Abstract
The finding that α1‐antitrypsin (AAT) deficiency, PiZZ, a well‐established genetic risk factor for COPD, is related to high levels of circulating AAT polymers, prompted us to measure serum levels of such polymers and selected markers of inflammation in age‐ and gender‐matched patients with stable COPD and control subjects with and without severe AAT deficiency, and to assess their relationship with each other and with the genetic AAT‐variant. We found that COPD individuals (n = 20), independent of AAT‐variant, had significantly higher serum levels of AAT and its polymers, MMP‐9, sICAM‐1, VEGF and sE‐selectin than controls (n = 30). Subjects with PiZZ COPD (n = 10) showed significantly elevated serum levels of AAT‐polymers, sE‐selectin and sICAM‐1, while patients with PiMM COPD (n = 10) showed higher levels of MMP‐9, VEGF, IL‐8 and MCP‐1 than controls. By using factor analysis we were able to split the analysed biomarkers into two independent components: the first containing MMP‐9, MCP‐1, IL‐8 and VEGF and the second—AAT and its polymers and sE‐selectin. The result from the binomial logistic regression showed that 95.2 percent of the control individuals and 94.7 percent of the COPD patients can be correctly classified on the basis of the measured serum biomarkers. These observations highlight the importance of the finding sets of biomolecules, which could offer new strategies for the diagnosis of COPD and may have value for monitoring progression of COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex and slowly progressing disease, which is influenced by genetic and environmental factors, and by genotype–environment interactions Citation[[1]]. COPD is most commonly seen in long‐term smokers and is usually associated with a progressive decline in pulmonary function, more rapid than that associated with normal ageing Citation[[2]]. Inherited AAT deficiency is the only proven genetic risk factor related to COPD. Individuals with homozygous Pi ZZ AAT deficiency have reduced baseline serum AAT levels by 90%, and attenuated acute phase response Citation[[3]]. Recent studies have also found that PiZ carriers have significant amounts of circulating AAT‐polymers Citation[[4]]Citation[[5]]. These may have important implications for the pathogenesis of the disease, since polymerization obscures the reactive center loop of AAT, rendering the protein inactive as an inhibitor of proteolytic enzymes Citation[[6]]. It is widely accepted that inherited Pi ZZ AAT deficiency results in proteinase–antiproteinase imbalance and that this is the proximal cause of obstructive lung disease. It is also known that a combination of inherited AAT deficiency and certain environmental factors, like cigarette smoking, results in COPD development in early years of life Citation[[1]]Citation[[2]].
The concept that endothelial activation plays an important role in COPD is supported by several independent studies. In 1986 Lieberman and Sastre demonstrated that AAT‐deficiency is associated with elevated serum levels of angiotensin converting enzyme (ACE) levels Citation[[7]]. A negative correlation was found between ACE activity and lung function in COPD patients Citation[[8]]. Because endothelial cells are the source of activated ACE Citation[[9]], one may consider endothelial activation as an important event in the development of COPD. Cella and co‐workers showed significantly decreased plasma levels of nitric oxide and thrombomodulin and increased levels of selectins in COPD patients Citation[[10]], pointing to endothelial cell damage. The idea that COPD may result from the progressive lung microvascular endothelium damage is supported by a rat emphysema model showing that blockage of the vascular endothelial growth factor (VEGF) receptor produces emphysema in rats in the absence of inflammatory cells Citation[[11]].
Findings obtained from various studies lead to the proposal that a combination of environmental factors, including smoking, air pollution, childhood respiratory infections and latent adenoviral infections, and/or genetic factors, such as AAT‐deficiency, are likely to be the essential contributors to the development of COPD. The identification of markers to detect the development of disease at an early stage is particularly important for the slowly progressing diseases. However, at early stages it is often not possible to rely on one or only a few markers. A more reliable prognosis can be achieved by using patterns of biomarkers representing different pathways, including inflammation, apoptosis, oxidative stress and endothelial activation, that contribute to pathogenetic mechanisms underlying COPD. However, to date, there are no good, measurable patterns of markers that correlate with COPD development and/or outcome.
The purpose of the current study was to measure the systemic levels of acute phase proteins (AAT and AAT‐polymers), chemokines (IL‐8 and MCP‐1), metalloproteinase (MMP‐9), adhesion molecule (sICAM‐1), angiogenic factor (VEGF) and endothelial cell activity biomarker (sE‐selectin) in serum of control subjects and COPD patients with normal PiMM AAT, and with severe PiZZ AAT‐deficiency, and to assess their inter‐relationship and relationship with the genetic AAT‐variant.
Materials and Methods
Subjects
The studied group consisted of 20 controls with PiMM AAT, 10 asymptomatic PiZZ AAT individuals and 20 patients with COPD: 10 PiZZ and 10 PiMM AAT cases. The characteristics of study participants are given in . All patients with COPD were in a clinically stable condition for at least 4 weeks. It must be pointed out, that COPD patients (both PiMM and PiZZ AAT) included in this study are well known out‐hospital patients at the Department of Respiratory Medicine, Malmo University Hospital, therefore HRCT was not performed specifically for this study. All asymptomatic PiZZ individuals had normal lung function. Three of them had mild asthma and were treated with inhaled corticosteroids. The controls showed no evidence of any disease and had no respiratory symptoms; none of them was on medication. The exclusion criteria were liver diseases, vasculitic and other extra‐pulmonary diseases and treatment with oral corticosteroids. The PiZZ individuals were recruited from the Swedish AAT Deficiency Register Citation[[2]]. The healthy volunteers were recruited from the hospital staff and their relatives. All the individuals gave a signed, informed consent to take part in this study, which has been approved by the research ethical committee of Lund University, Sweden.
Table 1. Clinical Characteristics of COPD Patients and Control Individuals with and Without AAT‐Deficiency
Lung Function Tests
Lung‐function tests included FEV1 and FVC. The measurements were performed in accordance with European recommendations Citation[[12]]. The results of FEV1 and FVC are expressed as percent of predicted values according to Berglund et al. Citation[[13]]. We used the reference values of Berglund and co‐workers because these reference values are considered as the most correct for a Swedish study population, and they are still used at the Department of Clinical Physiology at our hospital. We have used them in all our studies on lung function in PiZZ individuals included in the Swedish AAT Deficiency register. Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria were used for definition of COPD Citation[[14]]. The patients with COPD had moderate or severe COPD, i.e., FEV1 < 80% of predicted and the ratio FEV1/FVC < 70%. The asymptomatic PiZZ individuals had normal lung function with FEV1 > 80% of predicted and the ratio FEV1/FVC > 70%.
Blood Sample Collection
Blood was taken by venepuncture; serum was immediately separated by centrifugation and stored at − 80°C until assayed. AAT‐phenotyping was performed by isoelectric focusing at the Department of Clinical Chemistry, University Hospital, Malmö Citation[[15]].
Double Sandwich ELISA Assay of AAT‐Polymers
Nunc‐Immuno plates were coated with mouse monoclonal antibody ATZ11 available in our laboratory Citation[[16]] diluted 1:750 in coating buffer (0.05 mol NaCO3, pH 9.6) and incubated over night at 4°C. The plates were washed with PBS and non‐specific binding sites were blocked with 1% bovine serum albumin for 1 hour at room temperature. The wells were washed, the standards and samples were pipetted into them and incubated of 24 hours at 4°C or for 2 hours at room temperature. A stock solution of polymerized AAT Citation[[17]] was used to produce a dilution series from 0.5 mg/ml to 0.0039 mg/ml. The analyzed samples were diluted (1:200) in buffer containing polyclonal goat antibody to human elastase (4 µg/ml) (Santa Cruz Biotechnology, Inc.). This sample dilution buffer was used in order to eliminate ATZ11 antibody cross‐reactivity with AAT–elastase complexes. After incubation, the washing procedure was repeated and polyclonal rabbit antibody to human AAT, diluted 1:1000, was added and incubated for 2 hours on a shaker at room temperature. After washing as described above, peroxidase‐conjugated swine antibody to rabbit immunoglobulins, diluted 1:1000, was added for 2 hours at room temperature. Finally, the wells were washed as before and 2,2′‐azino‐bis3‐ethylbenzthiazoline‐6‐sulfonic acid substrate was added. The absorbance was estimated after 15 min at 405 nm in an ELISA Reader (Labsystems, Helsinki, Finland). All samples were analysed in triplicates. For the blank, washing buffer was substituted for the antibody. The AAT‐polymer concentration was obtained by interpolation from the standard curve (the inter‐assay coefficient of variation was less than 5 percent, detection limit was less than 2.4 µg/ml). The concentration of AAT‐polymers was calculated after subtraction of the blank. There was no cross‐reactivity with native AAT.
Quantitative Analysis of Serum AAT
Concentration of AAT in individual serum samples was determined by nephelometry at the Clinical Chemistry Laboratory, UMAS, Malmo Citation[[18]].
Assays of Inflammatory Markers
Serum samples were analysed for matrix metalloproteinase 9 (MMP‐9), interleukin 8 (IL‐8), sE‐selectin, monocyte chemoattractant protein (MCP‐1), soluble intercellular adhesion molecule‐1 (sICAM‐1) and VEGF levels by using quantitative sandwich enzyme immunoassay kits (R&D Systems Europe Ltd., Abingdon, UK) according to the manufacturer's instructions. The optical density was determined using a microplate reader (Labsystems). The detection limits for the assays were as follows: VEGF, 31.2 pg/ml, sICAM‐1, 7.8 pg/ml, sE‐selectin, 0.1 ng/ml, IL‐8, 15.6 pg/ml, MCP‐1, 7.8 pg/ml, MMP‐9, 0.16 ng/ml. Reproducibility of the results was tested by measuring the same sample in two or three repeats. For sICAM‐1, VEGF, IL‐8 and sE‐selectin two independent assays were performed from the same serum sample, in duplicates each time. The inter‐ and intra‐assay variance was lower than 5 percent.
Statistical Analysis
Statistical analysis was performed with use of SPSS software (version 11.0 for Windows, SPSS Inc., Chicago, IL). All variables, except serum AAT, were log‐transformed to achieve a normal distribution, and all calculations were performed with normally distributed variables. Data in are presented as a geometric mean (log‐transformed variables) or as a mean (non‐transformed variable) ± 95 percent confidence interval. The difference of means of variables was evaluated with independent two samples t‐test. The estimation of the bivariate correlation (linear association) between the variables was performed by computing the Pearson's correlation coefficient and the correlation matrix is shown in .
Table 2. Correlations Between Measured Parameters
Factor extraction was performed by principal component analysis and the correlation matrix was used as input to the analysis. Only factors with eigenvalues of 1.0 or higher were extracted and the Varimax rotation was used to improve the interpretability of the factors. The factor loadings of the rotated solution are shown in . We used binomial logistic regression to investigate whether the inflammatory biomarkers analyzed could be used as a diagnostic aid to discriminate between control individuals (dichotomous dependent variable = 0) and COPD patients (dichotomous dependent variable = 1). Our logistic regression model therefore predicted the probability of a subject to belong to the emphysema group in relation to measured inflammatory biomarkers. The Hosmer and Lemeshow's Goodness‐of‐Fit test was used to test the null hypothesis that there was no difference between the observed and predicted values of the dependent variable Citation[[19]].
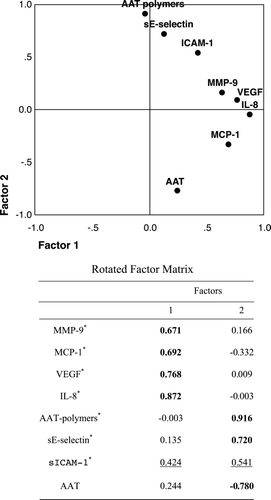
Figure 1. Factor loading plot. The plot displays the rotated two‐factor solution where each variable is plotted according to its factor loadings. The high factor loadings (> 0.6, bold) of IL‐8, VEGF, MCP‐1 and MMP‐9 indicate strong association with the first factor, while AAT‐polymers and sE‐selectin are strongly positively and AAT is strongly negatively associated with the second factor. sICAM‐1 (underlined), on the other hand, is intermediately associated with both factors. Extraction method: Principal Component Analysis. Rotation method: Varimax with Kaiser Normalization. *—presents natural logarithm of serum concentration.
Results
In this cross‐sectional study we assessed systemic levels of several biomarkers, including AAT‐polymers in COPD patients (n = 20) both with PiZZ AAT‐deficiency and wild type PiMM‐AAT and in controls (n = 30) who were carefully matched concerning sex, age, and AAT‐phenotype. The two COPD groups were similar in the degree of impairment of lung function. Care was also taken to avoid the confounding effects of smoking and therefore only a minority of the subjects analysed were current smokers. The characteristics of the individuals studied are summarised in .
The Inter‐correlations Between the Measured Serum Biomarkers of All Individuals Analysed
The pattern of the correlations reveals that all biomarkers analysed can be grouped into two sets: one consisting of MCP‐1, MMP‐9, VEGF and IL‐8, the other—of AAT polymers, sE‐selectin and AAT (). The variables within a selected set, but not between the two sets, are significantly correlated to each other. Because sICAM‐1 significantly correlated to IL‐8, a member of the first set of biomarkers, and to AAT‐polymers, a member of the second set, we were not able to attribute it to one of the sets determined. Factor analysis also confirms two sets of biomarkers identified, where the first factor is mainly determined by the IL‐8, VEGF, MCP‐1 and MMP‐9 and the second factor is determined by AAT polymers, sE‐selectin and AAT. The results of the factor analysis are shown as a rotated factor matrix solution (). In addition, factor analysis confirmed sICAM‐1 to be related to both components.
AAT‐Polymers and Inflammatory Biomarkers in COPD Patients vs. Controls
The mean levels of inflammatory mediators in sera from control subjects and COPD patients with M‐ and Z‐AAT are shown in . Compared with controls, COPD patients, independent of AAT‐phenotype, had higher concentrations of serum MMP‐9 (2‐fold, p < 0.001), VEGF (1.5‐fold, p < 0.05), sICAM‐1 (1.5‐fold, p < 0.01), AAT‐polymers (4.8‐fold, p < 0.001) and sE‐selectin (2.2‐fold, p < 0.01). Furthermore, the comparison of control M‐ and Z‐AAT carriers showed higher serum levels of sICAM‐1 (1.6‐fold, p < 0.01), AAT‐polymers (14.3‐fold, p < 0.001) and sE‐selectin (3.3‐fold, p < 0.01) in Z‐AAT compared to M‐AAT, while total AAT levels are (3.2‐fold, p < 0.001) higher in M‐ compared to Z‐AAT carriers. As expected, the levels of AAT and AAT‐polymers were found to be associated with AAT‐variant. Higher serum levels of AAT‐polymers (10.5‐fold, p < 0.001), despite lower levels of total AAT (4.1‐fold, p < 0.001), were observed in Z‐AAT compared to M‐AAT COPD patients.
Table 3. Measured Biomarkers in Serum From Control and COPD Individuals with and Without PiZZ AAT‐Deficiency
We used binomial logistic regression in order to evaluate whether the variables measured could be used to discriminate between the two groups—control subjects vs. COPD patients. The Hosmer and Lemeshow's Goodness‐of‐Fit test showed χ2(8) = 4.5, p = 0.809 and therefore we failed to reject the null hypothesis, which implied that the model's estimates fitted the data and that the logistic model was a good fit. The result of the classification by the binomial logistic regression based on all variables showed that 95.2 percent (1 of 21 misclassified) of the control individuals and 94.7 percent (1 of 19 misclassified) of the COPD patients were correctly classified. Thus, 95 percent of originally grouped patients were correctly classified.
AAT‐Polymers and Inflammatory Markers in COPD Patients with PiZZ and PiMM AAT vs. Controls
Biomarkers in the PiZZ AAT Group
Our data show significantly increased levels of AAT‐polymers (2.4‐fold, p < 0.001) and AAT (1.1‐fold, p < 0.05) in PiZZ COPD patients compared to PiZZ asymptomatic individuals. It must be pointed out that the mean levels of IL‐8 were 1.6‐fold higher in PiZZ patients than in asymptomatic PiZZ individuals. However, due to the wide distribution of the measured values the groups did not differ statistically.
Biomarkers in the PiMM AAT Group
We compared the profiles of proteins measured in healthy and COPD individuals with PiMM AAT. Our data show that levels of serum MMP‐9 (2.6‐fold, p < 0.001), sICAM‐1 (1.6‐fold, p < 0.01), AAT‐polymers (2.2‐fold, p < 0.0001), sE‐selectin (3.2‐fold, p < 0.01) as well as total AAT (1.4‐fold, p < 0.0001) are higher in PiMM COPD patients than in PiMM controls.
Discussion
This cross‐sectional study presents an attempt to perform a quantitative analysis of levels of circulating AAT and its polymers, and various inflammatory biomarkers as a means of distinguishing between PiMM and PiZZ AAT individuals with normal lung function and those who have COPD. All COPD patients included in this study were diagnosed several years ago and were previously thoroughly characterised as part of the Swedish AAT Registry. Our findings reveal that the levels of circulating AAT‐polymers, total AAT, MMP‐9, sE‐selectin, VEGF and sICAM‐1 are significantly elevated in COPD patients compared to age‐ and sex‐matched controls. Furthermore, we found that the levels of serum AAT‐polymers, sE‐selectin and sICAM‐1 are markedly increased in asymptomatic PiZZ subjects as compared to healthy PiMM controls. Consequently these findings led us to the suggestion that increased production of certain biomarkers, such as AAT‐polymers, sE‐selectin and sICAM‐1 in Z‐AAT individuals may reflect early, ongoing processes associated with the development of COPD.
Airway inflammation in patients with COPD is associated with an increased number of neutrophils and macrophages in the lungs Citation[[20]]Citation[[21]]. The presence of chemoattractants, such as leukotriene B4, MCP‐1 and IL‐8 and the expression of adhesion molecules, such as endothelin‐1, sICAM‐1 and sE‐selectin, on the neutrophil surface and on the endothelium suggest that these molecules play a role in the recruitment and activation of inflammatory cells in the airways of COPD patients Citation[[22]]Citation[[23]]Citation[[24]]. MMP‐9 is a product of both neutrophils and macrophages, having the capacity to degrade elastin and is probably the best candidate for causing lung tissue destruction in COPD Citation[[25]]. MMP‐9 may also enhance inflammation through the generation of chemokines and cytokines, and through the inactivation of protease inhibitors, leading to increased proteolytical activity Citation[[26]]Citation[[27]]. Recently Shapiro and co‐workers have suggested that the analysis of the expression of MMPs, including MMP‐9, could be used to predict a susceptibility to develop COPD Citation[[28]]. We show that the levels of serum total MMP‐9 are significantly increased in both PiZZ and PiMM COPD patients compared to controls. It was somewhat surprising that MMP‐9 levels were found to be significantly higher in individuals with COPD having M‐AAT variant than in those with Z‐AAT deficiency. We also observed a similar pattern for serum VEGF levels, which were found to be higher in M‐ than in Z‐AAT COPD patients. One cannot exclude the possibility that the parallel changes observed in serum levels of MMP‐9 and VEGF in Z‐ and M‐AAT COPD patients might be related to AAT‐phenotype. Further studies are needed to clarify this matter.
Several clinical studies have demonstrated that the expression of sICAM‐1 on the bronchial epithelium and sE‐selectin on bronchial mucosal vessels is increased in patients with COPD Citation[[29]]Citation[[30]]. sE‐selectin is a trans‐membrane glycoprotein expressed only on endothelial cells and only after cell activation by pro‐inflammatory cytokines or endotoxin Citation[[31]], while sICAM‐1 is an inter‐cellular adhesion molecule which promotes interactions between leukocytes and epithelial cells and is crucial for the development of airway inflammation Citation[[32]]. It is postulated that raised serum levels of these biomarkers may reflect endothelium activation in various pathological conditions. In this study we found that levels of circulating sE‐selectin and sICAM‐1 are significantly higher in COPD patients and in asymptomatic PiZZ subjects, which strengthens the argument that endothelial activation is playing a role in the pathogenesis of COPD.
The increased levels of systemic inflammatory markers, such as C reactive protein (CRP), α1‐antichymotrypsin (ACT) and α1‐antitrypsin (AAT) have also been demonstrated in COPD Citation[[20]]Citation[[33]]. Severe AAT‐deficiency, PiZZ, due to a substitution of Glu‐342 by Lys, does not occur from lack of AAT biosynthesis, but from a blockage of its secretion from the liver Citation[[34]]. This change of a single amino acid in a certain domain of the Z‐AAT molecule perturbs the folding and the structure of AAT, allowing its spontaneous polymerization Citation[[35]]. Wild type M‐AAT, depending on its concentration, temperature and pH, is also known to form polymers Citation[[6]]. Recently we have demonstrated that plasma obtained from Z‐AAT carriers contains significant amounts of polymerised AAT Citation[[4]]. Z‐AAT polymers have also been identified in the lungs of Z‐AAT subjects Citation[[5]]. These observations provide good evidence that Z‐AAT polymerization occurs not only within hepatocytes but also in the circulation. In the present study we show that levels of circulating AAT and its polymers are increased in COPD cases compared to controls. Whether AAT‐polymers are formed in circulation or secreted form the liver, how the profile of AAT polymers is influenced by the plasma composition, or whether these polymers can be disrupted once they are formed remains at present unknown. Moreover, we also found that the levels of AAT and its polymers in COPD cases significantly correlate with endothelium‐related markers, namely sE‐selectin and sICAM‐1. This may be another finding relevant to the endothelial dysfunction in COPD, particularly in Z‐, but also in M‐AAT patients. AAT is suggested to be a major protein component in the epithelial lining fluid of the lung, and its levels in non‐deficient individuals are typically 2 and 5 µmol Citation[[36]], while during intense inflammatory events it can exceed 10 µmol Citation[[37]]Citation[[38]]. There is increasing evidence that AAT not only regulate inflammation by inhibiting the proteolytic activity of serine proteinases, but also may directly or indirectly contribute to tissue repair reactions, functions of leukocyte and endothelial cells Citation[[38]]Citation[[39]]Citation[[40]]Citation[[41]]Citation[[42]]Citation[[43]].
In this study we measured circulating levels of several markers, which represent different pathways (e.g. oxidative stress, endothelial activation, inflammation and proteolysis) likely contributing to COPD. We had no prior idea how these markers would correlate with each other and if it will be possible to group them into two independent sets. However, the pattern of the correlations between all measured biomarkers have shown that they can be grouped into two independent sets: the first consisting of MCP‐1, MMP‐9, VEGF and IL‐8 and the second—of AAT polymers, sE‐selectin, and total AAT. Since sICAM‐1 was found to correlate significantly with biomarkers belonging to both sets, namely IL‐8 and AAT‐polymers, we were not able to attribute sICAM‐1 to one of the sets of the variables. By using factor analysis, we confirmed the possibility of dividing the biomarkers analysed into two independent components: the first, most likely related to inflammatory responses (MMP‐9, MCP‐1, IL‐8 and VEGF) and the second—reflecting endothelial activation (AAT and its polymers and sE‐selectin). Factor analysis also confirmed that sICAM‐1 is related to both components. Furthermore, by using binomial logistic regression we demonstrate that measured levels of circulating total AAT and its polymers, cytokines, gelatinase B and endothelial biomarkers can be used to distinguish control subjects and COPD patients. In our case, the result of the classification from the binomial logistic regression shows that 95.2 percent of the healthy individuals and 94.7 percent of the COPD patients are correctly classified. Thus, 95 percent of originally grouped patients can be correctly classified on the basis of the measured serum biomarkers. These observations highlight the importance of the finding sets of biomolecules, which could offer new strategies in identifying ways of early detection of COPD and may have an improved value in monitoring COPD progression.
Acknowledgments
The authors wish to thank Ewa Szemberg Rindahl for sample collection and preparation. This work was supported by grants from Astra Zeneca, Lund University (Sweden) and the Swedish Heart Lung Foundation.
| Abbreviations | ||
| AAT: | = | alpha‐1‐antitrypsin |
| ACT: | = | alpha‐1‐antichymotrypsin |
| PiZZ: | = | homozygous type Z deficiency variant |
| PiMM: | = | normal AAT gene variant |
| COPD: | = | chronic obstructive pulmonary disease |
| MMP: | = | matrix metalloproteinase |
| MCP: | = | monocyte chemoattractant protein |
| CRP: | = | C‐reactive protein |
| FEV1: | = | forced expiratory volume in 1 second |
| FVC: | = | forced vital capacity |
| ACE: | = | angiotensin converting enzyme |
| sICAM‐1: | = | soluble intercellular adhesion molecule‐1 |
| IL‐8: | = | interleukin 8 |
References
- Sandford A J, Silverman E K. Chronic obstructive pulmonary disease. 1: Susceptibility factors for COPD the genotype–environment interaction. Thorax 2002; 57: 736–741, [PUBMED], [INFOTRIEVE]
- Piitulainen E, Eriksson S. Decline in FEV1 related to smoking status in individuals with severe alpha1‐antitrypsin deficiency (PiZZ). Eur Respir J 1999; 13: 247–251, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Yoshida A, Lieberman J, Gaidulis L, Ewing C. Molecular abnormality of human alpha1‐antitrypsin variant (Pi‐ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A 1976; 73: 1324–1328, [PUBMED], [INFOTRIEVE]
- Janciauskiene S, Dominaitiene R, Sternby N H, Piitulainen E, Eriksson S. Detection of circulating and endothelial cell polymers of Z and wild type alpha 1‐antitrypsin by a monoclonal antibody. J Biol Chem 2002; 277: 26540–26546, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Elliott P R, Bilton D, Lomas D A. Lung polymers in Z alpha1‐antitrypsin deficiency‐related emphysema. Am J Respir Cell Mol Biol 1998; 18: 670–674, [PUBMED], [INFOTRIEVE], [CSA]
- Pauwels R A, Buist A S, Calverley P M, Jenkins C R, Lomas D A. Loop‐sheet polymerization: the mechanism of alpha1‐antitrypsin deficiency. Respir Med 2000; 94: S3–S6
- Lieberman J, Sastre A. Serum angiotensin converting enzyme levels in patients with alpha 1‐antitrypsin variants. Am J Med 1986; 81: 821–824, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ucar G, Yildirim Z, Ataol E, Erdogan Y, Biber C. Serum angiotensin converting enzyme activity in pulmonary diseases: correlation with lung function parameters. Life Sci 1997; 61: 1075–1082, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Mendelsohn F A, Kachel C. Production of angiotensin converting enzyme by cultured bovine endothelial cells. Clin Exp Pharmacol Physiol 1981; 8: 477–481, [PUBMED], [INFOTRIEVE]
- Cella G, Sbarai A, Mazzaro G, Vanzo B, Romano S, Hoppensteadt T, Fareed J. Plasma markers of endothelial dysfunction in chronic obstructive pulmonary disease. Clin Appl Thromb/Hemost 2001; 7: 205–208
- Kasahara Y, Tuder R M, Taraseviciene‐Stewart L, Le Cras T D, Abman S, Hirth P K, Waltenberger J, Voelkel N F. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000; 106: 1311–1319, [PUBMED], [INFOTRIEVE], [CSA]
- Viegi G, Pedreschi M, Pistelli F, Di Pede F, Baldacci S, Carrozzi L, Giuntini C. Prevalence of airways obstruction in a general population: European Respiratory Society vs. American Thoracic Society definition. Chest 2000; 117: 339S–345S, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Berglund E. The pathophysiology of chronic respiratory failure. Scand J Respir Dis, Suppl 1970; 72: 13–18
- Pauwels R A, Buist A S, Calverley P M, Jenkins C R, Hurd S S, GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163: 1256–1276, [PUBMED], [INFOTRIEVE], [CSA]
- Jeppson J O, Laurell C B, Franzen B. Agarose gel electrophoresis. Clin Chem 1979; 25: 629–638, [PUBMED], [INFOTRIEVE]
- Wallmark A, Alm R, Eriksson S. Monoclonal antibody specific for the mutant PiZ alpha 1‐antitrypsin and its application in an ELISA procedure for identification of PiZ gene carriers. Proc Natl Acad Sci U S A 1984; 81: 5690–5693, [PUBMED], [INFOTRIEVE], [CSA]
- Dafforn T R, Mahadeva R, Elliott P R, Sivasothy P, Lomas D A. A kinetic mechanism for the polymerization of alpha1‐antitrypsin. J Biol Chem 1999; 274: 9548–9555, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Parvez Z. Laser nephelometry: development, clinical application, and future prospects. Med Instrum 1984; 18: 127–130, [PUBMED], [INFOTRIEVE]
- Afifi A, Clark V. Computer‐Aided Multivariate Analysis. Life Learning Publication, California, USA 1984, 456 pp.
- Barnes P J. Chronic obstructive pulmonary disease. N Engl J Med 2000; 343: 269–280, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Grashoff W F, Sont J K, Sterk P J, Hiemstra P S, de Boer W I, Stolk J, Han J, van Krieken J M. Chronic obstructive pulmonary disease: role of bronchiolar mast cells and macrophages. Am J Pathol 1997; 151: 1785–1790, [PUBMED], [INFOTRIEVE]
- Gonzalez S, Hards J, van Eeden S, Hogg J C. The expression of adhesion molecules in cigarette smoke‐induced airways obstruction. Eur Respir J 1996; 9: 1995–2001, [PUBMED], [INFOTRIEVE], [CROSSREF]
- de Boer W I, Sont J K, van Schadewijk A, Stolk J, van Krieken J H, Hiemstra P S. Monocyte chemoattractant protein 1, interleukin 8, and chronic airways inflammation in COPD. J Pathol 2000; 190: 619–626, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Becker S, Quay J, Koren H S, Haskill J S. Constitutive and stimulated MCP‐1, GRO alpha, beta, and gamma expression in human airway epithelium and bronchoalveolar macrophages. Am J Physiol 1994; 266: L278–L286, [PUBMED], [INFOTRIEVE]
- Russell R E, Culpitt S V, DeMatos C, Donnelly L, Smith M, Wiggins J, Barnes P J. Release and activity of matrix metalloproteinase‐9 and tissue inhibitor of metalloproteinase‐1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2002; 26: 602–609, [PUBMED], [INFOTRIEVE], [CSA]
- Opdenakker G, Van den Steen P E, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol 2001; 69: 851–859, [PUBMED], [INFOTRIEVE], [CSA]
- Liu Z, Zhou X, Shapiro S D, Shipley J M, Twining S S, Diaz L A, Senior R M, Werb Z. The serpin alpha1‐proteinase inhibitor is a critical substrate for gelatinase B/MMP‐9 in vivo. Cell 2000; 102: 647–655, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Lanone S, Zheng T, Zhu Z, Liu W, Lee C G, Ma B, Chen Q, Homer R J, Wang J, Rabach L A. Overlapping and enzyme‐specific contributions of matrix metalloproteinases‐9 and ‐12 in IL‐13‐induced inflammation and remodeling. J Clin Invest 2002; 110: 463–474, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Qu Q, Zhao M, Gu G. sICAM and HLA‐DR expression in bronchial epithelial cells from patients with chronic obstructive pulmonary disease. Zhonghua Jiehe He Huxi Zazhi 1998; 21: 411–414, [PUBMED], [INFOTRIEVE], [CSA]
- Di Stefano A, Maestrelli P, Roggeri A, Turato G, Calabro S, Potena A, Mapp C E, Ciaccia A, Covacev L, Fabbri L M. Upregulation of adhesion molecules in the bronchial mucosa of subjects with chronic obstructive bronchitis. Am J Respir Crit Care Med 1994; 149: 803–810, [PUBMED], [INFOTRIEVE], [CSA]
- Gerritsen M E, Shen C P, McHugh M C, Atkinson W J, Kiely J M, Milstone D S, Luscinskas F W, Gimbrone M A, Jr. Activation‐dependent isolation and culture of murine pulmonary microvascular endothelium. Microcirculation 1995; 2: 151–163, [PUBMED], [INFOTRIEVE]
- Tang M L, Fiscus L C. Important roles for l‐selectin and sICAM in the development of allergic airway inflammation in asthma. Pulm Pharmacol Ther 2001; 14: 203–210, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hill A T, Campbell E J, Bayley D L, Hill S L, Stockley R A. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha (1)‐antitrypsin deficiency (PiZ). Am J Respir Crit Care Med 1999; 160: 1968–1975, [PUBMED], [INFOTRIEVE], [CSA]
- Foreman R C. Alpha 1‐antitrypsin deficiency—a defect in secretion. Biosci Rep 1987; 7: 307–311, [PUBMED], [INFOTRIEVE]
- Yoshida A, Lieberman J, Gaidulis L, Ewing C. Molecular abnormality of human alpha1‐antitrypsin variant (Pi‐ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A 1976; 73: 1324–1328, [PUBMED], [INFOTRIEVE]
- Wewers M D, Herzyk D J, Gadek J E. Comparison of smoker and nonsmoker lavage fluid for the rate of association with neutrophil elastase. Am J Respir Cell Mol Biol 1989; 1: 423–429, [PUBMED], [INFOTRIEVE]
- Abrams W R, Fein A M, Kucich U, Kueppers F, Yamada H, Kuzmowycz T, Morgan L, Lippmann M, Goldberg S K, Weinbaum G. Proteinase inhibitory function in inflammatory lung disease. I. Acute bacterial pneumonia. Am Rev Respir Dis 1984; 129: 735–741, [PUBMED], [INFOTRIEVE], [CSA]
- Wewers M D, Herzyk D J, Gadek J E. Alveolar fluid neutrophil elastase activity in the adult respiratory distress syndrome is complexed to alpha‐2‐macroglobulin. J Clin Invest 1988; 82: 1260–1267, [PUBMED], [INFOTRIEVE], [CSA]
- Parmar J S, Mahadeva R, Reed B J, Farahi N, Cadwallader K A, Keogan M T, Bilton D, Chilvers E R, Lomas D A. Polymers of alpha(1)‐antitrypsin are chemotactic for human neutrophils: a new paradigm for the pathogenesis of emphysema. Am J Respir Cell Mol Biol 2002; 26: 723–730, [PUBMED], [INFOTRIEVE], [CSA]
- Yao J, Baecher‐Allan C M, Sharon J. Serpins identified as cell growth inhibitors in human plasma. Molec Cell Biol Res Commun 2000; 3: 76–81, [CROSSREF], [CSA]
- Banda M J, Rice A G, Griffin G L, Senior R M. Alpha 1‐proteinase inhibitor is a neutrophil chemoattractant after proteolytic inactivation by macrophage elastase. J Biol Chem 1988; 263: 4481–4484, [PUBMED], [INFOTRIEVE]
- Joslin G, Griffin G L, August A M, Adams S, Fallon R J, Senior R M, Perlmutter D H. The serpin‐enzyme complex (SEC) receptor mediates the neutrophil chemotactic effect of alpha‐1 antitrypsin‐elastase complexes and amyloid‐beta peptide. J Clin Invest 1992; 90: 1150–1154, [PUBMED], [INFOTRIEVE]
- Forsyth K D, Talbot V, Beckman I. Endothelial serpins—protectors of the vasculature?. Clin Exp Immunol 1994; 95: 277–282, [PUBMED], [INFOTRIEVE], [CSA]