Abstract
Study Objectives. Compare hospitalization risk of various initial treatment regimens for COPD. Design. Retrospective observational cohort design. Setting. Patients enrolled in 24 different managed care plans across the United States during 1997–2000. Patients. Aged at least 45 years with a primary diagnosis of COPD identified. Initiation date was the date the first inhaler was dispensed. Patients were required to have filled this prescription within 60 days of the first documented COPD diagnosis in the database. Interventions. Five therapy cohorts were identified 1) ipratropium alone or in combination with albuterol (IPR), 2) long‐acting beta agonists (LABA), 3) inhaled corticosteroid (ICS), 4) ICS + IPR and 5) ICS + LABA. Measurements. Subjects were observed for 12 months or until a COPD‐related hospitalization was observed, whichever came first. A sensitivity analysis was conducted by varying the observation period from > 60, > 90 and > 180 days to determine if this would impact the results. Results. 3616 patients were identified, 1754 (49%) on IPR alone, 1032 (29%) on ICS alone, 357 (10%) on ICS + IPR, 266 (7%) on LABA alone and 207 (6%) on ICS + LABA. Compared with IPR alone, patients in the ICS alone and ICS plus LABA had groups a 36% and 47% reductions in the risk of a COPD hospitalization, (HR: 0.643; 95% CI 0.512, 0.808 and HR: 0.533; 95% CI 0.328, 0.865) respectively. Conclusions. The results of this analysis suggest that initial treatment with an ICS alone or in combination with a LABA, compared to IPR alone, was associated with a significant decrease in the risk of COPD hospitalization 12 months following the start of therapy independent of concomitant asthma diagnosis. Similar outcomes were observed when the observation period was varied from > 60, > 90 and > 180 days.
Introduction
In the United States, chronic obstructive pulmonary disease (COPD) affects an estimated 10 million adults. In addition, it is estimated that 24 million adults have evidence of lung function impairment Citation[[1]]. During 2000, COPD was responsible for 8 million physician office and hospital outpatient visits, 1.5 million emergency department visits, 726,000 hospitalizations, and 119,000 deaths. Respiratory conditions, including COPD, are the fourth highest cause of death in the United States, with COPD prevalence steadily increasing Citation[[1]]Citation[[2]]. In 1996, prevalence had increased 57% relative to 1979–1980 and increased 10% compared to 1986–1988 Citation[[2]]. Females have experienced the greatest increase in COPD prevalence where rates have doubled from 1980 to 1995 Citation[[3]].
Short‐acting anticholinergics and beta agonists have been the mainstay of COPD therapy, though they have not been shown to modify the long‐term decline in lung function. However, agents such as the long‐acting beta agonists and inhaled corticosteroids may have other effects such as reduced frequency and severity of COPD exacerbations Citation[[4]]Citation[[5]]Citation[[6]]Citation[[7]]. A long‐term clinical study known as TRISTAN was able to show that the use of an inhaled corticosteroid and the combination of an inhaled corticosteroid with a long‐acting beta agonist improved lung function symptoms and reduced exacerbations in patients with COPD compared to patients on placebo Citation[[11]]. In addition, airway inflammation may have some role in the pathogenesis of COPD, the use of orally administered corticosteroids has been observed to rapidly improve COPD symptoms experienced during exacerbations Citation[[8]]Citation[[9]]Citation[[10]].
Given the impact of COPD on patients' general health and well‐being, and the cost associated with treating acute exacerbations, which often require inpatient care, identifying treatment regimens that avoid such events is of particular interest to patients, physicians and third party payers. To date, observational studies of the role of ICS in COPD have been mixed: several studies have shown that ICS use was independently associated with a decreased risk of hospital admission, readmission and mortality in COPD patients Citation[[11]]Citation[[12]]Citation[[13]]. However, other analyses have not shown a positive effect of ICS in patients with COPD Citation[[14]]Citation[[15]]Citation[[16]]. In particular, an analysis by Suissa et al.,Citation[[16]]may have exposed a bias in the study design used in these analyses. This bias of ‘immortal time’ may limit the conclusions from these studies Citation[[16]].
In an attempt to clarify the role of ICS, alone or in combination with long‐acting beta agonists, in the treatment of COPD, we conducted a large observational study to compare treatment with inhaled corticosteroids, long‐acting beta agonists, or the combination of ICS and bronchodilators relative to ipratropium with respect to time from initial COPD‐related hospitalization in the first 12 months after initiation of therapy. This analysis was conducted using administrative claims data from 24 managed care plans across the United States in order to better understand the impact of these therapies in an actual clinical practice.
Methods
Data Source
Patient‐level administrative medical and pharmacy data were captured from the PharMetrics Integrated Outcomes Database (Waterton, MA), which contains administrative claims data from 24 managed care plans across the United States and encompasses inpatient and outpatient medical care, in addition to linked prescription claims and ancillary charges. Medical utilization in the database is grouped into episodes of care, based on Symmetry Health Data Systems' Episode Treatment Group (ETG) analytical methodology Citation[[17]]. Claims are grouped into episodes based on diagnosis, with clinically validated algorithms to ensure correct matching of drug and medical claims, and to clinically define the appropriate initiation and termination dates of each type of episode Citation[[12]]. All data was collected from claims submitted by providers (i.e. physicians, pharmacies, hospitals) to payers (i.e. managed care organizations) to receive payment for services rendered. All claims underwent an initial check by the providers and payers to ensure correct coding of elements related to billing, such as diagnosis codes for medical care, and national drug codes (NDC) for prescription claims. In addition, prior to inclusion in the Integrated Outcomes Database, the data underwent a further level of quality/validity check ensuring that all claims are checked for double billing/nonadjudicated claims, valid age range, and gender‐specific procedures for inappropriate gender. Studies using this data have been published previously in peer‐reviewed journals, and this data is currently used by the contributing plans for benchmarking and research purposes Citation[[18]]Citation[[19]]Citation[[20]].
Sample Selection
The study population was identified from the database for the period January 1, 1998 to December 31, 1999. Subjects with COPD were identified using International Classification of Diseases, Ninth Revision‐Clinical Modification (ICD‐9‐CM) codes 491.xx (“chronic bronchitis”), 492.x (“emphysema”), and 496.x (“chronic airways obstruction, not else‐where classified”) Citation[[21]]. To be eligible for the analysis, patients also must have received at least one prescription claim for one of the following agents at any time during this time period: ipratropium or fixed combination albuterol/ipratropium (IPR), any inhaled corticosteroid (ICS), or the long‐acting beta agonist (LABA). Any patients who received cromolyn, theophylline or a leukotriene receptor antagonist, or who had a COPD‐related hospitalization prior to their initial prescription claim were excluded from the analysis. Study patients were also required to be at least 45 years of age and to have been continuously eligible for benefits 12 months prior to and 12 months subsequent to the initial prescription claim during the study period. Treatment cohorts were based on the use of study agents (IPR, ICS or LABA) during the first 60 days after the index prescription claim: IPR only, ICS only, LABA only, ICS + IPR, ICS + LABA. These cohorts were then followed for 12 months using an intent‐to‐treat analysis. Patients who received all three agents (IPR, ICS and LABA) or IPR + LABA within the first 60 days were excluded from the analysis due to insufficient sample size (n = 32 for IPR + ICS + LABA; n = 41 for IPR + LABA). Patients who only received short‐acting beta agonist were excluded.
Collection of Data
Utilization data were collected and analyzed to assess medical and pharmaceutical utilization, emergency room (ER) visits, and hospitalizations. All medical claims were assessed for the 12 months prior to the initial COPD prescription claim to establish prior resource utilization and 12 months after the initial claim to establish postperiod utilization. Postutilization analyses included assessments of COPD‐related utilization as defined by the primary diagnosis of COPD on medical claims and prescription claims. Specifically, utilization was categorized as either COPD‐related inpatient, outpatient, ER, ancillary, or pharmaceutical. Additionally, all respiratory claims (COPD and non‐COPD related) were also identified and defined as any claim with a primary diagnosis code of ICD‐9 between 460.xx and 519.xx.
Outcomes
The main outcome of interest was the time to initial COPD hospitalization. The risk of respiratory‐related hospitalizations was also analyzed. The main exposure of interest was the initial COPD therapy regimen prescribed.
Statistical Analysis
All categorical variables (gender, presence of comorbid conditions, etc) are presented as percentages (%) and were compared using the test of proportions using independent samples. Since IPR was the largest cohort, it was used as the reference group for all measures of effect. For comparisons of COPD and all respiratory hospitalization rates between treatment cohorts, a Cox proportional hazards model was used, adjusting for differences across the cohorts with respect to gender, age, managed care plan, physician specialty associated with the initial COPD diagnosis, COPD subtype (emphysema, bronchitis, undefined), presence of comorbid asthma, and preperiod (12 months prior to first prescription) utilization of any hospital service (other than COPD) and oral corticosteroid use. As multiple univariate comparisons were conducted for each variable, Bonferroni's adjustment was used for the alpha (α = 0.05/5 = 0.01).
Sensitivity Analysis
Many of the medications commonly used for the treatment of COPD are also used to treat asthma. Since COPD patients may also have a concomitant diagnosis of asthma, the role of these agents in the treatment of COPD may be unclear. Therefore, a sensitivity analysis was conducted which compared risk of COPD and all respiratory hospitalizations for patients who had no asthma diagnosis during the 2‐year observation period in order to determine if the results observed in the overall analysis were robust to the exclusion of patients with concomitant asthma.
Additionally, three alternate study periods were used to compare hospitalization rates amongst the treatment cohorts: > 60 days postindex; > 90 days postindex; and > 180 days postindex to address the potential ‘immortal time bias’ suggested by Suissa et al.Citation[[16]] as the basis for potential protective effect of ICS observed in previous analyses. As the sample sizes for the combination cohorts and LABA were relatively small (∼ 200 patients per cohort), three separate time periods were selected in order to measure the stability of the point estimates as well as statistical significance.
Results
There were 3616 COPD patients included in the analysis. provides the baseline demographic information for these patients. Baseline is defined as the 12 month period prior to the initial prescription. Sample patients were, on average, 62 years of age, with little variation among the treatment groups (range: 61.9–63.6 years). Over half (55%) of the sample was female; however, ICS patients were significantly more likely to be female (p < 0.0001 vs. IPR, ). General practitioners/internists were the most common physician specialty associated with the COPD diagnosis for patients in the LABA group, while specialists were the most common in the ICS + LABA and ICS + IPR cohorts (p < 0.02) compared to IPR. The prevalence of comorbid chronic and respiratory conditions was similar across the cohorts. In addition, the proportion of patients with concomitant asthma diagnosis over the 2‐year observation period ranged from 21% in the IPR group to 55% in the ICS + LABA group.
Table 1. Baseline Demographics by Treatment Regimen
The baseline rate of any respiratory‐related hospitalization (excluding COPD) within the various treatment cohorts is presented in . Hospitalizations were fairly common, with 17% of all study patients having a respiratory illness related hospitalization (excluding COPD) in the 12‐month baseline period. However, there were no significant differences between the other study cohorts and IPR with respect to respiratory related hospitalization.
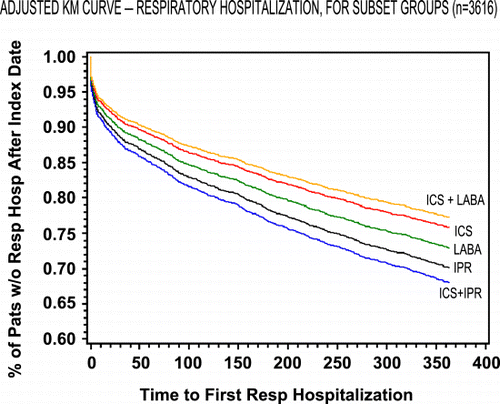
After adjusting for differences in baseline characteristics, compared to IPR only, the ICS and ICS + LABA cohorts had a lower risk of COPD related hospitalization during the 12‐month posttreatment follow‐up period (). Patients in the ICS only group had a 36% reduction in risk of COPD‐related hospitalization compared to IPR‐only patients (95% CI: 0.51, 0.81). The combination of ICS + LABA appeared to have an additive effect producing a 47% reduction in risk (95% CI: 0.33, 0.86) relative to IPR only. No significant differences in COPD related hospitalization rates could be discerned for the LABA or ICS + IPR cohorts. Similar trends were observed with all respiratory hospitalizations, with both ICS and ICS + LABA cohorts having statistically significant lower hospitalization rates in the 12‐month follow‐up period compared to IPR (). and provide a graphical depiction of COPD‐related and respiratory‐related hospitalizations over time for the cohorts after adjusting for differences in baseline characteristics.
Table 2. Adjusted Risk of Various COPD Treatment Regimens on COPD and All‐Respiratory Hospitalizations.
Figure 1. Adjusted probability of COPD hospitalization‐free survival in patients with chronic obstructive pulmonary disease by treatment cohort. (Full color version available online.)
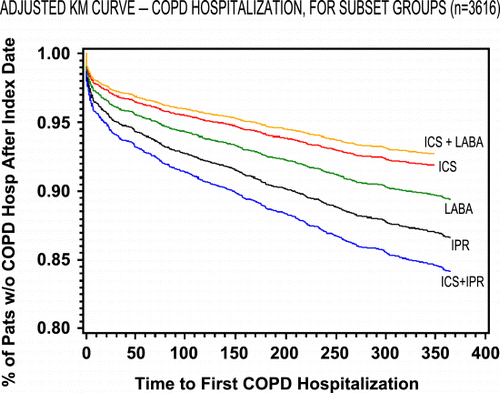
Figure 2. Adjusted probability of respiratory hospitalization‐free survival in patients with chronic obstructive pulmonary disease by treatment cohort. (Full color version available online.)
Sensitivity Analysis
To address the issue of concurrent asthma diagnosis, a model that included only those COPD patients without an asthma diagnosis (n = 2453) yielded reduced COPD‐ (HR: 0.696; 95% CI 0.519, 0.934) and respiratory‐related (HR: 0.796, 0.649, 0.976) hospitalizations for ICS (n = 590) relative to IPR (n = 1392). Hazard ratios for LABA and ICS + LABA were below 1 but 95% CI also included 1, which may be related to the low sample sizes: 94 and 168 patients, respectively.
To address the potential immortal time bias, additional comparisons were conducted using alternative time periods (). The first model excluded patients with any COPD/respiratory hospitalizations during the first 60 days postindex and compared hospitalization rates for days 61–360 only, the hazard ratios for ICS only vs. IPR for both COPD (HR: 0.658; 95% CI 0.474–0.915) and respiratory‐related hospitalizations (HR: 0.710; 0.610–0.826) remained statistically significant. In contrast, the comparison of ICS + LABA vs. IPR for COPD hospitalizations was no longer statistically significant (HR: 0.746, 0.415, 1.342). However, ICS + LABA vs. IPR for respiratory hospitalization remained statistically significant (HR: 0.669; 95% CI: 0.459–0.975).
Table 3. Adjusted Risk of Various COPD Treatment Regimens on COPD and All Respiratory Hospitalizations (Impact of Additional Exclusion Criteria on Statistically Significant Comparisons in Overall Analysis)
The second model extended the hospital‐free period to 90 days postindex. Again, treatment with ICS vs. IPR produced significant hazard ratios for both COPD (HR: 0.56; 95% CI: 0.385, 0.816) and respiratory‐related hospitalizations (HR: 0.60; 95% CI: 0.471, 0.764). This was also observed when the hospital‐free period was extended to 180 days (). The point estimates for the ICS + LABA vs. IPR comparisons were fairly stable across both these time periods, particularly for the respiratory‐related hospitalization measure (HR range of 0.7 to 0.8) however, the 95% CI crossed 1 which may be due to the low number of COPD‐ and respiratory‐related events for the combination group (range 6 to 29) ().
Discussion
The main findings of this study is that initial therapy with ICS alone (34% reduction) and in combination with maintenance bronchodilators (ICS + LABA: 47% reduction) was associated with a lower risk of COPD and all respiratory related hospitalizations compared to IPR therapy.
The positive impact of inhaled steroids on COPD outcomes is important, given the recent findings of several large trials that found inhaled‐steroid use in COPD did not effect the rate of decline in expiratory flow volumes Citation[[4]]Citation[[5]]Citation[[6]]Citation[[7]]Citation[[11]]Citation[[22]]. Although decline of lung function as measured by FEV1 is a significant and important determinant of COPD morbidity and mortality, FEV1 by itself has relatively weak predictive powers for these outcomes—in fact, clinically relevant changes in health status can occur in the absence of any measurable effects on lung function Citation[[5]]Citation[[23]]. Therefore, other measures, such as patient symptoms, rate of exacerbation, or patient health status, should also be determined in judging the efficacy of agents in the treatment of COPD.
Inhaled corticosteroids, beta agonists and anticholinergics, alone or in combination, have all been shown to reduce the frequency and severity of COPD exacerbations Citation[[4]]Citation[[5]]Citation[[24]]. It appears that IPR remains widely used as first‐line treatment of COPD patients, given the large number of patients (48%) with IPR as initial therapy in this analysis. However, ICS was also commonly used, even in patients without concomitant asthma. In addition, when ICS and/or LABA were prescribed to patients with COPD, they appeared to have a positive impact, as measured by utilization of medical services, both COPD‐specific and overall respiratory related. Even though patients started on ICS had an associated reduction in risk of a COPD‐specific and all respiratory hospitalizations during the 12 months after treatment initiation, patients receiving combination ICS + LABA experienced the greatest risk reduction for either COPD‐related or all respiratory hospitalizations compared to patients on IPR only. In addition, contrary to other studies, there was a higher proportion of females 55%, to males, 45% in all of the cohorts. This is consistent with the gender distribution of the underlying population, which was ∼ 60% female during the study period. In addition, self‐reported prevalence of COPD is higher in women than men Citation[[1]]Citation[[25]]. Furthermore, even though COPD‐related mortality and morbidity are increasing among women, in this observational analysis, gender was not a significant predictor of a COPD‐ or respiratory‐related hospitalization.
Recent studies have shown that COPD patients treated with ICS may have better health‐related quality of life, lower rates of exacerbation, fewer respiratory symptoms, and lower risk of rehospitalization or death within the first year after an initial hospitalization Citation[[5]]Citation[[11]]Citation[[22]]. Calverley et al. Citation[[11]] in the TRISTAN study found that the use of ICS in patients with COPD reduced the total exacerbation rate by 19% and exacerbations that required oral corticosteroids by 34% while the combination of an ICS and LABA had reductions of 25% and 39% respectively, which were all significant changes compared with placebo Citation[[11]]. The results of this analysis are consistent with those findings, where the use of an ICS was associated with a reduction in COPD‐ and respiratory‐related hospitalization with an additional benefit to the combination of an ICS and LABA in the reduction of risk of hospitalization in the first year after initiating pharmacotherapy.
Given the potentially high prevalence of comorbid asthma in this population, which is often unrecognized, the inclusion of these patients within the cohort increases the generalizability of these results, and further highlights the potential benefit of these agents in COPD Citation[[26]]. While attempts were made to control for comorbid asthma, there did appear to be a channeling bias against ICS and/or LABA with respect to asthma that may not have been adequately controlled in this analysis. To address this potential bias, a ‘sensitivity analysis’ was conducted which excluded all patients with a diagnosis of asthma anytime during the 24‐month observation period. The results of this sensitivity analysis were consistent with the overall results, which lend more credence to the potential benefit of ICS, alone or in combination with a long‐acting beta agonist, in the treatment of COPD.
A recent analysis by Suissa et al. Citation[[11]], suggested that observational cohort studies may be subject to ‘immortal time’ biases, i.e., that comparing treated cohorts to non‐treated cohorts is biased due to the necessity of treated patients to have survived long enough to receive treatment Citation[[16]]. In this analysis, we compared only treated cohorts, however patients on combination therapy may have been subject to this issue, due to their need to ‘survive’ to add the second agent. Therefore, several sensitivity analyses were performed comparing the treatment cohorts at alternative time periods. For all time periods, ICS vs. IPR contrasts remained statistically significant. The ICS + LABA vs. IPR cohort lost statistical significance when extending the time period; however, this may have been due more to sample size than immortal time bias, as evidenced by the statistical significance in respiratory hospitalizations for the 61–360‐day comparison and the relative stability of the point estimates across the time‐period comparisons.
Observational analyses, such as the analysis reported here, are not intended to replace randomized controlled studies, as the former are more susceptible to biases, nor should any causality be inferred from the results of such an analysis Citation[[27]]. A limitation of this analysis, as with any observational study using administrative claims, is the lack of information on clinical parameters such as symptoms and pulmonary function, which are clinical makers of severity. However, we did employ statistical techniques and careful study design to address differences in the observable variables which have been identified in other studies to be predictors of COPD morbidity and mortality Citation[[28]]Citation[[29]]. These included: physician specialty associated with the index COPD diagnosis, COPD diagnosis subtype, preperiod use of inpatient/ER services, comorbid asthma diagnosis, and oral steroid use (surrogate marker for exacerbation). In addition, to controlling for differences in practice patterns, the managed care plan in which the patient was enrolled was included as a covariate, as were patient age and gender. As expected, all covariates, except for gender and prior oral steroid use were significantly predictive of COPD and any respiratory related hospitalization.
In addition, the ability to characterize the impact of treatment under ‘actual practice’ conditions in populations not often included in clinical trials (i.e. the elderly, or those with multiple chronic conditions) is a benefit of these designs Citation[[12]]. Furthermore, another limitation of these kinds of analyses is that we cannot assess the patients' compliance with the medications. We were only able to identify the lack of compliance by failure to refill a prescription and/or the change in prescription by the health care provider. Thus, despite the limitations of observational studies, emerging evidence suggests that their findings for pharmacologic interventions are at times similar to those of large randomized controlled trials Citation[[30]]Citation[[31]].
In conclusion, the results of this retrospective observational study suggest that initial COPD treatment with an inhaled corticosteroid, alone or in combination with maintenance bronchodilators, was associated with a significant decrease in the risk of hospitalization, compared to ipratropium alone, during the first 12 months of therapy. This benefit was observed in patients with and without a concomitant asthma diagnosis. The combination of an inhaled corticosteroid and LABA, though apparently uncommon as first‐line therapy in COPD, was associated with the greatest decrease in risk of COPD or respiratory hospitalization. Further studies should focus on this combination to confirm these findings across health plans and populations.
| Abbreviations | ||
| IPR: | = | Ipratropium + Combivent |
| LABA: | = | Long‐acting beta agonist |
| ICS: | = | Inhaled corticosteroids |
| COPD: | = | Chronic obstructive pulmonary disease |
| ETG: | = | Episode Treatment Group |
| ICD‐9‐CM: | = | International Classification of Diseases, Ninth Revision‐Clinical Modification |
| ER: | = | Emergency room |
| SD: | = | Standard deviation |
| CI: | = | Confidence interval |
| HR: | = | Hazard ratio |
| FEV1: | = | Forced Expiratory Volume in 1 second |
References
- MMWR. Surveillance summaries. Morb Mort Wkly Rep August 2, 2002; 51(No. SS‐6)
- Collins J G. Prevalence of selected chronic conditions: United States, 1990–92; National Center for Health Statistics. Vital Health Stat 1997; 10(194)12–13
- Lacasse Y, Brooks D, Goldstein R S. Trends in the epidemiology of COPD in Canada, 1980 to 1995. COPD and Rehabilitation Committee of the Canadian Thoracic Society. Chest 1999; 116: 306–313, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Pauwels R A, Lofdahl C G, Laitinen L A, Schouten J P, Postma D S, Pride N B, Ohlsson S V, for the European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. Long‐term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med 1999; 340: 1948–1953, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Burge P S, Calverley P M, Jones P W, Spencer S, Anderson J A, Maslen T K. Randomized, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000; 320: 1297–1303, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long‐term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomized controlled trial. Lancet 1999; 353: 1819–1823, [PUBMED], [INFOTRIEVE], [CROSSREF]
- The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline of pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000; 343: 1902–1909, [CROSSREF], [CSA]
- Barnes P J. Chronic obstructive pulmonary disease. N Engl J Med 2000; 343: 269–280, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Davies L, Angus R M, Calverley P M. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999; 354: 456–460, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Niewoehner D E, Erbland M L, Deupree R H, Collin S D, Gross N J, Light R W, Anderson P, Morgan N A. The Department of Veterans Affairs Cooperative Study. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1999; 340: 1941–1947, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003; 361: 449–456, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Sin D D, Tu J V. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 580–584, [PUBMED], [INFOTRIEVE], [CSA]
- Soriano J B, Vestbo J, Pride N B, Kiri V, Maden C, Maier W C. Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Respir J 2002; 20: 819–825, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Fan V S, Bryson C L, Curtis J R, Fihn S D, Bridevaux P O, McDonell M B, Au D H. Inhaled corticosteroids in chronic obstructive pulmonary disease and risk of death and hospitalization: time‐dependent analysis. Am J Respir Crit Care Med 2003; 168: 1488–1494, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Bourbeau J, Ernst P, Cockcoft D, Suissa S. Inhaled corticosteroids and hospitalisation due to exacerbation of COPD. Eur Respir J 2003; 22: 286–289, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Suissa S. Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease. Am J Resp Crit Care Med 2003; 168: 49–53, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Symmetry Health Data Systems. Episode Treatment Groups Users Guide: Release 4.3. Symmetry Health Data Systems, Phoenix, AZ 2002
- Stempel D A, McLaughlin T P, Griffis D L, Stanford R H. Cost analysis of the use of inhaled corticosteroids in the treatment of asthma: a 1‐year follow‐up. Respir Med 2001; 95(12)992–998, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Yazdani C, McLaughlin T P, Smeeding J, Walt J. Prevalence of treated eye disease in a managed care population. Clin Ther 2001; 23(10)1672–1682, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lee J, Cummins G, Okamoto L. A descriptive analysis of the use and cost of new‐generation antihistamines in the treatment of allergic rhinitis: a retrospective database analysis. Am J Managed Care 2001; 7(suppl 4)S103–S112, [CSA]
- Department of Health and Human Services. The international classification of diseases, 9th rev. Clinical Modification: ICD‐9‐CM. Vol. 1. Diseases: Alphabetic Index2nd ed. Department of Health and Human Services, Washington, DC 1980
- Paggiaro P L, Dahle R, Bakran I, Frith L, Hollingworth K, Efthiniou J, on behalf of the International COPD Study Group. Multicentre randomized placebo‐controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disorder. Lancet 1998; 351: 773–780, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Anthonisen N R, Wright E C, Hodgkin J E. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 133: 14–20, [PUBMED], [INFOTRIEVE]
- Mahler D A, Donohue J F, Barbee R A, Goldman M D, Gross N J, Wisniewski M E, Yancey S W, Zakes B A, Rickard K A, Anderson W H. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999; 115: 957–965, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Strassels S A, Smith D H, Sullivan S D, Mahajan P S. The costs of treating COPD in the United States. Chest 2001; 119: 344–352, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Guite H F, Dundas R, Burney P G. Risk factors for death from asthma, chronic obstructive pulmonary disease, and cardiovascular disease after a hospital admission for asthma. Thorax 1999; 54(4)301–307, [PUBMED], [INFOTRIEVE]
- Wen S W, Hernandez R, Naylor C D. Pitfalls in nonrandomized outcomes studies. The case of incidental appendectomy with open cholecystectomy. JAMA 1995; 274: 1687–1691, [PUBMED], [INFOTRIEVE], [CROSSREF]
- D'Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative databases. J Clin Epidemiol 1996; 49: 1429–1433, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Sin D D, Tu J V. Lack of association between ipratropium bromide and mortality in elderly patients with chronic obstructive airway disease. Thorax 2000; 55: 194–197, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Benson K, Hartz A J. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000; 342: 1878–1886, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Concato J, Shah N, Horwitz R I. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000; 342: 1887–1892, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]