Abstract
In 2003, US poison centers were contacted regarding ingestion of β-blockers by 15,350 patients including 3766 (25%) under 6 years of age; 7415 (48%) were evaluated in healthcare facilities and 33 died.
An evidence-based expert consensus process was used to create this guideline. Relevant articles were abstracted by a trained physician researcher. The first draft of the guideline was created by the primary author. The entire panel discussed and refined the guideline before its distribution to secondary reviewers for comment. The panel then made changes in response to comments received.
The objective of this guideline is to assist US poison center personnel in the appropriate out-of-hospital triage and management of patients with suspected ingestions of β-blockers by describing the process by which a β-blocker ingestion might be managed, identifying the key decision elements in managing cases of β-blocker ingestion, providing clear and practical recommendations that reflect the current state of knowledge, and identifying needs for research.
This guideline applies to ingestion of β-blockers alone and is based on an assessment of current scientific and clinical information. The panel recognizes that specific patient care decisions may be at variance with this guideline and are the prerogative of the patient and health professionals providing care, considering all of the circumstances involved.
Recommendations are in chronological order of likely clinical use; the grade of recommendation is in parentheses. 1) Patients with stated or suspected self-harm or who are the victims of a potentially malicious administration of β-blocker should be referred to an emergency department immediately. In general, this should occur regardless of the dose reported (Grade D). 2) Patients without evidence of self-harm should have further evaluation, including determination of the precise dose ingested, history of other medical conditions, and the presence of co-ingestants. Ingestion of either an amount that exceeds the usual maximum single therapeutic dose or an amount equal to or greater than the lowest reported toxic dose (whichever is lower) warrants consideration of referral to an emergency department. Ingestion of any excess dose of any β-blocker in combination with a calcium channel blocker or the ingestion of any excess dose by an individual with serious underlying cardiovascular disease also warrants referral to an emergency department (Grade C). 3) Do not induce emesis. Consider the oral administration of activated charcoal if it is available and no contraindications are present but do not delay transportation to administer charcoal (Grade A). 4) Asymptomatic patients who ingest more than the referral dose should be sent to an emergency department if the ingestion occurred within 6 hours of contacting the poison center for an immediate-release product other than sotalol, within 8 hours of contacting the poison center for a sustained-release product, and 12 hours if they took sotalol (Grade C). 5) Ambulance transportation is recommended for patients who are referred to emergency departments because of the potential for life-threatening complications of β-blocker overdose. Provide usual supportive care en route to the hospital, including intravenous fluids for hypotension (Grade D). 6) Follow-up calls should be made to determine outcome at appropriate intervals for up to 12–24 hours based on the judgment of the poison center staff (Grade D). 7) Asymptomatic patients who are referred to healthcare facilities should be monitored for at least 6 hours after ingestion if they took an immediate-release preparation other than sotalol, 8 hours if they took a sustained-release preparation, and 12 hours if they took sotalol. Routine 24-hour admission of an asymptomatic patient who has unintentionally ingested a sustained-release preparation is not warranted (Grade D).
Introduction
Scope of the Problem and Importance of this Guideline
Drugs that antagonize the β-adrenergic receptor (β-blockers, β-adrenergic antagonists) are potentially lethal when taken in overdose. According to the Toxic Exposure Surveillance System (TESS) of the American Association of Poison Control Centers, United States (US) poison centers were contacted regarding ingestion of β-blockers by 15,350 patients in 2003, including 3766 patients (25%) under 6 years of age. A total of 7415 patients (48%) were evaluated in healthcare facilities, 1761 exhibited moderate effects, 407 exhibited major effects, and 33 died Citation[1]. A review of all intentional and unintentional fatalities reported by US poison centers for the years 1985–2002 revealed 41 deaths in which a β-blocker was the only ingested drug. The age range of this β-blocker-only fatality cohort was 14 to 80 years. There were no reported β-blocker deaths in patients under 6 years of age Citation[2].
The evaluation and management of possible β-blocker poisoning has medical, economic and social costs. Because β-blocker ingestion can cause severe toxicity including hypotension, bradycardia, cardiovascular collapse and death, the usual dose threshold for medical evaluation and observation has been quite low. It is critical to decide on a strategy that could be used to determine which patients need referral to healthcare facilities for medical evaluation. Poison centers usually recommend emergency department evaluation of any patient who becomes symptomatic or intentionally ingests a β-blocker to cause self-harm. The most important decision is to select a critical threshold dose that requires emergency department evaluation after an unintentional ingestion in either an adult or a child. Typical scenarios include a β-blocker-naïve child who is found handling a β-blocker medication container who might have ingested one or more tablets or capsules, or an adult or child who already takes a β-blocker and inadvertently doubles or triples their dose. Referring every β-blocker ingestion to the emergency department regardless of dose is expensive and labor intensive Citation[3]. However, the therapeutic index of β-blockers is low. In some cases the reported toxic dose may overlap with the maximum therapeutic dose. Patients with significant underlying cardiovascular disease might be especially vulnerable to the toxic effects of these drugs. Unintentional double doses in this group might be more likely to cause toxicity than comparable ingestions in healthy patients.
This guideline provides recommendations on the duration of observation an asymptomatic patient requires if the patient's ingestion exceeds a threshold dose. Deciding on how long to observe after the suspected ingestion of a sustained-release preparation can be particularly perplexing. Currently, several of the commonly prescribed β-blockers are sustained-release preparations. Given the potential for prolonged symptoms, many poison centers automatically recommend a 24-hour admission for patients with suspected ingestions of sustained release preparations. A somewhat different scenario occurs when a caller to the poison center inquires about an unintentional ingestion in an asymptomatic patient that had occurred many hours (e.g., 12–48 hours) prior to the call. In these cases, the ingested dose may have exceeded the referral dose but, given the time lapse, hospital referral is no longer necessary.
The potential costs of ambulance transport, emergency department evaluation, and intensive care unit observation are substantial, especially considering that most patients develop no symptoms as a result of these exposures. In the report of Belson et al. Citation[4], only eight of 378 potential β-blocker exposures in children (2%) actually resulted in symptoms (four with minor and four with moderate symptoms).
There is a paucity of data from studies with high levels of evidence that address these issues. Randomized clinical trials have never been done and very few cohort or case-control studies on acute β-blocker overdose have been performed to date. The expert consensus panel utilized the available data—mainly case series, case reports, abstracts, poison center experiences—along with its own clinical experience and expertise to create these recommendations.
Background and Definitions
The β-blocker drugs are used for a variety of medical disorders including angina pectoris, hypertension, congestive heart failure, tachydysrhythmias, reduction of post-myocardial infarction mortality, thyrotoxicosis, pheochromocytoma, migraine headache, glaucoma, tremor, and anxiety. These drugs competitively block β-adrenergic receptors resulting in decreased cyclic AMP production and a blunting of catecholamine effects. The resulting negative inotropic, chronotropic, and dromotropic effects are manifested by decreases in blood pressure and pulse rate.
Individual β-blockers vary with regard to their β-receptor selectivity, intrinsic stimulatory activity, lipid solubility and membrane-stabilizing (sodium channel) effects (). Stimulation of β1 receptors increases the inotropy, chronotropy, and automaticity in the heart. β2 receptor stimulation results in bronchodilation, enhanced gluconeogenesis, and potassium movement into cells. Propranolol is a nonselective β-adrenergic antagonist that demonstrates equal affinity for β1 and β2 receptors and lacks intrinsic activity. Its high lipid solubility facilitates entry into the brain resulting in the increased likelihood of neurological effects such as seizures at toxic doses. Atenolol and metoprolol are selective β1 antagonists. β-adrenergic receptor cardioselectivity is diminished after overdose. Labetalol is a β- and an α1-adrenergic antagonist. Sotalol is a nonselective β-adrenergic antagonist that is particularly toxic because is also antagonizes the potassium channel. Timolol, a nonselective β-adrenergic antagonist, is often dispensed as a liquid ophthalmic preparation used to treat glaucoma.
TABLE 1. Properties of β-adrenergic receptor blockers.
Toxicity from β-blockers can result in an excessive decrease in blood pressure or pulse rate. Other toxic manifestations of β-blocker toxicity include seizures, coma, and occasional bronchospasm and hypoglycemia. In most reported cases, toxicity resulted from an intentional, single, acute ingestion. Chronic toxicity is less commonly reported.
Critical factors that need to be considered in the assessment of β-blocker ingestion include the specific β-blocker, type of product (immediate- vs. sustained-release), the presence of synergistic coingestants, the dose, presence of pre-existing medical conditions, whether the patient is currently taking β-blockers, and the intent of the patient. Patients with underlying cardiovascular disease such as cardiac conduction disturbances or patients on other cardioactive medications such as calcium channel blockers could be particularly vulnerable to β-blocker toxicity.
For the purpose of this guideline, age groups are defined as 1) children under 6 years of age and 2) older children and adults. The older age group is much more likely to attempt self-harm and to conceal an ingestion. Acute exposures are defined as those occurring over a period of no more than 8 hours, and chronic exposures are those that occur over a period of 8 or more hours.
Intended Users of the Guideline
The intended users of this guideline are personnel in US poison centers. This guideline has been developed for the conditions prevalent in the US. While the toxicity of β-blockers is not expected to vary in a clinically significant manner in other nations, the out-of-hospital conditions could be much different. Some β-blockers available outside the US are not currently marketed in the US. These β-blockers are not addressed in this document. This guideline should not be extrapolated to other settings unless it has been determined that the conditions assumed in this guideline are present.
Objective of this Guideline
The objective of this guideline is to assist poison center personnel in the appropriate out-of-hospital triage and initial management of patients with suspected ingestions of β-blockers by 1) describing the process by which a β-blocker ingestion might be managed, 2) identifying the key decision elements in managing cases of β-blocker ingestion, 3) providing clear and practical recommendations that reflect the current state of knowledge, and 4) identifying needs for research. This guideline applies to ingestion of β-blockers alone. Co-ingestion of additional substances could require different referral and management recommendations depending on the combined toxicities of the substances.
This guideline is based on an assessment of current scientific and clinical information. The expert consensus panel recognizes that specific patient care decisions may be at variance with this guideline and are the prerogative of the patient and the health professionals providing care, considering all of the circumstances involved.
Methodology
The methodology used for the preparation of this guideline was developed after reviewing the list of key elements of guidelines described by Shaneyfelt et al. Citation[5]. An expert consensus panel was established to oversee the guideline development process (Appendix 1). The American Association of Poison Control Centers (AAPCC), the American Academy of Clinical Toxicology (AACT), and the American College of Medical Toxicology (ACMT) appointed members of their organizations to serve as panel members. To serve on the expert consensus panel, an individual had to have an exceptional track record in clinical care and scientific research in toxicology, board certification as a clinical or medical toxicologist, significant U.S. poison center experience, and bean opinion leader with broad esteem. Two Specialists in Poison Information were included as full panel members to provide the viewpoint of the end-users of the guideline.
Literature Search
The National Library of Medicine's MEDLINE database was searched (1966–February 2003) using adrenergic beta-antagonists (exploded as a MeSH term) with the subheadings poisoning (po) or toxicity (to), limited to humans.
The MEDLINE and PreMEDLINE (1966–February 2003) were searched using a list of 42 β-blockers as textwords (title, abstract, MeSH term, CAS registry) plus either poison* or overdos* or tox*, limited to humans. This same process was repeated in International Pharmaceutical Abstracts (1970–February 2003, excluding abstracts of meeting presentations), Science Citation Index (1977–February 2003), Database of Abstracts of Reviews of Effects (accessed February 2003), Cochrane Database of Systematic Reviews (accessed February 2003), and Cochrane Central Register of Controlled Trials (accessed February 2003). A similar search was conducted in EMBASE (1990–March 2003). MEDLINE was searched again for all articles describing β-blocker use in children from 1 through 5 years of age. Reactions (1980–March 2003), the β-blocker poisoning management in POISINDEX Citation[6], and the bibliographies of recovered articles were reviewed to identify previously undiscovered articles. Furthermore, North American Congress of Clinical Toxicology abstracts published in the Journal of Toxicology-Clinical Toxicology (1995–2003) were reviewed for original human data. The chapter bibliographies in four current major toxicology textbooks were reviewed for citations of additional articles with original human data Citation7-10. Finally, The Toxic Exposure Surveillance System (TESS) maintained by the American Association of Poison Control Centers, was searched for deaths resulting from unintentional β-blocker poisoning or any deaths from β-blocker poisoning in children. These cases were abstracted for use by the panel.
Criteria Used to Identify Applicable Studies
The recovered citations were entered into an EndNote library and duplicate entries were eliminated. The abstracts of these articles were reviewed, looking specifically for those that dealt with: 1) estimations of mg/kg or ingested doses with or without subsequent signs or symptoms, and 2) management techniques that might be suitable for out-of-hospital use (e.g., gastrointestinal decontamination). Articles excluded were those that didn't meet either of the preceding criteria, didn't add new data (e.g., some reviews, editorials), or that described inpatient-only procedures (e.g., dialysis).
Data Extraction Process
All articles that were retrieved from the original search were reviewed by a single abstractor. Each article was assigned a level of evidence score from 1 to 6 (Appendix 2); the complete paper was reviewed for original human data regarding the toxic effects of β-blockers, or original human data directly relevant to the out-of-hospital management of patients with β-blocker toxicity or overdose. Relevant data (e.g., dose of β-blocker, resultant effects, time of onset of effects, therapeutic interventions or decontamination measures given, efficacy or results of any interventions, and overall patient outcome) were compiled into a table and a brief summary description of each article was written. This evidence table is available at http://www.aapcc.org/DiscGuidelines/BetaBlockerEvidenceTable.pdf. The completed table of all abstracted articles was then forwarded to the panel members for review and consideration in developing the guideline. Every attempt was made to locate foreign language articles and have their crucial information extracted, translated and tabulated. In addition to this evidence table, several brief sub-tables were generated that included all of the articles and data relating to a particular topic (e.g., dose of β-blockers in acute pediatric ingestions reported to cause toxicity). These were also forwarded to the author and guideline panel members. A written summary of the data was created and distributed by the abstractor. Copies of all of the abstracted articles were made available for reading by the panel members on a secure AAPCC website.
Criteria Used to Evaluate Studies and Assign Levels of Evidence
The articles were assigned level-of-evidence scores based on the Grades of Recommendation table developed by the Centre for Evidence-Based Medicine at Oxford University (Appendix 2). Single case reports were classified along with case series as level 4.
Guideline Writing and Review
A guideline draft was prepared by the primary author. The draft was submitted to the expert consensus panel for comment. Using a modified Delphi process, comments from the expert consensus panel members were collected, copied into a table of comments, and submitted to the primary author for response. The primary author responded to each comment in the table and, when appropriate, the guideline draft was modified to incorporate changes suggested by the panel. The revised guideline draft was again reviewed by the panel and, if there was no strong objection by any panelist to any of the changes made by the primary author, the draft was prepared for the external review process. External review of the second draft was conducted by distributing it electronically to AAPCC, AACT, and ACMT members and the secondary review panel. The secondary review panel consisted of representatives from the federal government, public health, emergency services, pediatrics, pharmacy practice, and consumer organizations (Appendix 3). Comments were submitted via a discussion thread on the AAPCC web site or privately through email communication to AAPCC staff. All submitted comments were stripped of any information that would identify their sources, copied into a table of comments, and reviewed by the expert consensus panel and the primary author. The primary author responded to each comment in thetable and his responses and subsequent changes in the guideline were reviewed and accepted by the panel. Following a meeting of the expert consensus panel, the final revision of the guideline was prepared.
Review of Current Practice
Recommended Therapeutic Doses of β-Blockers
To determine which patients need to be referred in to a healthcare facility many factors need to be considered. A review of current poison center guidelines suggests that a threshold dose is often used to facilitate this referral decision. At present, the referral threshold dose varies widely between poison centers ().
TABLE 2. Summary of pediatric β-blockers guidelines obtained from 17 US poison control centers.
The decision to refer an asymptomatic patient to a healthcare facility is based on many factors including the inherent toxicity of the drug, the potential for clinical deterioration, underlying medical conditions, and concomitant medical therapy. For patients with pre-existing medical conditions or for those patients taking other cardioactive medications, such as calcium channel blockers, the threshold referral dose might need to be reduced. In addition, minimal toxic symptoms such as transient nausea might be well tolerated at home and not require medical evaluation but more significant symptoms such as repetitive vomiting, syncope, orhypotension might merit medical evaluation and might require intervention with intravenous fluids or pressor agents.
Although some patients might develop adverse effects at therapeutic doses, the vast majority of patients who develop toxicity have ingested a supratherapeutic dose. An understanding of the range of therapeutic doses is important to arrive at what constitutes a supratherapeutic dose. References may cite a number of different dosing concepts including therapeutic dose, initial dose, maximum single dose, and maximum daily dose. Some β-blockers are dosed once per day (e.g., atenolol) while others are dosed as often as 4 times per day (e.g., propranolol). In addition, some therapeutic dosing regimens include initial starting doses that can be considerably less than typical therapeutic doses.
In each case, the recommended dose may not be a single dose but a range of doses. In addition, the dose may vary depending on the medical indication. As an example, the therapeutic dose range for propranolol in the treatment of hypertension is 80–240 mg/day (although doses as high as 640 mg/day have been used); while for pheochromocytoma the recommended dose is 30 mg/day. Given these ranges, establishing a threshold using therapeutic dose or maximum therapeutic dose is problematic.
For the purpose of developing this guideline, USP DI, Drug Facts and Comparisons, and DRUGDEX were consulted to determine therapeutic dosing of β-blockers in adults and their available dosage forms Citation11-13. Unfortunately, these references provide limited information on pediatric therapeutic dosing. This is because in many cases the safety and efficacy of β-blockers in children have not been established. Nonetheless, except for timolol, most of these β-blockers are utilized in children. To define appropriate β-blocker therapeutic dosing in children, five standard drug and pediatric reference textbooks were also reviewed Citation14-18. provides information on the usual lowest single therapeutic dose, usual maximum single therapeutic dose, usual maximum therapeutic daily dose, dosing frequency and oral dosage forms. The range of maximum daily therapeutic doses can be quite wide. For example, three of the references suggest that the maximum daily dose for propranolol is 16 mg/kg in children. Three otherreferences listed either 5 mg/kg or 6 mg/kg as the 24-hour maximum pediatric dose. Such differences in maximum dose added to the challenge of guideline development.
TABLE 3. Therapeutic doses of β-blockers in adults and children over 6 years of age.
A key question that needs to be considered is which supratherapeutic dose constitutes a toxic dose. Depending on the therapeutic index, a supratherapeutic dose need not be a toxic dose. Current practice patterns (see below) indicate that some poison centers refer patients to healthcare facilities when they have ingested a dose above a therapeutic dose or maximum therapeutic dose or maximum therapeutic daily dose. Ideally, the most important factor to consider should be the dose that causes toxicity, not the therapeutic dose.
Given the lack of controlled trials assessing overdose and toxicity, the extant evidence is largely limited to case reports and case series. These data have many limitations including the dose history. A report may state that a certain dose was ingested but this information might have been obtained over the telephone from a healthcare provider and not directly from a patient. Even if obtained directly from the patient, recall bias regarding exact dose may be a problem. With pediatric exposures in particular, many uncertainties arise in determining the exact dose ingested. Without a direct patient history, such information could be derived from estimates of tablet counts. Since the vast majority of case reports do not have confirmatory analytical data—either quantitative or qualitative—in some cases the suspected ingestion might have not occurred or it might be an ingestion of another product.
Current Poison Control Center Practice
Not surprisingly, current prehospital guidelines regarding out-of-hospital management of β-blocker ingestions vary considerably. In 2000, Belson et al. Citation[4] surveyed medical directors at 49 AAPCC-certified regional poison control centers about poison center recommendations for pediatric β-blocker exposures. Thirty-three poison centers responded to the survey. Of these respondents, 14 had triage guidelines for referring children to healthcare facilities following β-blocker exposures. Thirty-six percent of respondents referred all β-blocker exposures to heathcare facilities regardless of the dose. Referral patterns varied depending on the specific β-blocker ingested. Twenty-two percent of centers referred all atenolol ingestions to healthcare facilities and half referred all metoprolol SR (sustained-release) ingestions to healthcare facilities. For those centers that were more selective in their referral pattern, the threshold dose for referral also varied considerably. Some centers used any amount greater that a single therapeutic dose as their threshold for referral while other centers used any amount greater than a daily therapeutic dose, or any amount greater than a certain mg/kg dose. For the centers using mg/kg thresholds, the threshold dose also varied. For propranolol IR (immediate-release) and metoprolol IR ingestions, 7% of centers used 2 mg/kg or more as their threshold while 22% of centers used 8 mg/kg or more as a threshold. Belson's study clearly showed that one standard for referral did not exist.
Belson also reported the length of observation period recommended by poison centers for patients who ingest β-blocker products. For immediate-release products, suggested observation periods ranged from 0–4 hours to 12–23 hours, with 5–8 hours being the most common recommendation. Longer observation was recommended after ingestions of sustained-release products, ranging from 5–8 hour to 24 hours or more; 20% of the 33 centers surveyed recommended 24 or more hours of observation.
In 2003, during the preparation of this guideline, the expert consensus panel decided to further investigate poison center referral patterns for β-blocker ingestions. All U.S. poison centers were solicited by the AAPCC to forward a copy of their β-blocker guideline, if available, for review. One state poison center system (four poison centers) and 16 other individual poison centers responded. Three responding centers did not have any specific β-blocker guidelines. Consistent with Belson's study, a review of these guidelines shows that referral patterns to healthcare facilities were highly variable (). Referral thresholds were based on mg/kg thresholds, the maximum daily therapeutic doses, initial daily doses, lowest single therapeutic doses, or one tablet. Some centers recommended referral for any amount. Other centers used twice the prescribed dose or maximum therapeutic dose (whichever is larger). In some cases a center defined the maximum therapeutic dose (e.g., 3 mg/kg propranolol) while other centers did not define this maximum dose. The mg/kg referral thresholds for propranolol included 2, 3, 4, 6, and 8 mg/kg. The scope of the guidelines also varied regarding the individual specificity of β-blocker information. Some guidelines provided generic information regardless of the specific β-blocker (e.g., refer in all β-blocker ingestions greater than the maximum daily dose) while others provided specific threshold referral points on 19 different β-blocker products. Some of the guidelines also provided specific information about suggested observation times after β-blocker ingestions. For immediate-release preparations, the typical recommendation was to observe for at least 6 hours after ingestion but for the sustained-release preparations the recommended observation time was often considerably longer. Several centers recommended hospital admission and observation for up to 24 hours.
Such a wide range of approaches suggests the lack of a clear evidence-based standard of care. A more consistent approach to β-blocker ingestion would offer an opportunity to reduce over- and under-referral to healthcare facilities as well as to identify areas for further research and analysis.
Review of the Medical Literature
Dose of the β-Blocker Causing Toxicity after Overdose
Of the 42 β-blockers that have been developed, 15 are commercially available in the U.S. During the years 2000–2002,the most common β-blocker ingestions reported to US poison centers were atenolol 36%, metoprolol 32%, propranolol 16%, and carvedilol 4% Citation[2]. This guideline develops specific recommendations for the nine β-blockers for which there are the most data: acebutolol, atenolol, carvedilol, labetalol, metoprolol, nadolol, propranolol, sotalol, and timolol. Based on the literature review, provides a summary of the lowest reported toxic dose and longest reported delay to onset of toxicity for these drugs.
TABLE 4. Maximum recommended single therapeutic dose, lowest reported toxic dose, and longest reported delay in onset of toxicity for β-blockers.
Adult Acute Supratherapeutic Ingestion
Most of the medical literature regarding β-blocker toxicity in humans consists of case reports and a few case series. Studies designed to specifically investigate a threshold dose for the development of acute β-blocker toxicity have not been performed. There are no level 1 studies that evaluate threshold dose.
One level 2b/3b study looked at acute β-blocker overdose data in both cohort and case-control fashions, to compare the relative toxicity of certain β-blocker agents Citation[25]. Although a specific dose-toxicity relationship for each agent was not investigated, certain information can be inferred regarding such relationships. The authors found that acute ingestion of greater than 2 g of propranolol by adults was associated with a higher likelihood of seizures than ingestions of less than 2 g. No patient ingesting less than 1.2 g of propranolol in this report developed seizures. The patients with seizures had greater cardiovascular toxicity than the group that did not have seizures. Therefore, it can be inferred that adults with acute ingestions of more than 1.2 g of propranolol, and particularly those with ingestions of more than 2 g are more likely to experience seizures and a greater degree of toxicity. What is not clear, however, is whether there was a threshold dose below which no one developed toxicity.
A large number of level 4 reports were identified with specific dose-toxicity information. It should be stressed that in the vast majority of cases, it was not possible to gauge the accuracy of the estimated dose. By reviewing these reports, the expert consensus panel attempted to determine the lowest doses to cause significant toxicity. Many of these cases were confounded by concomitant ingestions. For example, the lowest dose of propranolol alleged to cause significant toxicity was reported by Chen et al. Citation[26]. In this case a 21-year-old woman developed hypotension (blood pressure decreased from 118/76 to 80/50 mmHg) and bradycardia (pulse dropped from 82 to 59/minute) after taking 280 mg of propranolol. Her hypotension improved without treatment. However, she had also ingested thioridazine 350 mg and diazepam 42 mg. Henceit is very difficult to determine how much of the hypotension is directly related to the propranolol. The case with the next lowest propranolol dose to cause toxicity was also reported by Chen et al. in the same paper Citation[26]. In this case, a 17-year-old woman presented with a blood pressure of 120/70 mmHg and pulse of 45/minute 6 hours after ingesting 500 mg of propranolol. Soon after admission her blood pressure dropped to 80/60 mm Hg. She was treated with glucagon and her blood pressure improved. In this case the patient also took 500 mg of oxazolom, a long-acting benzodiazepine. The next lowest dose to cause toxicity was reported by Ducret et al. Citation[27]. In this case, a 65-year-old woman took 800 mg propranolol alone and developed severe hypotension. Her plasma propranolol concentration of 1536 ng/mL was markedly elevated above the therapeutic range (14–90 ng/mL). This case more convincingly supports the contention that an 800-mg dose has the potential to cause severe toxicity. Such a literature analysis, however, does not provide information on what dose causes little or no toxicity.
Pediatric Acute Supratherapeutic Ingestion
No level 1, 2, or 3 data were found evaluating the threshold dose for the development of acute clinical toxicity after β-blocker overdoses in children less than 6 years of age.
Several level 4 reports provided specific dose-toxicity information for pediatric β-blocker exposures. In a 1973 report Citation[28], two toddlers together ingested a total of 150 mg of propranolol. The exact amount ingested by each child was uncertain. At 7 hours after ingestion the two children were found to have blood glucose concentrations of 14 mg/dL and 50 mg/dL. In another case, a 3-year-old ingested 400 to 1200 mg of propranolol. The plasma concentration was 2289 ng/mL at 4 hours after ingestion. Despite this dose and the high serum concentration, the only observed effect was a diminished heart rate response to crying and activity Citation[29]. Belson et al. Citation[4] performed a retrospective study of 411 cases of acute β-blocker exposures in children less than 7 years of age during a 7-year period. Three hundred seventy-eight patients were included in the final analysis. Forty-one percent were managed at home and 59% were evaluated in healthcare facilities. Fifty percent of the 348 who had documentation about GI decontamination actually underwent decontamination. Only eight of 378 (2%) patients, six of whom underwent decontamination, became symptomatic. Four patients developed mild symptoms and four developed moderate symptoms. None developed severe toxicity and there were no deaths. Of the symptomatic patients, the smallest toxic dose of atenolol was 5.3 mg/kg and the smallest toxic dose of propranolol was 5 mg/kg.
Adult and Pediatric Chronic Supratherapeutic Ingestion
No level 1, 2, or 3 data were found regarding chronic (over more than 8 hours) supratherapeutic β-blocker ingestions in either age group. Only a few level 4 reports could be found on chronic dose-toxicity. The interpretation of the medical literature is complicated by the effects of conditions that are thought to lower the threshold for toxicity such as pre-existing cardiovascular disease, concomitant calcium channel blocker use, or co-ingestion of other cardiovascular agents. One case report described a 4-year-old boy who was reported to develop a seizure and hypoglycemia while taking propranolol at 10 mg/kg/day for renovascular hypertension, a dosage that exceeds the manufacturer's usual recommended dosage of 2–4 mg/kg/day. The child had been maintained on this dosage for some time previously without problems and only developed hypoglycemia and a seizure after 3 days of fasting due to facial trauma Citation[29].
Time of Onset of Toxicity after Overdose
Clinical effects were defined as any sign, symptom, or laboratory/electrocardiographic finding consistent with β-blocker toxicity. It is important to note that the actual onset of effects likely occurred earlier than reported in many cases, because many patients appear to have presented well into the course of their poisoning. Case reports generally refer only to the time of onset of the first toxic effect. They do not give information on the time-to-peak-effects or the total duration of effects. In several instances of overdose with sotalol, patients clinically deteriorated many hours into the course of their poisoning, implying a peak dysrhythmic effect that could occur quite late in a patient's course (e.g., 4–20 hours) Citation[30].
Adult Acute Supratherapeutic Ingestion
There were no level 1 studies investigating the time of onset of clinical effects after β-blocker overdose in adults. There was one level 2b paper that contained prospective observational information on the time of onset Citation[31]. This 6-year review of cases of β-blocker overdose that were reported to two poison centers found that 92% (22/24) patients who developed clinical toxicity and in whom a time was recorded had the onset of effects within 3 hours of ingestion. Eight percent (2/24) developed effects between 3–6 hours, and none had an onset of effects later than 6 hours after ingestion. A level 2b study of β-blocker overdoses found that all patients developed effects within 6 hours of ingestion Citation[25].
With the exception of sotalol, the onset of clinical effects occurs within 6 hours in most cases of β-blocker overdose. There are a few cases in which patients presented late (after 6 hours) in the course of poisoning and in whom demonstrable clinical effects were evident at the time of presentation Citation32&33. Exactly when these patients initially developed effects is not known. In each of these late presentating cases, the ingested β-blocker was an IR formulation. Cases involving late presentation after ingestion of SR preparation were not found. There was one level 4 report of a patient with an acute sotalol overdose who developed symptoms at 12 hours after ingestion Citation[34]. In this 1985 case, a 25-year-old woman presented 6 hours after ingesting 1.2 g of sotalol. At presentation, her blood pressure was 100/70 mmHg and her pulse was 64/minute. At12 hours after ingestion her blood pressure was 90/60 mm Hg and her pulse was 45/minute. In a case series (level 4) of sotalol overdoses, it was noted that the onset of dysrhythmias occurred 4–20 hours after ingestion. However, it is unclear from the report if these patients had other clinically evident effects prior to the onset of dysrhythmias Citation[30].
Patients have been reported to deteriorate quite rapidly (within minutes) once the onset of effects began Citation[35]. In other cases (level 4), deterioration was directly preceded by the induction of emesis or gastric lavage Citation35-37.
Pediatric Acute Supratherapeutic Ingestion
There were no level 1, 2, or 3 studies investigating the time of onset of clinical effects after acute β-blocker overdose in children less than 6 years of age. Three level 4 reports cited above were reviewed that contained information on onset of effects Citation28&29. Belson's retrospective study of pediatric β-blocker ingestions provided the most useful data Citation[4]. In this report, the onset of symptoms for the seven patients who ingested an immediate-release β-blocker was 45 minutes to 3.5 hours with a median of 3 hours.
Sustained-Release Products
Concerns have arisen over the potential for a prolonged duration of toxic effects after the ingestion of sustained-release products. Both propranolol and metoprolol are available as sustained-release as well as immediate-release products.
The review of the literature did not find any specific information regarding time of onset of symptoms after the ingestion of these long-acting preparations. Peak serum concentrations following dosing with SR propranolol occur at about 6 hours compared to 2 hours with IR propranolol Citation[38]. For sustained-release metoprolol, the time to peak concentration was 3.3 hours compared to 1.5–2 hours for immediate-release metoprolol Citation[39]. While prolonged effects would be expected after the ingestions of these sustained-release products, time to symptom onset should not be markedly delayed.
Potential Out-of-Hospital Treatments
Gastrointestinal Decontamination
There were no studies specifically looking at out-of-hospital decontamination measures. The articles were therefore reviewed for information regarding those in-hospital decontamination measures that could reasonably be expected to be instituted in an out-of-hospital setting. These were limited to induction of emesis and administration of activated charcoal.
One level 1b study found that ipecac-induced emesis was less effective than activated charcoal in reducing absorption after a therapeutic β-blocker ingestion. The study was a randomized, crossover study in healthy volunteers that found that 50 g activated charcoal given 5 minutes after the ingestion of therapeutic pindolol doses (10 mg) and 1 hour after 20 mg metoclopramide, reduced subsequent pindolol absorption by 99% compared to control. In contrast, ipecac syrup given 5 minutes after the pindolol dose reduced absorption of the drug by about 60% Citation[40].
The rest of the data on activated charcoal are from case reports and case series (level 4) in which it was used. It was not possible to detect any benefit or lack of benefit from activated charcoal administration in such cases. However, no significant detrimental effects were reported with its use.
A number of case reports and case series (level 4) were reviewed in which ipecac-induced emesis was used for β-blocker overdose. It was impossible to detect any benefit or lack of benefit from ipecac administration in these level 4 data Citation41-44. Several authors noted that ipecac-induced emesis caused what appeared to be a severe vagal response (e.g., emesis immediately precipitated asystole or bradycardia and hypotension) in some patients with β-blocker overdose Citation35-37.
Other Treatments
There were no studies addressing the efficacy of any other treatments for β-blocker overdose that would be suitable for out-of-hospital use.
Limitations of the Published Data
Overall, the level 4 data were extremely difficult to interpret and summarize for a number of reasons. The case reports and case series varied widely in the level of clinical detail presented and the cases themselves varied widely in the severity and clinical effects of poisoning and in the timing, combination, dose, and routes of various treatments used.
The lack of precision in dose reporting is a major limitation of this data analysis. The estimates that were used are subject to many assumptions and guesswork. Data for amount ingested are often inaccurate or incomplete. The history may be obtained from an intoxicated patient or an emotionally stressed or elderly caregiver. Parents might underestimate or overestimate the ingested dose because of denial or anxiety. Poison center staff often record the dose taken as the worst-case scenario in order to provide a wide margin of safety. Tablet counts from bottles are often unreliable. The suspect tablets might be simply missing, with only a possibility that it was ingested. In most case reports and case series the histories of β-blocker exposure were not independently verified or confirmed by laboratory testing. Poor correlation between reported estimated doses and subsequent serum concentrations or toxicity has been documented for children with unintentional ingestions of other drugs, such as acetaminophen, for which quantitative laboratory confirmation is routine Citation45-47.
For the purpose of these analyses the expert consensus panel concentrated on cases of β-blocker-only overdoses. Even when the authors present a history of β-blocker-only toxicity, the lack of analytical confirmation of the presence of the β-blocker and the lack of analytical confirmation of the absence of other possible confounding drugs, such as calcium channel blockers, weakens the data culled from these case reports and case series. In addition, an unrecognizedunderlying medical condition might decrease a patient's tolerance to a particular dose.
In most of the case reports and case series reviewed the exact time of ingestion was not reported or was not known. The time of onset of toxicity usually can only be estimated as occurring within a range of hours after the suspected ingestion. The unclear time interval from ingestion to onset of toxicity is confounded by a lack of definition of consequential toxicity. For instance, after a β-blocker overdose the development of mild drowsiness in a child could indicate toxicity onset or could represent the approach of nap time.
Another problem encountered was a lack of data on a number of potentially important prehospital interventions and approaches. Studies on prehospital gastrointestinal decontamination of patients with β-blocker toxicity have not been performed. Likewise, studies on the use of glucagon and other pressor agents to treat β-blocker toxicity in the prehospital setting have not been reported.
Even the rather straightforward issue of determining the most appropriate mode of transport to an emergency department for the patient with β-blocker toxicity has not been studied. Given the potential for serious toxicity, expeditious transport by EMS might be the most appropriate approach.
Conclusions
Key Decision Points for Triage
The expert consensus panel chose to emphasize the importance of information that would be needed in order to make a sound triage decision for the patient with a known β-blocker ingestion. These variables include the patient's intent, the dose and formulation of the specific product ingested, the presence of symptoms, and the patient's underlying medical condition, other medications used. The expert consensus panel agreed that in each case, the judgment of the specialist in poison information or the poison center medical director might override any specific recommendation from this guideline.
Patient Intent
The expert consensus panel concluded that all patients with suicidal intent or in whom a malicious intent was suspected (e.g., child abuse or neglect) should be expeditiously transported by EMS to an emergency department that offers critical care services, regardless of the dose ingested. Patients without these characteristics (e.g., adults with definite unintentional ingestion or children below the age of 6 years in whom abuse is not suspected) are candidates for more selective referral to healthcare facilities.
Presence of Symptoms
In patients with demonstrated unintentional β-blocker ingestion, medical evaluation in an emergency department is warranted if the patient is significantly symptomatic. Symptoms such as syncope, generalized weakness, CNS depression, seizures, chest pain, shortness of breath, or other signs of poor perfusion might individually or together suggest evidence of significant β-blocker toxicity. All patients with any of these or other symptoms attributed to the β-blocker should be referred to an emergency department and transported preferably by EMS, regardless of dose ingested. The importance of each of these variables can be difficult to judge in a telephone conversation but a low threshold for emergency department evaluation is considered prudent at this time.
Underlying Medical Condition and Other Medications Used
The expert consensus panel concluded that some patients with serious underlying medical conditions (e.g., end-stage cardiomyopathy) should be referred to a health care facility regardless of the dose ingested.
Dose and Formulation of the Specific β-Blocker
The estimation of dose is based largely on the patient's history and the type of product and its packaging (when available for evaluation). If precise data for the ingestion are unknown or unclear (package size, unit size, number of units ingested), poison centers in the United States often utilize a method in which the maximum potential dose is calculated. For example, if the actual dose ingested cannot be ascertained, the amount of the drug product that is missing from the container is multiplied by the concentration of the formulation. Sustained-release products often contain larger total quantities of the drug but their rate of absorption into the systemic circulation could be much slower and toxicity might be prolonged.
For asymptomatic patients with an acute, unintentional ingestion of a β-blocker, the expert consensus panel concluded that home observation might be suitable for some low-dose exposures. However, the panel recognized that a definite threshold dose for toxicity, based on a confirmed history of exposure, has not been established. After a thorough review of published case reports, recommended therapeutic dosage regimens, current poison control center practice, and expert experience, the panel concluded that ingestion of an amount that exceeds the usual maximum therapeutic dose would warrant consideration of referral to an emergency department.
For each of the specific β-blockers considered by this guideline, the maximum single therapeutic dose in adults is less than the lowest reported dose causing significant toxicity (). Compared to adult data, there are relatively few reports of β-blocker toxicity with dose information in young children. In the case of propranolol, the maximum daily therapeutic dose (16 mg/kg according to some references) is greater than the lowest dose reported to cause significant toxicity. Hence, the panel chose to use the maximum single therapeutic dose as the referral threshold rather than the maximum daily therapeutic dose. Nonetheless, the paucity of reported cases suggests that serious β-blocker toxicity in children is uncommon. Furthermore, the lack of any β-blocker deaths in young children in the TESS database dating back to 1983 supports the contention that severe β-blocker toxicity in young children is rare.
This recommendation applies to both patients who are naïve to the specific β-blocker and to patients currently taking β-blockers who take extra doses. Using this approach, for patients currently taking a β-blocker, a double dose or even a triple dose does not mandate healthcare facility referral if the total dose is at or below the maximum therapeutic single dose.
It also recognized that the thresholds chosen for this guideline are more conservative than some current poison center protocols and less conservative than others. Further prospective study might provide more definitive data and could result in adjustments of the recommended threshold doses.
Time of Onset of Toxicity after Overdose
The panel concluded that asymptomatic patients who unintentionally ingest more than the referral dose of an immediate-release β-blocker within 6 hours of contacting the poison center (12 hours for sotalol) require medical evaluation in a healthcare facility. However, if the ingestion occurred more than 6 hours before contacting the poison center and the patient had never been symptomatic, the patient could stay at home with poison center follow-up since the chance of delayed toxicity is small.
Asymptomatic patients who unintentionally ingest more than the referral dose of a sustained-release β-blocker should be referred to a health care facility if the call is received within 8 hours of ingestion. An extra 2 hours was added as a safety factor because of the lack of time to onset of toxicity data after sustained-release product ingestions. Since symptom onset after the ingestion of a sustained-release product would be expected within 6 hours, if more than 8 or more hours has lapsed since ingestion and the patient has never been symptomatic, the patient could stay at home with poison center follow-up since the chance of delayed toxicity is small.
Duration of Observation
The survey of poison centers revealed that a patient who ingests a β-blocker is often referred to a hospital for overnight or 24-hour admission, especially when a sustained-release product has been ingested. The expert consensus panel concluded that this common practice is not routinely needed. It is very unlikely that symptoms will first develop more than 6 hours after an ingestion of a β-blocker. Furthermore, a delay in toxicity is unlikely to occur regardless of whether the patient ingests an immediate-release or sustained-release product. Although the time to peak concentration for sustained-release propranolol (Inderal LA™) is 6–10 hours, there are no reports in the medical literature suggesting that the onset of toxicity occurs beyond 6–7 hours. The panel concluded that asymptomatic patients should be monitored for at least 6 hours after ingestion if they took an immediate-release product and at least 8 hours if they took a sustained release product. Routine 24-hour admission of an asymptomatic patient who has unintentionally ingested a sustained-release product is not warranted. An exception to this strategy is the ingestion of sotalol for which an observation period of at least 12 hours is warranted.
Potential Out-of-Hospital Management
Gastrointestinal Decontamination
The expert consensus panel concluded that out-of hospital gastrointestinal decontamination offered potential benefit but the potential risks and overall benefit to the patient were difficult to determine. Induced emesis with syrup of ipecac was concluded to carry the potential risk of pulmonary aspiration of gastric contents if the patient becomes hypotensive, has a seizure, or loses consciousness and would not provide sufficient benefit to warrant its use. Moreover, ipecac would likely delay or prevent the use of alternative, potentially more effective treatments such as activated charcoal. Activated charcoal was determined to be a potentially useful treatment that could be administered orally in an ambulance or at the home. However, the panel agreed that transport to a healthcare facility should not be delayed in order to attempt charcoal administration.
Specific Pharmacological Therapy
The panel concluded that although the available literature on in-hospital management of β-blocker poisoning supports the use of intravenous glucagon, which is often available to paramedics, no studies were found addressing the effectiveness or safety of glucagon for the out-of-hospital treatment of β-blocker-induced hypotension and bradycardia.
Recommendations
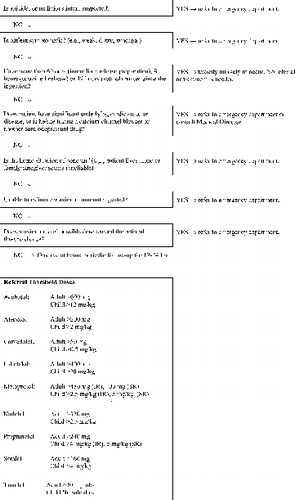
Patients with stated or suspected self-harm or who are the victims of a potentially malicious administration of β-blocker should be referred to an emergency department immediately. This referral should be guided by local poison center procedures. In general, this should occur regardless of the dose reported (Grade D).
Patients without evidence of self-harm should have further evaluation, including determination of the precise dose ingested, history of other medical conditions, and the presence of co-ingestants. Ingestion of either of the following amounts (whichever is lower) warrants consideration of referral to an emergency department: (see ).
TABLE 5. Recommended referral to an emergency department based on dose.
An amount that exceeds the usual maximum single therapeutic dose or
An amount equal to or greater than the lowest reported toxic dose.
Ingestion of any excess dose of any β-blocker in combination with a calcium channel blocker or the ingestion of any excess dose by an individual with serious underlying cardiovascular disease (e.g., end-stage cardiomyopathy) also warrants referral to an emergency department (Grade C).
Do not induce emesis. Consider the oral administration of activated charcoal if it is available and no contraindications are present. However, do not delay transportation in order to administer charcoal (Grade A).
Asymptomatic patients who ingest more than the referral dose should be sent to an emergency department if the ingestion occurred within 6 hours of contacting the poison center for an immediate-release product other than sotalol, within 8 hours of contacting the poison center for a sustained-release product and 12 hours if they took sotalol (Grade C).
Ambulance transportation is recommended for patients who are referred to emergency departments because of the potential for life-threatening complications of β-blocker overdose. Provide usual supportive care en route to the hospital, including intravenous fluids for hypotension (Grade D).
Depending on the specific circumstances, follow-up calls should be made to determine outcome at appropriate intervals for up to 12–24 hours based on the judgment of the poison center staff (Grade D).
Asymptomatic patients who are referred to healthcare facilities should be monitored for at least 6 hours after ingestion if they took an immediate-release preparation other than sotalol, 8 hours if they took a sustained-release preparation, and 12 hours if they took sotalol. Routine 24-hour admission of an asymptomatic patient who has unintentionally ingested a sustained-release preparation is not warranted (Grade D).
These recommendations are summarized in Appendix 4.
Implications for Research
Prospective validation of these guidelines is strongly recommended. In particular, future studies should collect data that could better define the most appropriate referral threshold dose and observation times. A large-scale prospective study of unintentional β-blocker ingestions is needed, with a careful attempt to confirm the estimate of the dose taken, the specific formulation, the presence or absence of underlying illness, the use of other medications, the presence or absence of symptoms, the time of onset of any toxicity, the duration of medical observation, and outcome. Given the relatively low incidence of serious toxicity after unintentional ingestion, especially in children, a multi-center and multi-year study will be needed. An additional need is better correlation between the estimated ingested dose, clinical symptoms, and serum concentrations of the β-blockers in patients with serious overdoses. Further investigation is also warranted to determine the utility of these guidelines for special populations such as patients with cardiovascular disorders and those who are naïve to β-blockers. Prehospital use of glucagon, and other measures should be studied.
Disclosure
Dr. Booze's husband is employed by AstraZeneca Pharmaceuticals. There are no other potential conflicts of interest reported by the panel members or authors regarding this guideline.
Notes
IR—immediate release; SR—sustained release.
References
- Watson, W A, Litovitz, T L, Rodgers, G C Jr, Klein-Schwartz, W, Youniss, J, Reid, N, Rouse, W G, Rembert, R S, Borys, D. 2002 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 2003; 21:353–421. [PUBMED], [INFOTRIEVE], [CSA]
- AAPCC TESS Fatality Abstracts 1985–2002. Washington, (DC): American Association of Poison Control Centers.
- Miller, T R, Lestina, D C. Costs of poisoning in the United States and savings from poison control centers: a benefit-cost analysis. Ann Emerg Med 1997; 29:239–245. [PUBMED], [INFOTRIEVE], [CSA]
- Belson, M G, Sullivan, K, Geller, R J. Beta-adrenergic antagonist exposures in children. Vet Hum Toxicol 2001; 43:361–365. [PUBMED], [INFOTRIEVE], [CSA]
- Shaneyfelt, T M, Mayo-Smith, M F, Rothwangl, J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature. JAMA 1999; 281:1900–1905. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Poisindex system. R K Klasco, ed. Greenwood Village (CO): Thomson Micromedex, edition expires March 2003.
- In: M J Ellenhorn, ed. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore: Williams & Wilkins, 1997.
- Ford, M D, Delaney, K A, Ling, L J, Erickson, T. Clinical Toxicology. Philadelphia: WB Saunders, 2000.
- In: L R Goldfrank, N E Flomenbaum, N A Lewin, M A Howland, R S Hoffman, L S Nelson, eds. Goldfrank's Toxicologic Emergencies. 7th ed. New York: McGraw-Hill, 2002.
- In: L M Haddad, M W Shannon, J F Winchester, eds. Clinical Management of Poisoning and Drug Overdose. 3rd ed. Philadelphia: WB Saunders, 1998.
- USP DI. Drug Information for the Health Care Professional. 24th ed. Englewood (CO): Micromedex, 2004.
- Drug Facts and Comparisons. 58th ed. St. Louis: Facts and Comparisons, 2004.
- Drugdex Drug Evaluations. T A Hutchison, ed. Greenwood Village (CO): Thomson Micromedex, edition expires September 2004.
- In: R E Behrman, R M Kliegman, H B Jenson, eds. Nelson Textbook of Pediatrics. 17th ed. Philadelphia: WB Saunders, 2004.
- The Harriet Lane Handbook: A Manual for Pediatric House Officers. 16th ed.Philadelphia: Mosby, 2002.
- Park, M K. Pediatric Cardiology for Practitioners. 4th ed. Philadelphia: Mosby, 2002.
- Takemoto, C K, Hodding, J H, Kraus, D M. Pediatric Dosage Handbook. 9th ed. Hudson (OH): Lexi-Comp, 2002.
- In: A Garson, J T Bricker, D J Fisher, S R Neish, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore: Williams & Wilkins, 1998.
- Nicolas, F, Villers, D, Rozo, L, Haloun, A, Bigot, A. Severe self-poisoning with acebutolol in association with alcohol. Crit Care Med 1987; 15:173–174. [PUBMED], [INFOTRIEVE]
- Abbasi, I A, Sorsby, S. Prolonged toxicity from atenolol overdose in an adolescent. Clin Pharm 1986; 5:836–837. [PUBMED], [INFOTRIEVE]
- DeLima, L G, Kharasch, E D, Butler, S. Successful pharmacologic treatment of massive atenolol overdose: sequential hemodynamics and plasma atenolol concentrations. Anesthesiology 1995; 83:204–207. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Korzets, A, Danby, P, Edmunds, M E, Feehally, J, Walls, J. Acute renal failure associated with a labetalol overdose. Postgrad Med J 1990; 66:66–67. [PUBMED], [INFOTRIEVE]
- Lindvall, K, Personne, M, Sjogren, A. High-dose prenalterol in beta-blockade intoxication. Acta Med Scand 1985; 218:525–528. [PUBMED], [INFOTRIEVE]
- Gupta, K. Hazards of B-blockers in the elderly: severe bradycardia due to Sotacor overdose. Br J Clin Pract 1985; 39:116–117. [PUBMED], [INFOTRIEVE]
- Reith, D M, Dawson, A H, Epid, D, Whyte, I M, Buckley, N A, Sayer, G P. Relative toxicity of beta blockers in overdose. J Toxicol Clin Toxciol 1996; 34:273–278.
- Chen, T W, Huang, T P, Yang, W C, Hong, C Y. Propranolol intoxication: three cases' experiences. Vet Hum Toxicol 1985; 27:528–530. [PUBMED], [INFOTRIEVE], [CSA]
- Ducret, F, Zech, P, Perrot, D, Moskovtchenko, J F, Traeger, J. Intoxication volontaire par le propranolol. Evolution des taux séiques. Nouv Presse Med 1978; 7:27–28. [PUBMED], [INFOTRIEVE]
- Hesse, B, Pedersen, J T. Hypoglycaemia after propranolol in children. Acta Med Scand 1973; 193:551–552. [PUBMED], [INFOTRIEVE]
- Artman, M, Grayson, M, Boerth, R C. Propranolol in children: safety-toxicity. Pediatrics 1982; 70:30–31. [PUBMED], [INFOTRIEVE]
- Neuvonen, P J, Elonen, E, Vuorenmaa, T, Laakso, M. Prolonged Q-T interval and severe tachyarrhythmias, common features of sotalol intoxication. Eur J Clin Pharmacol 1981; 20:85–89. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Love, J N, Howell, J M, Litovitz, T L, Klein-Schwartz, W. Acute beta blocker overdose: factors associated with the development of cardiovascular morbidity. J Toxicol Clin Toxciol 2000; 38:275–281. [CROSSREF], [CSA]
- Khan, M I, Miller, M T. Beta-blocker toxicity–the role of glucagon. Report of 2 cases. S Afr Med J 1985; 67:1062–1063. [PUBMED], [INFOTRIEVE], [CSA]
- Hantson, P, Lambermont, J Y, Simoens, G, Mahieu, P. Carvedilol overdose. Acta Cardiol 1997; 52:369–371. [PUBMED], [INFOTRIEVE]
- Baliga, B G. Beta-blocker poisoning: prolongation of Q-T interval and inversion of T wave. J Indian Med Assoc 1985; 83:165. [PUBMED], [INFOTRIEVE]
- Wilkinson, J. Beta blocker overdoses. Ann Emerg Med 1986; 15:982. [PUBMED], [INFOTRIEVE]
- Smith, R C, Wilkinson, J, Hull, R L. Glucagon for propranolol overdose. JAMA 1985; 254:2412. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Soni, N, Baines, D, Pearson, I Y. Cardiovascular collapse and propranolol overdose. Med J Aust 1983; 2:629–630. [PUBMED], [INFOTRIEVE]
- Leahey, W J, Neill, J D, Varma, M P, Shanks, R G. Comparison of the efficacy and pharmacokinetics of conventional propranolol and a long acting preparation of propranolol. Br J Clin Pharmacol 1980; 9:33–40. [PUBMED], [INFOTRIEVE]
- Darmansjah, I, Wong, E, Setiawati, A, Moeloek, D, Irawati, D, Siagian, M, Muchtar, A. Pharmacokinetic and pharmacodynamic properties of controlled release (CR/ZOK) metoprolol in healthy oriental subjects: a comparison with conventional formulations of metoprolol and atenolol. J Clin Pharmacol 1990; 30(2 suppl):S39–45. [PUBMED], [INFOTRIEVE]
- Neuvonen, P J, Olkkola, K T. Activated charcoal and syrup of ipecac in prevention of cimetidine and pindolol absorption in man after administration of metoclopramide as an antiemetic agent. J Toxicol Clin Toxciol 1984; 22:103–114.
- Buiumsohn, A, Eisenberg, E S, Jacob, H, Rosen, N, Bock, J, Frishman, W H. Seizures and intraventricular conduction defect in propranolol poisoning. A report of two cases. Ann Intern Med 1979; 91:860–862. [PUBMED], [INFOTRIEVE]
- Salzberg, M R, Gallagher, E J. Propranolol overdose. Ann Emerg Med 1980; 9:26–27. [PUBMED], [INFOTRIEVE]
- Shore, E T, Cepin, D, Davidson, M J. Metoprolol overdose. Ann Emerg Med 1981; 10:524–527. [PUBMED], [INFOTRIEVE]
- Tynan, R F, Fisher, M M, Ibels, L S. Self-poisoning with propranolol. Med J Aust 1981; 1:82–83. [PUBMED], [INFOTRIEVE]
- Rumack, B H. Acetaminophen overdose in young children. Treatment and effects of alcohol and other additional ingestants in 417 cases. Am J Dis Child 1984; 138:428–433. [PUBMED], [INFOTRIEVE], [CSA]
- Gee, P, Ardagh, M. Paediatric exploratory ingestions of paracetamol. N Z Med J 1998; 111:186–188. [PUBMED], [INFOTRIEVE]
- Caravati, E M. Unintentional acetaminophen ingestion in children and the potential for hepatotoxicity. J Toxicol Clin Toxicol 2000; 38:291–296. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Oxford Centre for Evidence-based Medicine Levels of Evidence. 2001. http://www.cebm.net/levels_of_evidence.asp#levels.
Appendix 1
Expert Consensus Panel Members
Lisa Booze, PharmD
Certified Specialist in Poison Information
Maryland Poison Center
University of Maryland School of Pharmacy
Baltimore, Maryland
E. Martin Caravati, MD, MPH, FACMT, FACEP
Professor of Surgery (Emergency Medicine)
University of Utah
Medical Director
Utah Poison Center
Salt Lake City, Utah
Gwenn Christianson, RN, MSN
Certified Specialist in Poison Information
Indiana Poison Center
Indianapolis, Indiana
Peter A. Chyka, PharmD. FAACT, DABAT
Professor, Department of Pharmacy
University of Tennessee Health Science Center
Memphis, Tennessee
Daniel C. Keyes, MD, MPH
Medical Director
Pine Bluff Chemical Demilitarization Facility
Associate Professor, Southwestern Toxicology Training Program
Dallas, Texas
Anthony S. Manoguerra, PharmD, DABAT, FAACT
Professor of Clinical Pharmacy and Associate Dean
School of Pharmacy and Pharmaceutical Sciences
University of California San Diego
Former Director, California Poison Control System, San Diego Division
San Diego, California
Kent R. Olson, MD, FACEP, FAACT, FACMT
Medical Director
California Poison Control System, San Francisco Division
Clinical Professor of Medicine & Pharmacy
University of California, San Francisco
San Francisco, California
Elizabeth J. Scharman, Pharm.D., DABAT, BCPS, FAACT
Director, West Virginia Poison Center
Professor, West Virginia University School of Pharmacy
Department of Clinical Pharmacy
Charleston, West Virginia
Paul M. Wax, MD, FACMT
Managing Director
Banner Poison Center
Professor of Clinical Emergency Medicine
University of Arizona School of Medicine
Phoenix, Arizona
Alan Woolf, MD, MPH, FACMT
Director, Program in Environmental Medicine
Children's Hospital, Boston
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Appendix 2
Appendix 3
Secondary Review Panel Organizations
Ambulatory Pediatric Association
American Academy of Breastfeeding Medicine
American Academy of Emergency Medicine
American Academy of Pediatrics
American Association for Health Education
American College of Clinical Pharmacy
American College of Emergency Physicians
American College of Occupational and Environmental Medicine
American Pharmacists Association
American Public Health Association
American Society of Health System Pharmacists
Association of Maternal and Child Health Programs
Association of Occupational and Environmental Clinics
Association of State and Territorial Health Officials
Canadian Association of Poison Control Centres
Centers for Disease Control—Injury Bureau
Consumer Federation of America
Consumer Product Safety Commission
Department of Transportation
Emergency Medical Services for Children
Emergency Nurses Association
Environmental Protection Agency
European Association of Poisons Control Centres and Clinical Toxicologists
Food and Drug Administration
National Association of Children's Hospitals and Related Institutions
National Association of Emergency Medical Services Physicians
National Association of Emergency Medical Technicians
National Association of School Nurses
National Association of State Emergency Medical Services Directors
National Safe Kids Campaign
Teratology Society
World Health Organization International Programme on Chemical Safety