 Zilker Th.1
1Toxikologische Abteilung, II. Medizinische Klinik, Technische Universität München, München, 2GSF-Forschungszentrum, Neuherberg, Germany
Objective: Acute poisonings with hexavalent chromic acid are very rare. Penetrating the skin easily, chromic acid causes severe burns and clinical features of chromium poisoning. Extracorporal elimination procedures show rather inconsistent results. Case Report: A 43-year-old man immersed his legs accidentally in a chromic acid solution. Initial toxicological analysis revealed a chromium in serum of 4900 μg/L and a chromium in urine of 7550 μg/l. In the clinical course the patient developed bloody diarrhoea, fever, respiratory insufficiency, acute renal failure, and II°–III° burns of both feet and lower legs. 16 days later, when the patient was transferred to our department, toxicological analysis revealed a chromium in blood of 2440 μg/l, a chromium in serum of 328 μg/l and a chromium in urine of 1310 μg/l. Extracorporal elimination procedures including high flux hemodialysis without any chelating agent, hemodialysis with EDTA, hemofiltration without any chelating agent and hemofiltration with EDTA and N-acetylcystein have been started. Hemodialysis (HD) was performed with a dialysis fluid flow of 800 ml/min. Hemofiltration (HF) was performed with a filtration rate of 6 l/h over a period of 5 hours. Five weeks after the accident respiratory and renal functions began to improve and mechanical ventilation and renal replacement therapy could be stopped. The burns at both feet and lower limbs needed a skin grafting. Three months after the accident the patient could be discharged in good condition. Two HD without chelating agents revealed only during the first three hours a clearance of 27.4 and 24.2 ml/min respectively, afterwards and during the whole period of the third HD there was no chromium measurable in the dialysis fluid. In two HD with EDTA only the first one showed a clearance of 28.9 ml/min during the first three hours, after these three hours and during the whole period of the second HD with EDTA there was no chromium detectable in the dialysis fluid. Two HF with EDTA showed a clearance of 11.9 ml/min and 14.7 ml/min respectively. One HF with N-acetylcysteine had a clearance of 19.8 ml/min. Five HF without chelating agents revealed a clearance of 9.8, 9.9, 9.4, 8.7 and 6.8 ml/min respectively. These elimination procedures have been performed over a period of three weeks. During this time the half-life for chromium concentration in serum was 39 days. Conclusion: Cutaneous exposure of chromium acid may cause a severe life-threatening chromium intoxication. High flux dialysis and hemofiltration with and without EDTA had only minor influence on the decrease of chromium concentration in serum.
188 ACUTE ARSENIC TRIOXIDE POISONING: NONSERIOUS COURSE OF ILLNESS BECAUSE OF HIGH DOSAGE CHELATION THERAPY
Horn HJ, Eicher H, Mühlberg W, Schmitt-Rüth R
Toxikologische Intensivstation, Medizinische Klinik 2, Klinikum Nürnberg Nord, Nürnberg, Deutschland
Case report: A 21-year old man, in a suicide attempt, swallowed 600 mg arsenic trioxide in crystal form. After 7 hours with no therapy, the only symptoms were gastrointestinal (i.e. diarrhoea, intestinal colic, vomiting), as well as a small ulcer in the stomach. Because of rapid and high volume intravenous infusion, hypovolaemic shock was avoided. Due to high dosage chelation therapy with DMPS (dimercaptopropane sulfonate), arsenic was eliminated by renal clearance (Figure 1). After testing the arsenic urinary concentration over a period of more than 8 days, we estimate that the total amount of poison swallowed was more likely to be 1000 mg or over. This is five times over the lethal dosage for one human being. There is no doubt that because of the high dosage of medication given (15.25 g DMPS over 12 days), along with the enormous amounts of fluids (27.5 l in 5 days, an average of 5.5 liters/day), severe complications were avoided. The huge amounts of intravenous infusions were given for volume substitution and “forced diuresis”. It is important to note, that there was no cardiovascular, respiratory, renal, neurological, haematological or muscular damage present. Perhaps because of slight liver toxicity either for arsenic trioxide or for DMPS, we found a small increase in transaminases GOT and GPT. This was however completely reversible. The antidote therapy, intravenous in the beginning and then followed on orally, was tolerated very well by the patient. There were no severe side effects. After increasing daily nutrition and discussing the patients welfare and stability with the psychologist, we released him after 12 days into the care of his parents. Conclusion: The report shows the importance and effectiveness of high dosage chelation therapy with DMPS, which today is the best known antidote for metal poisoning in Europe.
References: Hill, L.T.; Hurlbut, K.M.; Phillips, S.D. Poisindex, Micromedix; Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, 2001.
Table 1. Arsenic Serum- and Urinary Concentration as Well as Arsenic Clearance During Chelation Therapy with DMPS (Dimercaptopropane Sulfonate) in Acute Arsenic Poisoning (Abstract 188)
189 CHRONIC ARSENIC POISONING: PROBABLY NO EFFECT FROM CHELATING THERAPY USING DMSA
Midtdal K,1 Solberg K,1 Stenehjem A,2 Lierhagen S,3 Jacobsen D1,2
1National Poisons Information Centre, Oslo, Norway. 2Medical Division, Ullevaal University Hospital, Oslo, Norway, 3Foundation for Nature Research and Cultural Heritage Research, University of Trondheim, Trondheim, Norway
Objective: The role of DMSA (2,3-dimercaptosuccinic acid) treatment in patients with chronic arsenic intoxication is still unclear. Although animal data have demonstrated a favourable effect of DMSA treatment, one controlled clinical study did not show any beneficial effect of such therapy. The aim of this study was to evaluate DMSA treatment and arsenic excretion in a patient with an unusual type of arsenic poisoning. Case report: A 39-year-old woman was admitted to hospital with nausea, diarrhoea, vomiting and weakness of unknown aetiology. The patient's condition progressively deteriorated with the development of tetraplegia, respiration insufficiency and multiple system organ failure. The diagnosis was established by arsenic assays of urine and plasma starting at 1146 μg/L and 245 μg/L respectively. Chelating therapy with DMSA (600 mg orally three times daily) was given for a period of 45 days with 3 treatment intervals of totally 13 days included. The clinical manifestations of arsenic toxicosis disappeared very slowly. Serum arsenic and urinary excretion of arsenic were monitored during the entire course of treatment with DMSA (Fig. 1). Conclusion: Although DMSA treatment was associated with a significant decrease in blood arsenic concentrations and an increase in arsenic urinary excretion, DMSA treatment probably had no significant effect on the total body clearance of arsenic in our patient. The role of DMSA as an effective antidote in chronic arsenic poisoning should therefore be questioned.
190 PERMANENT PARALYSIS AT SITE OF DERMAL EXPOSURE TO CHLORPYRIFOS
Meggs WJ
Division of Toxicology, Department of Emergency Medicine, Brody Medical School at East Carolina University, Greenville, North Carolina, USA
Objective: Acute poisoning with organophosphate pesticides causes fasciculations and reversible paralysis due to cholinesterase inhibition at the neuromuscular junction. A mixed sensory and motor neuropathy can also develop, and permanent paralysis can result from damage to nerve fibers and peripheral neuropathy. Permanent paralysis is most commonly seen with systemic poisoning. In this case, persistent focal paralysis at the site of dermal exposure is reported. Case Report: A 61 year old carpenter removed a section of wallboard that had been damaged by water. A nest of termites was found in the wall beneath the wall board. The carpenter went to a local hardware store and purchased a product marketed as a termiticide. The active ingredient was chlorpyrifos. He used a sprayer to apply the product to the nest of termites. No protective equipment was used. In particular, he did not wear gloves and reported that his hands became soaked with the pesticide. About 30 minutes after applying the pesticide, he became ill. He developed nausea, abdominal cramping, and weakness in his arms and legs which resulted in a fall. Bilateral shoulder pain and chest pain developed. He reported numbness in his left hand and arm. On arrival at a rural community hospital, pulse was 58, blood pressure was 120/72, and pupils were 2 to 3 mm. No anticholinergic symptoms were noted. He was treated with atropine 1 mg IV and pralidoxime 2 grams IV. Motor examination was recorded as 0/5 strength in the hands and wrists and 3/5 elsewhere. He was transferred to a tertiary care hospital where weakness persisted. EMG revealed prolonged sensory latencies in the upper extremities and prolonged median motor distal latencies, with widespread peripheral neuropathy. On the third day there was continued progression of neuropathy, and pralidoxime was continued. By day 12, there was dramatic improvement of motor strength except for the hands. Right interosseous muscle strength was 1/5, and left was 0/5. Right hand grip was 2/5, and left hand grip was 0/5. He was transferred to a rehabilitation center. He never regained use of his hands, and he was disabled from employment as a carpenter. Marked atrophy of the intrinsic muscles of the hands developed and persisted. Conclusions: Dermal exposure of the hands to chlorpyrifos led to atrophy and permanent paralysis of the intrinsic muscles of the hands. This association with local exposure is likely because the neurons of the upper extremities received the greatest dose of chlorpyrifos. This case demonstrates the importance of using protective equipment including gloves impervious to organophosphates when handling these compounds.
191 SECONDARY EXPOSURE TO MALATHION IN EMERGENCY DEPARTMENT HEALTH-CARE WORKERS
Butera R, Locatelli C, Barretta S,1 Arrigoni S, Bernareggi G, Casorati P, Carli M, Manzo L
Pavia Poison Center, IRCCS Maugeri Foundation and University of Pavia; 1Emergency Department, C. Poma Hospital, Mantova, Italy
Background: Emergency Department (ED) staff caring for grossly pesticide-contaminated patients are at risk for developing toxicity from secondary contamination. Objective: To describe the development of symptoms and plasma pseudocholinesterase (PChE) levels in a group of health-care workers providing care to a patient contaminated with the organophosphate compound malathion. Methods: Health-care workers suffering from secondary exposure to malathion in the ED were studied. The source of exposure was a patient brought to ED by emergency medical services after a large malathion ingestion in suicidal attempt. Despite cardio-pulmonary resuscitation, performed by medical staff, the patient died in the ED 45 minutes after the admission. The body was left in an ED room for 2 hours (as requested by law) and then removed by an undertaker's technician. Health-care workers did not wear appropriate respiratory or skin protective equipment while caring for the patient nor while the body remained in the ED. A questionnaire was given to health-care workers in order to obtain information about health professional category, location in the ED, contact with the contaminated patient (for caring or moving), onset and duration of symptoms if any. PChE levels were measured at the end of exposure and then re-measured after a mean interval of 4.7 hours (range: 4–7 hours). Results: Fifteen health-care workers were on duty when the poisoned patient was brought to the ED: two subjects were physicians, twelve were nurses and one was an undertaker's technician. Ten people (66.7%) were in the same room with the contaminated patient, two (13.3%) worked in the next room and three (20.0%) were somewhere else in the ED. Eight subjects (53.3%) cared for or moved the patient. Fourteen health-care workers (93.3%) were symptomatic and only one was asymptomatic. The most frequently reported symptoms were eye irritation (11 cases), pharyngodynia (7 cases), nausea (6 cases), lacrimation (5 cases), headache (4 cases), cough (4 cases) and excessive salivation (2 cases). Symptoms began during the exposure and lasted for several hours. Mean PChE levels (reference values: 3500–11000 UI/l) were 7620±1592 UI/l (range: 4400–10300 UI/l) at the end of the exposure and 7580±1790 UI/l (range: 4400–11400 UI/l) in the following control. Conclusion: Secondary exposure affected both health-care workers who cared the contaminated patient, and those who did not. Diagnostic reduction in PChE levels was not observed, suggesting that signs and symptoms in health-care workers were local in their nature and did not come from systemic illness.
192 ORGANOPHOSPHATE POISONED PATIENTS: CHOLINESTERASE LEVELS, OXIMES THERAPY AND CLINICAL COURSE
Reis E, Aragão I, Martins H, Prelada F
Emergency Department, Intensive Care Unit and Clinical Chemistry Department, Hospital Santo Antonio, Porto, Portugal
Objectives: Organophosphate Poisoning (OP) remains the leading cause of ICU admission and mortality by poisoning in Portugal. To evaluate the morbidity and hospital outcome of OP patients admitted to Emergency Department of a University Hospital. Methods: Analysis of all consecutive admissions between Jan/2000 and Aug/2001. Registration of demographics, severity of illness, morbidity, antidotes use (atropine and obidoxime), plasma cholinesterase (PChE) and erythrocyte cholinesterase (EChE) activity and final outcome were performed. Results: 26 patients were included, with OP of high toxicity in 11 cases (Chlorfenvinphos), moderate toxicity in 11 cases (Quinalphos 5, Chlorpyrinphos 3, Dimethoate 2, Demethon-methyl 1) and not identified in 4 cases. These patients were submitted to general treatment procedures. Muscarinic features were the predominant clinical manifestations (49%), followed by SNC (27%) and nicotinic manifestations (24%). A group of 16 patients needed ICU treatment and the others 10 remained in the Medical Intermediate Unit (MIU). Mechanical ventilation (MV) was required by 58% of patients. The overall mortality was 19% (ICU 5/16, MIU 0/10). We classified the patients by severity grade based on PChE activity (severe <500 U/L; moderate 500–1000 U/L; mild >1000 U/L). Twenty-one patients in severe group had SAPS II score of 36.9±19.6, with 13.2±9 days of MV. Four patients in moderate group had SAPS II score of 24.2±3.5. Only one patient had mild intoxication with a SAPS II score of 15, not requiring MV. In the survival group (n=21), we found an association between PChE and EChE levels at the first day and 48 h previous to the discharge. The evolution of PChE activity was not associated with the clinical status in all patients (p=0.16), in opposite to the EChE evolution (p=0.00004). The 5 patients who died were all in the severe group and the cause of death was: OP (n=2), ARDS/pneumonia (n=2) and sepsis (n=1); in this group, we observed the same evolution of PChE and EChE. Conclusions: Although most patients had ingested high toxicity compounds, obidoxime treatment associated with intensive care measures may reduce mortality. PChE levels are most useful to prove the diagnosis of OP poisoning; EChE had a better association with outcome.
References: 1. Burtis, C.A. Textbook of Clinical Chemistry, 3rd Ed.; Saunders: Philadelphia, PA, 1999; 939–940. 2. Fenton, J. The Laboratory and the Poisoned Patient. AACC 1999, 271–275. 3. Lee, P. Clinical Features of Patients with Acute Organophosphate Poisoning Requiring Intensive Care. Intensive Care Med. 2001, 27, 694–699.
193 INTOXICATION BY ORGANOPHOSPHATE PESTICIDES: A GROWING PROBLEM IN MEXICO
Sandoval JL, Gonzalez-Kladiano D,1 Lijstszain C,1 Martinez J
Emergency Room1 and Critical Care Unit, The American British Cowdray Medical Center, Mexico City, Mexico
Introduction: It is estimated that 40,000 people living in the rural areas of Latin-America die each year because of pesticide intoxication. In Mexico some 12,000 pesticide intoxications and 700 pesticide-related deaths are reported annually. 22% of these intoxications are caused by organophosphates. Case Series: We present several cases of organophosphate poisonings treated at our hospital. Of these two were accidental poisonings and three were suicide attempts. All these patients presented with nausea, respiratory failure, diarrhoea, abdominal cramps, bronchorrhoea and miosis. The patients were initially treated with bolus doses of atropine with subsequent use of pralidoxime. Two patients needed mechanical ventilation for 7 days. All the patients were ultimately discharged in stable condition. Conclusions: Organophosphate poisoning is a potentially lethal condition, and is a public health problem in undeveloped countries like Mexico. Use of many of these pesticides is now prohibited in developed countries.
References: 1. Salud Publica Mex. 1999, 41, 55–61. 2. Salud Publica Mex. 2000, 42, 53–55.
194 TIME-COURSE OF MORPHOLOGICAL CHANGES AFTER REPEATED EXPOSURE TO LOW-DOSE PARAOXON IN RATS. AN ULTRASTRUCTURAL STUDY
Voicu VA, Hinescu ME, Pociu A
“Carol Davila” University of Medicine & Pharmacy, Army Center for Medical Research, Bucharest, Romania
Objectives: The purpose of this study was to evaluate the ultrastructural consequences of repeated exposure to low-dose paraoxon in rats. Emphasis was on features of cell death type, as ultimate consequence of toxic insult. The main target organs examined were the liver and the central nervous system. Methods: Male Sprague–Dawley rats divided in several groups of six, were repeatedly exposed to paraoxon (1 mg/kg body weight/day i.p.) for up to ten days. The time course of morphological changes were examined after pre-scheduled periods, in the following groups: 6 hours, 1, 2, 3, 4, 5, 6, 8, and 10 days after first exposure. At the end of each experiment, animals in a group were euthanased, and tissue slices were obtained, fixed in glutaraldehyde, and then classically processed for electron microscopy. Electron micrographs were recorded with a Leo 912 Omega transmission electron microscope. Results: Data presented confirm our previous studies showing a significant increase of the apoptotic index, evaluated with Apoptag, in situ apoptosis detection kit (Appligene Oncor). Ultrastructural changes were mainly in liver, and more evident in the early period after exposure to paraoxon. The apoptotic index was decreased when compared with that recorded after first exposures, although sometimes remained higher than in controls. In the central nervous system ultrastructural changes were less characteristic for apoptosis, even in the early periods after exposure. However, a large array of ultrastructural changes was present. After exposures of more than two days morphological changes recorded were a mixture of apoptosis, “apoptosis-like programmed cell death”, “necrosis-like programmed cell death” (1), “oncosis”, necrosis/cell lysis, or vacuolar cell death. Zeiosis (dynamic plasma membrane blebbing of dying cells) was recorded less frequent than expected in tissues examined. Conclusion: In a search to identify morphological changes provoked by exposure to paraoxon, we observed a bi-modal tissue response to the toxic insult: a significant early increase of apoptosis, followed, in the subsequent days of exposure, by a large diversity of cell death patterns.
References: 1. Leist, M.; Jäättelä, M. Four Deaths and a Funeral: From Caspases to Alternative Mechanisms. Nat. Rev. (Mol. Cell. Biol.) 2001, 2, 589–598.
195 SEVERE LEG GANGRENE AFTER AMITRIPTYLINE SELF-POISONING OVERDOSE
Roche Campo F,1 Civeira Murillo E,1 Royo Hernández R,2 Ferrer Dufol A2
1Servicio de Medicina Intensiva. 2Unidad de Toxicología, Hospital Clínico Universitario “Lozano Blesa,” Zaragoza, Spain
Objective: Despite the widespread use of the much safer, selective serotonin reuptake inhibitor antidepressants, cyclic antidepressants are still a leading cause of drug-overdose in Europe and amitriptyline is the drug most commonly used, alone or in combination. The aim of this study was to present a case of self-poisoning by this drug associated with benzodiazepines which presented with an unusual severe soft tissue complication. Case Report: A 39-year-old female with a history of depression treated with amitriptyline and benzodiazepines and two previous self-poisoning episodes presented to the emergency department. She was found unresponsive on the floor at home. Some empty bottles of amitriptyline and benzodiazepines were also found. It was not possible to establish how long she was unconscious. She was admitted to the Intensive Care Unit in a severe coma (GSS 3) requiring endotracheal intubation and mechanical ventilation the first 2 days. Her vital signs included blood pressure 140/90 mmHg, heart rate 110 bpm and temperature 35.8°C. A frontal head contusion and cutaneous lesions were observed in abdominal (lineal hematomas) and lower left member. A comprehensive toxicologic screen showed serum levels of tricyclic antidepressants over 1000 ng/mL and amitriptyline and benzodiazepines positive in urine. Acute renal failure (urea 1.5 g/L and creatinine 3.2 mg/dL) by rhabdomyolysis (CK 28590 U/L) and metabolic acidosis was associated. Coagulation was altered: prothrombin time 16 seconds, partial thromboplastin time 66 seconds and INR 1.5. Head CT scan was normal. ECG showed sinus tachycardia, QT prolongation and unspecific repolarization alterations. After initial stabilization the evolution of muscle-cutaneous lesions shown a severe edema of the whole member with blisters and large epidermolysis of the leg, a compartmental syndrome with respiratory distress syndrome requiring reintubation, and infection of the leg lesions. In spite of the intensive antimicrobial therapy a gangrene ensued that required the member amputation one month after the admission. Without further complications the patient was discharged two months after admission. Conclusion: Severe soft tissue lesions, such as epidermolysis and rabdomyolysis traditionally associated to barbiturate poisoning are not specific events but can complicate other toxic coma.
196 TOXICITY OF RECTALLY APPLIED FORMALIN/MERCURIC CHLORIDE SOLUTION
Hahn IH, Hoffman RJ, Hoffman RS, Nelson LS
St. Luke's-Roosevelt Hospital Center, Maimonides Medical Center, New York City Poison Control Center, New York, New York, USA
Case Report: A 63 year old non-English speaking Haitian woman with non-bloody diarrhea and no other constitutional signs or symptoms presented to her health care provider who supplied her with a stool collection kit, containing formalin and mercuric chloride for the purpose of sampling stool for ova and parasites. The patient misunderstood the instructions for stool collection and applied the formalin/mercuric chloride solution as a rectal enema. One day later she presented with the complaint of bright red bloody stool. Her physical examination was normal except for bright red rectal bleeding. A focused neurological examination was normal without signs or symptoms suggestive of systemic mercury toxicity. Her complete blood count, serum electrolytes, BUN and creatinine were normal. Stool assays were negative for Clostridium difficile toxin as well as ova and parasites. Her serum mercury level was 717 mcg/L. A lower gastrointestinal endoscopy revealed severe bowel wall edema from the rectum to mid-transverse colon and ulcerations in the rectum. The patient was treated with dimercaptosuccinic acid (DMSA) 700 mg PO TID for 5 days followed by dosing of 700 mg PO BID for 14 days. The patient did not develop any untoward effects attributable to mercury toxicity or chelation therapy. A repeat serum mercury level was not available; however, the patient's lack of systemic symptoms and resolution of her rectal bleeding prompted discontinuation of chelation therapy and discharge from medical care. Discussion: This is a case of rectally applied formalin/mercuric chloride solution causing local toxicity. Mercuric salts and formaldehyde are both caustic agents. Symptoms experienced by this patient appear to be limited to the bowel, specifically bowel edema, ulceration, and rectal bleeding. She did not experience other common symptoms of mercury toxicity including neurologic or renal morbidity. This case is one of many in New York City in which non-English speaking patients have been poisoned as a result of misuse of mercury-containing stool collection kits. This case demonstrates the need for caution and thorough education when dispensing these and other potentially toxic products to patients with language barriers.
197 LEAD CONTENT IN SCALP HAIR OF CHILDREN FROM URBAN AND RURAL REGIONS IN THE UNITED ARAB EMIRATES
1Hasan MY, 1Adem A, 1Kosanovic M, 1Stephen S, 3Shaheen H, 2Fahim MA
1Department of Pharmacology and 2Department Physiology, Faculty of Medicine, 3Department of Biology, Faculty of Science, United Arab Emirates University, Al Ain, United Arab Emirates
Objectives: Lead is one of the common pollutants in the environments and is potentially dangerous to the whole population. It is a toxic substance that has profound effects on heme biosynthesis and may cause neurotoxicity particularly in young children. The most important source of lead pollution in oil producing countries is predominantly leaded petrol. Previous studies have utilized blood levels as indicator for lead exposure. The current study investigated lead exposure in children from urban and rural regions in the United Arab Emirates utilizing samples from scalp hair. Methods: Hair samples were randomly collected from 42 children (aged 6–18 years) representing urban and rural areas. The rural regions were defined as at least 50 km away from the heavy traffic sites. Immediately after cutting, hair samples were stored in plastic bags and attached to a questionnaire with the relevant background information. Samples were dried, weighed and sealed with polyethylene envelopes. Following the extraction procedures with nitric acid, ICP-MS instrument was utilized for lead determination. A psychological battery test was also performed on all individuals. Results: The analytical instrument showed a high degree of sensitivity for measuring many heavy metals including lead. The study revealed significant differences between lead levels in scalp hair of children from urban and rural areas. Mean lead levels for rural and urban regions were 0.79±0.10 and 3.47±0.47 μg/g respectively (Confidence Intervals 0.57–1.01 and 2.48–4.46; P<0.001). Battery test did not indicate significant behavioral abnormalities in any of these children. Although mean lead levels were below 5 in both regions significant differences were noticed between the two. Conclusion: Measuring metals concentration in scalp hair could be a useful method for monitoring exposure to various metals and lead in children. Since local fuel is not restricted to unleaded, the potential for environmental lead exposure is still considered to be high in heavy traffic areas. Furthermore, the introduction of policies such as the use of only unleaded fuel will reduce the potential risk for heavy metals and lead intoxication particularly in oil producing countries. Such policies would contribute positively to the overall reduction of environmental pollution (This study was supported by a research grant from the United Arab Emirates University).
198 TESTING FOR KETOBEMIDONE AND ITS MAIN METABOLITES IN URINE FROM DRUG ADDICTS USING GC/MS
Angelo HR, Hausner B
Department of Clinical Biochemistry, H:S Bispebjerg Hospital, Copenhagen NV, Denmark
Objective: Ketobemidone is an opioid agonist and a potent narcotic analgesic, which has been used for decades in the treatment of severe pain. It has also become a frequently abused drug in the Scandinavian countries. One of the most common used formulations, Ketogan®, also contains a spasmolyticum named A29 (N,N-dimethyl-4,4-diphenyl-3-buten-2-amine). Since no immunoassay method is yet developed for ketobemidone and its metabolites the initial screening has to be performed by alternative methods. Until now ketobemidone has been included in our TLC screening method for narcotic analgesics, but after the purchase of a GC/MS we wanted to develop a simple and robust method for ketobemidone testing using this equipment. Methods: 500 μl urine was deconjugated by treatment with 5 μl β-glucoronidase and 40 μl acetate buffer, pH 4.8 at 60°C for 1.5 h. After addition of 125 μl ammonium chloride buffer (pH 9.5) with 4% triethylamine, the internal standard (β-methylphenethylamine) and 500 μl heptane–chloroform–propylchloroformate (3:1:0.02), ketobemidone and the metabolites were derivatized and extracted by horizontal shaking for 20 min. After centrifugation for 20 min at 1300 g about 125 μl organic phase was pipetted into GC autosampler vials. The capillary gas chromatography was performed on a HP 6890/5973 GC/MS system. The HP-5MS (30 m×0.25 mm) 5% phenyl methyl siloxane column was operating at 120°C for 1 min, increased by 10°C to 290°C (final time 4 min). 2 μl was injected into the injection port, operating in pulsed splitless mode (1.7 min) at 250°C. Carrier gas (helium) flow was 1.2 ml/min. The MS operating in SIM mode with target (qualifying ions) as follows: m/e 116 (105) for the internal standard, 276 (333,70) for ketobemidone and 349 (262,147) for norketobemidone. Results: The retention times for the internal standard, ketobemidone, norketobemidone and the spasmolyticum were 8.4, 14.9, 18.8 and 8.5, respectively. 9 TLC positive addict urine samples were also tested positive with this GC/MS method even without deconjugation. The unconjugated/conjugated ratio for ketobemidone and norketobemidone was 1–3 and 5–30, respectively. A29 was further detected in all these addict samples. For ketobemidone and norketobemidon a satisfactory cut off, 1 μmol/l (0.25 μg/ml) was demonstrated. Conclusion: A simple GC/MS testing method for ketobemidone has been developed based on derivatization during the extraction procedure and with no evaporation needed. The deconjugation step might be omitted, but the detection of especially norketobemidone hereby was significantly decreased.
199 SIMULTANEOUS DETERMINATION OF AMPHETAMINE DERIVATIVES IN HUMAN URINE BY HPLC-UV
Soares ME, Carvalho M, Carmo H, Carvalho F, Bastos ML
CEQUP, Laboratory of Toxicology, University of Porto, Porto, Portugal
Objectives: Amphetamine derivatives are a class of compounds increasingly abused in various regions of the world, with high incidence in the USA and Occidental Europe, specially by young people (1). In spite of the clinical and forensic interest in the identification and quantification of these compounds in biological fluids, the development of adequate techniques is not yet fully accomplished. This is specially true for 4-Bromo-2,5-dimetoxyphenethylamine and 4-Methylthioamphetamine. The aim of this work was to develop and validate a HPLC method for the simultaneous quantification, in human urine, of Amphetamine and its main metabolite p-Hidroxyamphetamine, 3,4-Methylenedioxymethamphetamine and its metabolites 3,4-Methylenedioxyamphetamine, 4-Bromo-2,5-dimetoxiphenethylamine and 4-Methylthioamphetamine. Methods: Urine was acidified and hydrolysed for one hour. After cooling, the hydrolysed sample was purified through an OASIS MCX column, being the amphetamines eluted with 5% ammonium hydroxide in methanol. The eluate was injected in a HPLC-UV system equipped with a C18 column being the eluent constituted by methanol:acetate buffer 0.05 M containing 0.1% triethylamine, pH 3.9. The separation of compounds was achieved with a gradient elution (19 minutes at methanol/acetate buffer, 23+77 and 16 minutes at methanol/acetate buffer, 35+65). The detection was made at 210 nm. Results: A coefficient of variation between 3.3% and 5.9% was obtained for the precision for all the compounds for the overall procedure. The accuracy, evaluated by the standards addition method, was better than 85% for all the compounds. The limits of detection for the established conditions were 14.0, 5.3, 100.0, 34.0, 47.0, 33.0 ng for Amphetamine, p-Hidroxyamphetamine, 3,4-Methylenedioxy-N-methylamphetamine, 3,4-Methylenedioxyamphetamine, 4-Bromo-2,5-dimetoxiphenethylamine and 4-Methylthio-amphetamine, respectively, in 20 μl of injected sample. The range of linearity was between 0.69 and 100 μg/ml for Amphetamine, 0.26 and 25 μg/ml for p-Hidroxyamphetamine, 5.0 and 200 μg/ml for 3,4-Methylenedioxy-N-methylamphetamine, 1.72 and 200 μg/ml for 3,4-Methylenedioxyamphetamine, 2.38 and 200 μg/ml for 4-Bromo-2,5-dimetoxiphenethylamine and 1.66 and 200 μg/ml for 4-Methylthioamphetamine. Although the literature records several chromatographic methods for quantification in biological samples of amphetamine and other related compounds (2), to our knowledge this is the first HPLC method that enables the simultaneous determination of Amphetamine, Methamphetamine, their principal metabolites and the new drugs 4-Bromo-2,5-dimetoxiphenethylamine and 4-Methylthioamphetamine in human urine. Conclusion: The implemented method is precise and accurate and enables the monitoring in urine of six drugs of abuse with clinical and forensic interest.
References: 1. Christophersen, A.S. Amphetamine Designer Drugs—An Overview and Epidemiology. Toxicol. Lett. 2000, 112–113, 127–131. 2. Kraemer, T.; Maurer, H.H. Determination of Amphetamine, Methamphetamine and Amphetamine-Derived Designer Drugs or Medicaments in Blood and Urine. J. Chromatogr. B 1998, 713, 163–187.
200 USE OF FERRIC CHLORIDE TO IDENTIFY SALICYLATE-CONTAINING POISONS
Hoffman RJ, Nelson LS, Hoffman RS
Maimonides Medical Center, Brooklyn, New York, USA. New York City Poison Control Center, New York, New York, USA
Objective: Ferric chloride (FeCl3) is used to qualitatively test the urine of patients with presumed salicylate exposure. FeCl3 testing of an unidentified poison might provide confirmation of salicylate exposure in situations where FeCl3 urine testing cannot be used. Such situations include the absence of a urine sample, immediately after ingestion before urine contains a detectable quantity of salicylate, or for patients chronically using salicylates for which FeCl3 testing is unhelpful. This study seeks to determine if FeCl3 can be used to identify salicylate-containing products. Methods: This descriptive, blinded study assessed the reactivity of FeCl3 with commercially available salicylate-containing products. A 0.1 mL of 10% FeCl3 solution was applied to 15 various salicylate-containing products including: acetylsalicylic acid, bismuth subsalicylate, methylsalicylate, physostigmine salicylate, salicylic acid, trolamine salicylate, and herbal tablets with salicin-containing white willow bark (Salix sp.) These products tested were: regular and enteric-coated pills (n=4), powder (n=1), topical creams (n=5), topical liquids (n=4), and intravenous solution (n=1). FeCl3 was applied to crushed tablets and added directly to liquids and creams. Fifteen salicylate-free controls including liquids, pills, and creams similar in appearance to experimental samples were also tested. Three blinded physicians familiar with FeCl3 testing independently observed the addition of FeCl3 to each sample and rated a positive or negative result. Results: All salicylate-containing products were interpreted to be clearly FeCl3 positive and all control samples were interpreted to be clearly FeCl3 negative. Conclusion: Salicylate-containing products may be identified using FeCl3. When using FeCl3 testing as described herein, only a positive test result should be applied: any negative result should be considered inconclusive.
201 PATIENT HISTORY AND TOXSCREEN ANALYSIS IN PATIENTS ADMITTED FOLLOWING INGESTION OF RECREATIONAL DRUGS
Strachan FE, Chalmers CD, Kelly CA, Oliver JJ, Bateman DN
National Psoisons Information Service, Edinburgh Centre, Royal Infirmary of Edinburgh, Scotland, United Kingdom
Background: Recreational drug use can result in admission to hospital for a number of reasons including respiratory depression, depressed conscious level, agitation and psychotic behaviour. As a result patients are often unable or unwilling to give an accurate history of drug ingestion. Laboratory analysis of drug ingestion is often used to aid diagnosis and clinical management1. Objective: We assessed whether the history given by patients admitted to our unit following recreational drug use was confirmed by TOXSCREEN urine analysis. Methods: TOXSCREEN results for amphetamine, benzodiazepine, cannabis, cocaine, methadone and opioid were compared with history given on admission for patients admitted over a 6 month period. Data were collected retrospectively. Samples that tested positive for cannabis were not judged to affect history or management significantly. Results: Tests for 81 patients (24 female, 57 male; age 26±1.0 yrs) were included from patients admitted as a result of recreational drug use from January to July 2001. Three (4%) samples were negative for all tested substances, benzodiazepine was the most common positive result (57 samples, 70%) and cocaine the least common (3 samples, 4%). Sixty-two (77%) samples were positive for the drugs detailed in the patient history, but 38 of these samples also tested positive for additional drugs. A total of 49 samples (61%) tested positive for drugs not included in the patient history. Patient history was completely confirmed by TOXSCREEN in 24 samples (30%), partially confirmed in 43 samples (53%) and unconfirmed in 14 samples (17%). Summary: Although the majority of samples tested positive for the drugs detailed in the patient history, additional drug exposure was not reported in over half of the patients admitted. As the TOXSCREEN results are not quantified, this inaccuracy may result from differences between chronic and acute drug exposure. However, these results may be important in patient management. Conclusion: In this study, patient history alone was inaccurate in identifying drug exposure in patients admitted following ingestion of recreational drugs. Laboratory testing provides a useful aid to diagnosis and clinical management in patients presenting with a history of exposure to recreational drugs.
References: 1. Bateman, D.N.; Lee, D.S.; Herdman, J.; Jarvie, D. Drugs of Abuse and Self-Harm in Edinburgh: 1999. J. Toxicol. Clin. Toxicol. 2000, 38, 173–174.
202 OVERDOSE OF DISULFIRAM AND ETHANOL TREATED WITH FOMEPIZOLE
Krogh A von,1 Rygnestad T,1 Iversen H2
1National Poisons Information Centre, Oslo; 2Medical Department, Hammerfest Hospital, Hammerfest, Norway
Objectives: Disulfiram irreversibly inhibits aldehyde dehydrogenase and is used in the treatment of alcoholism. Intake of ethanol during disulfiram therapy will lead to accumulation of acetaldehyde, which is considered the main contributing factor to the disulfiram-ethanol reaction (DER). Death has followed overdose with disulfiram alone after acute intake of 10–30 g. Fomepizole (4-methylpyrazole) is a potent inhibitor of alcohol dehydrogenase and inhibits the conversion of ethanol to acetaldehyde, and has been suggested as an antidote in severe DER to inhibit further accumulation of acetaldehyde. Fomepizole is a relatively non-toxic compound with few adverse reactions at therapeutic doses. We report the first DER case in Norway treated with fomepizole. Case report: A male of 31 years had been drinking 330 g of ethanol over 12 hours and thereafter ingested 20 g disulfiram. Four hours later he had BP 99/44, frequent vomiting and a weak pulse of 100/min. He was treated with 7.5 g activated charcoal, promethazine as anti-emetic and i.v. fluids. He was admitted to the local hospital 8 hours after the disulfiram intake with BP 110/40 and frequent vomiting. He was hydrated with i.v. fluids, but 10 hours after the ingestion the BP was 90/57. He was then given 1000 mg fomepizole i.v. During the next hour and a half the emesis ceased, he became more alert and BP increased to 110/75. He remained circulatory stable thereafter. The patient was given two more 700 mg doses of fomepizole during the next 24 hours after which he could be discharged. At follow-up 4 months later he had no sequelae. Discussion and Conclusion: In the reported case the patient had a blood-ethanol of 1.3% 8 hours after taking disulfiram. Assuming an ethanol clearance of 0.15% per hour, he had a blood-ethanol of 2.5% or higher at the time when he took the potentially lethal dose of 20 g disulfiram. The large dose of both disulfiram and ethanol, the symptoms and the low blood pressure indicates a serious intoxication. Compared to a similar case not treated with fomepizole who became seriously ill and recovered with severe neurological sequelae1, our case treated with fomepizole had a less dramatic course. This favourable outcome suggests further studies of the use of fomepizole in serious DER and in DER complicated by other diseases, e.g. severe cardiovascular disease.
References: 1. Ellenhorn, M.J. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisioning, 2nd Ed.; Williams and Wilkins: Baltimore, 1997; 1361.
203 PHEOCHROMOCYTOMA MASKED AS SUSPECTED METHAMPHETAMINE ABUSE
Lynton RC, Marquardt K, Albertson T, Tharratt RS
Section of Clinical Pharmacy and Medical Toxicology, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, University of California at Davis, and the Sacramento Division of the California Poison Control System, California, USA
Objective: We present a case of pheochromocytoma thought secondary to methamphetamine overdose and show the importance of keeping medical conditions in the differential diagnosis of suspected drug toxic patients. Case Report: A 37 y/o male presented to a community hospital with a history of epigastric and back pain after vomiting all night. His history included methamphetamine use 2 days prior to presentation. There was no history of hallucinations or diaphoresis on presentation, however the patient was in respiratory distress. Pupils were 2 mm reactive. Initial blood pressure was 122/56 mmHg with a heart rate of 134 beats/min. His blood pressure increased to 200/160 mmHg. ECG showed ischemic changes in the lateral leads. An anion gap of 22 mOsm was noted, otherwise electrolytes were normal. Urine drug screen was only positive for opiates. Initial approach was to treat the patient with benzodiazapines for agitation and use labetolol to control blood pressure. A CT scan of the chest and abdomen to rule out esophageal or diaphragm rupture was ordered. After 2 hrs he was on a lorazepam drip at 6 mg/hr and diazepam 10 mg every 4 hours. No labetolol or nitroprusside was given. He became agitated with a tachycardia up to160 beats/min and blood pressure of 165/90 mm/Hg. New ECG changes prompted transfer to another hospital for an emergent cardiac evaluation. In that ER he had a cardiac arrest and was taken to the cath lab during which he was placed on nitroprusside for hypertension. He subsequently needed norepinephrine to maintain blood pressure. A balloon pump was place and cardiac catherization showed pulmonary hypertension, cardiomyopathy and poor ejection fraction. Hospital course included a fever and pneumonia requiring intubation and broad-spectrum antibiotics and SVT treated with diltiazem. He was eventually weaned off norepinephrine and the balloon pump removed. Despite the ECG changes and cardiac arrest no myocardial infarction was diagnosed. Retrospective review of the CT scan done on initial presentation showed a mass on the adrenal gland consistent with a pheochromocytoma. Catecholamines levels were 30 times the normal range. Conclusion: This case demonstrates the importance of maintaining a differential diagnosis of possible medical conditions that can mimic the actions of drugs or chemicals when managing a drug/chemical abuse or overdose case. Laboratory drug analysis in isolation is a poor diagnostic indicator. Methamphetamines certainly can cause many of the symptoms seen in this patient, and patients using methamphetamines who have underlying conditions of catecholamine excess may have life threatening consequences.
204 TRANYLCYPROMINE INTOXICATION WITH HYPERTHERMIA, HYPOTENSION, RENAL FAILURE AND DISSEMINATED INTRAVASCULAR COAGULATION
Riel AJHP van,1 Berkel M van,2 Meulenbelt J1,3
1National Poisons Control Centre, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; 2Beatrix Hospital, Gorinchem, The Netherlands; 3University Medical Center Utrecht, The Netherlands
Background: Tranylcypromine (Parnate®) is a non-selective irreversible inhibitor of monoamine oxidase (MAOI) recommended only for treatment of depressive patients that are unresponsive to other antidepressants. Tranylcypromine is not a drug of first choice because of its potential for causing serious interactions in combination with other drugs that increase serotonin-levels and tyramine-containing foods. We present a case of tranylcypromine-overdose in a 50-year old psychiatric patient. Case report: On admission the patient presented as a sick and unresponsive woman with searching eye-movements. She was sweating heavily, muscles were spastic, extremities showed tremors, there was slight respiratory failure, pulse rate was 150 bpm and body-temperature was 39.6°C. It became clear that several hours earlier she had taken 20 tablets of tranylcypromine 10 mg and possibly 10 tablets of 50 mg chlorthalidone. In the following hours she developed shock (systolic BP 60 mmHg), tachycardia (150 bpm), seizures, disseminated intravascular coagulation, PTT 24 sec (control 10.6–13.3 sec), APTT 45 sec (control 23–33 sec), fibrinogen 1.6 g/l (normal range 2.0–4.5 g/l), AT III 54% (normal range 80–120%), rhabdomyolysis (CK 18039 U/l) and renal failure (creatinine 188 μmol/l). The body-temperature rose to 43.1°C in the first two hours after admission, in spite of initial treatment. In order to reduce muscle metabolism and heat production, the patient was paralysed and intubated (using etomidate and rocuroniumbromide i.v.), furthermore she was kept under sedation (using midazolam i.v.) and mechanically ventilated. Additionally, the patient was cooled with icepacks in the armpits and groins and wet wrappings covering the whole body. This resulted in a drop of the body-temperature to 39.2°C within 1 hour and subsequently to 37.7°C. Because of shock an intravenous infusion and noradrenaline (i.v.) were administered. The patient was mechanically ventilated for three days and recovered within one week without physical sequelae. She was referred back to a psychiatric unit for further treatment. Conclusion: Symptoms in this patient are consistent with earlier reports of tranylcypromine intoxications. The symptoms resemble those seen in case of serotonin syndrome. Many authors report initial hypertension, presumably caused by release of serotonin and noradrenaline. In severe MAOI-overdose subsequent hypotension is reported frequently, while hypotension is seldom observed in serotonin syndrome. Therefore, initial treatment of hypertension caused by MAOI-overdose should involve short-acting agents such as nitroprusside at titrated doses. Sympathomimetic agents that interact directly with the postsynaptic receptors, especially noradrenaline and adrenaline, are the drugs of choice for treatment of hypotension caused by MAOI-overdose. These agents do not cause the release of noradrenaline from the peripheral sympathetic nerve-terminal and therefore carry less risk of overcorrection. Physical cooling can reduce hyperthermia; additional treatment with muscle-relaxants and perhaps dantrolene can also be effective.
205 BELIEVE IT OR NOT: SILVER STILL POISONS
Hori K, Martin TG, Rainey P, Robertson WO
Washington Poison Center, Seattle, Washington, USA
Background: For centuries, silver—as with gold—has been endowed with therapeutic benefits. Fortunately, in small amounts, it seems to kill almost no one but many persistent users end up with a peculiar, morbid discoloration of the skin. Clinical usage by allopaths has plummeted in the last half century but it is still used today as a “caustic” for superficial bleeding. Over 45 years of its existence, our Center had never been involved with a single case of “argyria” but within 7 days, we had three such instances—and over the most recent two months—3 more. Case Examples #1. Mrs X, a 66 year old otherwise entirely healthy woman, was visited by her friend—a nurse—who urged her to go to an ED because she was so “cyanotic”. When she did, some 36 hours later, heart disease and lung problems were quickly dismissed and the physician called us for the latest about methemoglobinemia. He sent off for a level and immediately gave methylene blue—but with no response. Then, the level came back normal. At that particular point, her husband was also noted to be “dusky”. While he was being examined, Mrs X was ushered out into the hallway where there were no chairs–so she hopped up on a gurney for a nap. Then, a visitor came by and proceeded to loudly object to the charge nurse that no dead body should be kept uncovered in the hallway. Mrs X—the “dead body”—sat up and exclaimed “This is not a dead body” terrorizing both the visitor and the charge nurse. Shortly thereafter, a three year exposure to naturopathic hydrolyzed silver treatment was revealed and confirmed by urine testing. Both husband and wife have finally discontinued their “habit.” #2. A 37 year old male confined to a state psychiatric facility was noted to have darkly “discolored” skin. Again, heart and lung disease were easily excluded and two methemoglobinemia tests were negative so we were called about how to treat “argyria.” His diagnosis was confirmed by a silver level of 89 mcg/liter of urine–“probably” obtained from an herbal tea. Discussion: Too much of a “good thing”—silver—still makes for argyria. The US's 1930s epidemic stemmed from Argyrol—a nose drop solution. Our current epidemic—attributable to what today is referred to as “alternative medicine”—may only get worse as rumor reveals its gain in popularity in Florida as prophylaxis against anthrax. Conclusion: The skin's grayish discoloration, made distinctly worse by sunlight, may persist for life. These walking corpses can end up as social outcasts. If you've seen one, you've seen them all!
206 PROSPECTIVE STUDY OF LARYNGEAL COMPLICATIONS FOLLOWING TRACHEAL INTUBATION IN PATIENTS ADMITTED FOR ACUTE POISONING
Mégarbane B, Be Hong T, Herman P, Goldgran-Tolédano D, Delahaye A, Gueye PN, Baud F
Réanimation Médicale et Toxicologique, Hôpital Lariboisière, Paris, France
Objectives: Tracheal intubation and mechanical ventilation largely contributed to the improvement of acute poisonings prognosis. Tracheal intubation represents an invasive technique, with potential induced laryngeal injuries. The objectives of our study were to evaluate the importance and to define predictive factors of early injuries. Methods: We prospectively collected data of all patients admitted in our toxicological intensive care unit (ICU) and intubated for acute poisoning. A complete NTE examination including fiberscopy was performed within the 24 hours following extubation. Results were expressed as mean±SD [extremes]. Sub-group comparisons were done using Chi2 and Mann–Whitney tests. Results: 112 poisoned and intubated patients off the 637 patients admitted in our ICU on a 9 month-period were included in this study. They were 44 men and 68 women, with a mean age of 43±17 years [16–86], a mean Glasgow Coma Score on admission of 5±3 [3–15] and a mean SAPS II score of 43±14 [17–80]. Patients were intubated for a neurological (93%), a hemodynamic (38%) and/or a respiratory failure (31%). The mean length of intubation was 64±112 hours [5–792] and the mean length of ICU stay 4±10 days [2–57]. Intoxicants involved in these poisonings were benzodiazepines in 54% of the cases, ethanol in 27%, neuroleptics in 24%, carbamates in 23% and tricyclic antidepressants in 21%. 75/112 patients (67%) presented multidrug intoxication. 27/112 patients (24%) extubated themselves and only 4/112 patients (4%) were reintubated, of which 3 for a laryngeal dyspnea. 7/112 patients (6%) presented dyspnea 51/112 (46%) dysphagia and 73/112 (65%) dysphonia. A complete NTE examination, including a fiberscopy during the 24 hours following extubation was performed in 67/112 patients (60%). Only 12/67 patients (18%) had a normal examination. 47/67 patients (70%) presented glottis injuries, mainly edema, ulcerations and/or granulomas, 17/67 (24%) subglottis and 9/67 (13%) epiglottis injuries. Comparisons between the subgroups of patients with and without laryngeal injuries showed only significant differences for the length of intubation (p=0.01) and of ICU stay (p=0.01). Only the presence of dysphagia the day after extubation (p=0.05) was predictive of the existence of laryngeal injuries whereas the kind of intoxicant did not modify the incidence. Conclusion: Incidence of tracheal intubation-induced laryngeal injuries is elevated in case of poisoned patients. It is independent from the ingested intoxicants but is related to the length of intubation. Such a high incidence may be explained by the circumstances of intubation of poisoned patients: most frequently at home for an acute vital failure and in the absence of an optimal anesthesia induction protocol.
207 DEPENDENCY “DOSE-OUTCOME” IN THE CASES OF ACUTE ACETIC ACID POISONING
Sarmanaev SKh, Yamanaeva IE, Aidarova LPh.
Toxicological Center, Ufa, Russia
Objectives: Acetic acid is a frequently used corrosive agent in Russia. The purpose of our research is a dependency study of fatal outcome due to ingestion of 70% acetic acid. Materials and methods: The medical charts of 196 cases with acute acetic acid poisoning have been retrospectively analysed for the period of 1996–2000. These are the cases that were hospitalised in the Toxicological Center of Ufa: 34 patients died and 162 survived. The statistical comparison was calculated by means of Fisher's angular transformation, Student's t-coefficient and variation coefficient [1]. The results are shown in Table 1. Results: Significant dose related differences were found between the groups of the dead and survivors (114.06±10.81 vs 44.00±2.38 ml; p<0.001), but the data were highly variable (variation coefficients in the group of survivors was 65.9% vs 53.6% in the group that died). Other factors such as age and the intention of poisoning influenced the outcome between the two groups. Conclusions: The lethal outcome of poisoning depends not only on the dose of the substance taken, but also on such factors as age and intention of poisoning. Factors such as the prompt availability of medical aid, the existence of food in the stomach, concomitant diseases and other factors can influence the effects of a given dose of the substance.
References: Lakin, G.F. Biomethria Moscow 1990, 352.
Table 1. (Abstract 207)
208 EFFECTS OF EARLY ENTERAL NUTRITIONAL SUPPORT IN PATIENTS WITH MODERATE AND SEVERE ACETIC ACID LESIONS OF DIGESTIVE TRACT
Leiderman IN, Sentsov VG, Kirichenko AV
Ural State Medical Academy, Regional Toxicological Center, Yekaterinburg, Russia
Objectives: Patients with poisonings from chemically aggressive liquids account for 14.2 to 23.7% of all our poisonings. The agent most commonly implicated in adult poisoning cases of this type is an acetic acid—from 50 to 78.5% of all cases. Lethality in this group of patients today remains high at about 10%. This situation is closely connected with a high rate of differing serious complications of the poisonings: severe SIRS, acute renal failure, acute GI bleeding, acute necrotizing pancreatitis, MODS. Very often the development of severe malnutrition due to the lesions of esophagus and stomach leads to the progression of SIRS and MODS and is closely connected with poor clinical outcome. Methods: We performed a prospective controlled randomized study on 130 patients with moderate and severe acute acetic acid poisonings. 28 patients who developed acute renal failure were excluded from this study. In the study group (n=70) we began to infuse isocaloric lactose-free enteral diet (25–50 ml/h) during first 24–48 h of treatment in ICU, after fibrogastroduodenoscopy and insertion of the thin feeding tube (3–4 F) into stomach or duodenum or jejunum under visual control. In control group (n=32) we provided the traditional variant of intravenous infusions of natural colloids and partial parenteral nutrition during first 7 days and then oral feeding diets. Significance was tested using Students “t” test. Results: As expected the levels of nosocomial pneumonia were significantly higher (p<0.05) in the control group 13/32–40.63% in comparison with the study group 18/70–25.71%. Also we determined significantly elevated (p<0.05) levels of serum albumin on 5 and 7 day after the poisoning in the study group, and reduction of infused (for 1 patient) average natural colloids volumes in the study group—149.20±22.45 ml vs 209.36±18.15 ml—in control. No differences were found in GI complication rates, LOS in ICU and lethality among study and control groups. Conclusion: Early enteral nutritional support may be used in the treatment of moderate and severe acute acetic acid poisonings as safer and, in some aspects, more effective treatment than massive infusions of natural colloids and partial parenteral nutrition.
209 SPECIALTIES OF A TOXICOLOGICAL INFORMATION CENTRE IN AN INDUSTRIAL REGION OF RUSSIA
Nozhkina NV, Sentsov VG, Brusin KM, Brovkin MV, Yentus VA
Sverdlovsk Regional Toxicological Center, Ural State Medical Academy, Yekaterinburg, Russia
Background: Sverdlovsk region is a large industrial area in the centre of the Russian Federation covering 195,000 km2 and a population of about 4,425,400 people. In 1990s we recorded an increasing number of hospitalised patients with acute poisonings: from 7376 patients in 1990 to 11280 in 2000. There are 5 specialist toxicological treatment centres located in the biggest cities of the region. An information center operates from the regional toxicological treatment center providing phone and on-site consultations 24 hours per day, making it possible to provide optimum toxicological care. Objectives: The investigation of the patient groups treated at the service centre, and examination of the departmental structure for the pathology speciality, to improve effective decision-making and the functioning of the centre. Methods: The case database of the centre for 2000 was analysed using 24 parameters. Results: 386 consultations were studied. 42.3% were from the towns, 33.4% from the rural territories and the remainder originated from Yekaterinburg (the main city of the region). The distribution of the calls: 53.8% were from reanimatologists, 37.5% general physicians, 2% emergency physicians, 0.9% toxicologists, 1.4% chiefs of the hospitals, and 3.8% members of the public. The clinical status of most patients was either severe (52.8%) or moderate (31.2%). The circumstances of the poisoning: 29% were suicide attempts, 20.1% alcohol usage, 7% opiate or toxic inhalant overdoses, 14.2% unknown. 35% of cases were poisonings with medicines, 16.7% with chemically aggressive fluids, 14.6% with alcohol and glycols, 9.4% with industrial toxins, 9% with household products, 5.3% with opiates, and in 4.5% the type of the toxin was unknown. Before consultation 45% of the patients were treated symptomatically, 40% by stimulating diuresis and 13.9% of patients had no medical intervention. 46% of consultations were to determine an appropriate method of treatment, 27.4% to determine the need for hospitalization at the regional centre, and in 23.2% of cases the callers needed help in diagnostics. After consultation hospitalization was recommended for 62.6% of cases, treatment plans were determined for 46% of cases, for 24% chemical and toxicological investigation in the laboratory of toxicological center was arranged, and in 24.2% there was a specialist referral. Conclusion: The existence of the information center provides the possibility to improve the quality of healthcare for the toxicological patients with severe poisoning, to centralize the information about most serious cases of poisoning, and to assess and improve the level of knowledge of physicians handling toxicological emergencies.
210 WEAPONS OF MASS DESTRUCTION: ARE WE ADEQUATELY PREPARING OUR FUTURE PHYSICIANS? AN OPPORTUNITY FOR POISON CONTROL CENTERS
McFee RB, Caraccio TR, Howell J
The LI Regional Poison Control Center, Winthrop University Hospital-Mineola, New York and Nova-Southeastern University COM, Davie, Florida, USA
Background: The attacks of September 11th and recent anthrax events have raised awareness and concern about weapons of mass destruction (WMD). In addition to poison control centers (PCC) and emergency responders, community physicians and physicians in training may take on a new role and be called upon to address WMD. An extensive literature search failed to identify articles discussing the preparedness or training of medical students, and few described such issues concerning residents and attending physicians. A phone survey of regional medical schools failed to identify any with WMD training in the curriculum. Most PCC provide educational programs primarily for emergency providers and trainees. Objective: To identify the general knowledge of WMD, including toxidromes and general public health management of such events, among medical students at a university in South Florida; a region that has been directly involved in WMD/anthrax events. Also, to assess the potential role of PCC to provide educational programs at medical schools. Method: A validated survey was administered. Twenty-five questions covered two broad categories: 1. General knowledge about WMD agents including basic management and 2. general public health preparation. Demographics, including prior military training were ascertained. Results: n=166. Forty-seven percent (78) thought inhalational anthrax was contagious. Less than 50% could identify the signs and symptoms of nerve agents (NA/organophosphates), cyanide or botulinum. Less than 50% knew the appropriate sheltering procedure for a gas exposure; <50% could identify the toxidrome/biodrome© for NA, arsine or smallpox, or the characteristic chest xray findings in inhalational anthrax. Over 50% correctly identified the appropriate authorities to handle a WMD event. Conclusion: To our knowledge this study is the first to describe the knowledge level of medical students, especially ones in a community directly affected by WMD. Virtually every communications medium presents information about WMD. In spite of the increasing availability of information, our medical students are neither knowledgeable nor being trained about WMD. PCC are in a unique position to provide the leadership and training in WMD yet remained underutilized. Many PCC are university affiliated and work closely with public health departments. Such collaboration, until recently, has been uncommon outside the PCC arena. PCC need to take a great role in training the health care community, especially future physicians. It is unlikely we can turn back the clock—the threat of WMD should always be taken seriously. As a result of this study, a WMD course has been developed in concert with PCC and is being provided to the medical college.
211 SUICIDAL POISONING WITH CASTOR BEAN (RICINUS COMMUNIS) EXTRACT INJECTED SUBCUTANEOUSLY—CASE REPORT
Targosz D, Winnik L, Szkolnicka B
Department of Clinical Toxicology Jagiellonian University Medical College, Krakow, Poland
Objective: Castor bean (Euphorbiaceae family) contains ricin and ricinine (toxalbumins), one of the most toxic substances known. This plant is cultivated in Poland as a decorative bush. Although ricin is present through the plant, the seeds contain the highest concentration. Reported cases involve ingestion of well-masticated seeds, whole seeds are nontoxic. Ricin ranks among the three most toxic parenteral substances by weight in the world. A biphasic toxicity is described consisting of acute and potentially fatal gastroenteritis followed by damaged viscera several days later. We report a 20-year-old man who injected subcutaneously castor bean extract in suicide attempt. Case Report: The patient was admitted to the clinic 36 hours after injecting the extract. He was experiencing severe weakness, nausea, dizziness, headache, compression in chest, abdominal pain and muscular pain of extremities with numbness. Tachycardia, hypotension, anuria, and metabolic acidosis were stated on admission. The patient was sent to a toxicological intensive care unit. Suggilation and edema at the site of needle insertion were seen. Fresh blood was seen in per rectum examination. No coagulopathy, elevated activity of transaminases, serum urea, creatine and leucocytes were noted on laboratory examination. During observation the patient deteriorated with hemorrhagic diathesis and liver, kidney, cardiovascular and respiratory systems failure. Endotracheal intubation and artificial ventilation were required. The maximal doses of pressor amines and hemorrhagic diathesis treatment were ineffective. Despite symptomatic intensive care the patient developed symptoms of multiorgan failure. After 18 hours he developed asystolic arrest. Resuscitation was not effective. Hemorrhagic foci in the brain, myocardium, and pleura were seen in postmortem examination.
212 BUPROPION—THE EXPERIENCE OF THE NATIONAL POISONS INFORMATION SERVICE (LONDON)
Colbridge MG, Dargan PI, Jones AL
National Poisons Information Service (London) (NPIS (London)), Guy's and St Thomas' NHS Trust, London, United Kingdom
Objectives: Bupropion (Zyban®) was introduced in the UK in June 2000 as an adjunct to cessation of smoking. It has been used both for this indication and as an antidepressant in the USA since 1989. The risk of convulsions with overdose of bupropion is well documented and there are isolated case reports of ECG abnormalities1 and reported fatalities2 after bupropion overdose. Our aim was to investigate the incidence of these and any other severe adverse events in a series of bupropion overdoses in the UK. Methods: We prospectively monitored all enquiries to the NPIS (L) involving bupropion ingestion following the first twelve months of its introduction Follow-up information regarding the clinical course was obtained by means of a postal questionnaire. Results: We received 224 calls about bupropion, involving 190 patients. Of these, 93 (49%) were accidental therapeutic errors and 97 (51%) were overdose cases. Of 109 follow-up questionnaires sent, there was a return of 57 (52%) of which 7 patients couldn't be traced. Of the remaining 50, the age range was 2–75 years. The range of stated doses was 150 mg–18.75 g (median 1.8 g) and the male: female ratio was 1:1.27. The clinical effects were tachycardia in 20 patients (40%), ECG abnormalities in 5 (10%), vomiting in 6 patients (12%), tremor and slurred speech in 5 patients (10%) and confusion in 4 patients (8%); 8 patients (16%) remained asymptomatic; these included the 3 paediatric cases (ages 2, 2.5 and 4 years) who had ingested 150–600 mg. Twelve patients (24%) had convulsions, the dose ingested in these was 1.5–18.75 g (median 4.7 g). The convulsions were self-limiting in 9 (75%) patients and one required 5 mg intravenous diazepam. The remaining 2 had prolonged convulsions followed by an EMD arrest after ingestion of 1.5 g and 5.4 g. Both patients were stabilized with intubation and ventilation, epinephrine 1 mg and treatment with thiopentone and lorazepam; they both developed hypotension requiring inotropic support, but made a full recovery and were extubated on day 2. Conclusions: Bupropion was well tolerated in the majority of cases but all patients who have ingested more than 1.5 g should be admitted for observation because of the risk of convulsions and / or cardiac arrest.
References: 1. Paris, P.A.; Saucier, J.R. ECG Conduction Delays Associated with Massive Bupropion Overdose. J. Toxicol. Clin. Toxicol. 1998, 36, 595–598. 2. Harris, C.R.; Gualtieri, J.; Stark, G. Fatal Bupropion Overdose. J. Toxicol. Clin. Toxicol. 1997, 35, 321–324.