Abstract
Question of the Study. To assess the impact of prescribing pulmonary drugs according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines on direct costs in stable chronic obstructive pulmonary disease (COPD). Patients and Methods. A total of 560 ambulatory COPD patients completed a specific questionnaire that included data regarding drug therapy. Severity was graded according to the British Thoracic Society (BTS) criteria and appropriateness of pharmacological treatment according to GOLD guidelines. Results. Annual direct costs were 1,657 EUR in stage I, 2,425 EUR in stage II, and 3,303 EUR in stage III. The mean direct costs was 2,061 EUR (38% corresponded to drug therapy). Medication accounted for 43%, 37.6%, and 28.4% of total direct costs for stage I, II, and III, respectively. Inhaled steroids and long‐acting β2‐agonists accounted for 78%, 76%, and 75% of total drugs costs in stages I, II, and III, respectively. Drug therapy which was not in accordance with guidelines accounted for 78.7% and 54% of total drug costs in stages I and II, respectively. Most patients with severe disease were treated adequately. Answer to the Question. Pharmacologic treatment has a great impact on direct medical costs in stable COPD. According to GOLD guidelines, patients with mild or moderate COPD are frequently treated with nonrecommended drugs.
Introduction
Chronic obstructive pulmonary disease (COPD) is recognized as a major cause of morbidity and mortality as well as a great health care burden in developed countries Citation[[1]]. According to the National Heart, Lung, and Blood Institute, the economic impact of COPD in 1993 was estimated to be $23.9 billion in USA, of which $14.7 billion were direct medical costs Citation[[2]]. In response to the overwhelming economic burden that COPD imposes on medical systems and on society, consensus guidelines to improve its diagnosis and management have been developed Citation[[3]]Citation[[4]]Citation[[5]]Citation[[6]]Citation[[7]]. In addition to improving management of COPD, these recommendations are directed reducing uncertainty among health care professionals about which medications are most effective. New drug classes such as long‐acting β2‐agonists and inhaled steroids have been recently recommended as usual treatment in a stepped‐care pharmacological approach for patients with COPD. These recommendations are not based on cost analyses and, therefore, may not be the best approach from a cost‐effectiveness point of view. However, implementation of guidelines in clinical practice may be an important step for a more rational management of patients with COPD.
In Spain, as in other countries worldwide, a limited number of studies have attempted to quantify direct and indirect costs of COPD Citation[[8]]Citation[[9]]Citation[[10]]. To obtain information on country‐specific impact of COPD is important, not only for increasing the recognition of the relevance of COPD on different health care systems, but also for identifying which interventions can improve the management of patients and reduce the burden of the disease. This study was conducted with the following objectives: 1) to assess the effect of pulmonary drug treatment according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines on direct costs in patients with stable COPD and 2) to determine how much of the total direct costs was related to drug treatment not in accordance with recommended practices.
Materials and Methods
The population of the current study was derived from a large‐scale, cross‐sectional multicenter study [the IDENTEPOC project (Spanish acronym of Identification of Chronic Obstructive Pulmonary Disease)]. This observational, descriptive, cross‐sectional and multicenter study enrolled a stratified sample from each autonomous Spanish community from the practices of primary care and outpatient clinics of pneumology in secondary and tertiary care hospitals Citation[[11]].
Recruitment of patients and calculation of the sample size corresponded to the IDENTEPOC project. Briefly, the sample size was calculated under the assumption of an expected COPD prevalence of 10% in the primary care setting and 30% in the outpatient clinics of pneumology. A representative sample of physicians was randomly selected from two national databases,—the Spanish Society of Primary Care and the Spanish Society of Respiratory Diseases (SEPAR)—, together with other databases from the pharmaceutical industry to avoid selection bias. COPD patients visited by the participating physicians were selected at random according to a computer‐generated table of random numbers. Noneligible subjects included those living outside the study area for the previous 6 months. In order to obtain the required study sample, a total of 12 patients had to be recruited by each of the 32 general practitioners and a total of 15 patients by each of the 44 pneumologists. The IDENTEPOC sample consisted of 898 patients, but for the purpose of this study only patients with a confirmed diagnosis of COPD by spirometry and for whom reliable data for all variables were obtained were included. The study population consisted of 560 patients, of whom 460 were recruited by pneumologists and 100 by general practitioners. The study protocol was approved by a local ethical committee and informed consent was obtained from all patients.
Data were collected from January 1 to June 30, 2000, during an interview with the patient using a questionnaire that included demographic data, respiratory symptoms, cigarette smoking, spirometric data, quality of life, pharmaceutical treatment, and use of medical care resources for the management of COPD. Use of pulmonary drugs and use of medical care resources in the past 12 months was checked by cross‐linking the patients' records. Spirometric data were obtained from the patients' medical records. Patients were stratified according to severity of FEV1 impairment using criteria of the SEPAR Citation[[7]] and the British Thoracic Society (BTS) Citation[[3]]. Stage I included patients with FEV1 between 60 and 80% of predicted; stage II, FEV1 between 40 and 59% of predicted; and stage III, FEV1 < 40% of predicted. In addition, persons with an FEV1/forced vital capacity ratio < 70% and FEV1 ≥ 80% of the predicted value, which corresponds to the GOLD criteria for mild COPD Citation[[6]], were also included in the study. Functional dyspnea was assessed using the Medical Research Council (MRC) scale Citation[[12]]. Health‐related quality of life was evaluated using a Spanish validated version of the St Georges' respiratory questionnaire Citation[[13]].
Pulmonary drug therapy was considered in accordance with GOLD recommendations Citation[[6]] for the management of patients with stable COPD as follows: inhaled steroids in patients with FEV1 < 50% of the predicted value or with more than two episodes of acute exacerbation of chronic bronchitis per year; long‐acting β2‐agonists in those with score 2 or greater of the MRC scale; theophylline in those with respiratory symptoms despite the use of β2‐agonists or anticholinergic drugs; and mucolytics/antioxidant agents in those with more than two episodes of acute exacerbation per year or in whom sputum was an almost daily complaint.
For each drug class used for pulmonary drug therapy, the agent and dosage was standardized as follows: short‐acting β2‐agonists (salbutamol 200 µg as required), anticholinergics (ipratropium bromide 40 µg q.i.d.), long‐acting β2‐agonists (salmeterol 50 µg b.i.d.), inhaled steroids (fluticanose 500 µg b.i.d.), theophylline 300 mg b.i.d., and N‐acetylcysteine 600 mg once daily. Drug prices were obtained from the 2000 Spanish Pharmacopoeia Citation[[14]]. Costs of outpatient visits, hospitalizations, emergency room visits, episodes of exacerbation, and long‐term oxygen therapy were estimated using the reference prices of the Spanish National Institute of Health (INSALUD) at the time of the study ().
Table 1. Average Registered Prices at the Time of the Study
The prevalence of different groups of severity was estimated according to data from a population‐based study conducted in seven areas of Spain (the IBERPOC study) in subjects aged between 40 and 69 years, in which the prevalence of COPD was 9.1% Citation[[15]]. Although this figure increases to 16% in subjects older that 60 years, a conservative prevalence of 9% was accepted for subjects aged 70 years or older. Smoking rates were used to estimate the frequency of COPD in the general population Citation[[16]]. It was calculated that between 1.5 and 1.7 million people would have suffered from the disease Citation[[17]].
Statistical Analysis
Student's t test and one‐way analysis of variance were used for the comparison of means. When data departed from normality, the Kruskal–Wallis test and the Wilcoxon rank–sum test were used. Categorical variables were compared with the chi‐square (χ2) test. Statistical significance was set at P < 0.05. The SPSS statistical software package for Windows was used for the analysis of data.
Results
Demographic and clinical characteristics of the patients are summarized in . Annual direct costs according to severity of COPD were estimated as 1657 EUR in stage I, 2425 EUR in stage II, and 3303 EUR in stage III. Pharmaceutical treatment and hospitalizations were the main costs in patients with moderate and severe disease, whereas drug therapy had a higher effect in patients with moderate and mild disease. The mean direct medical costs was 2,061 EUR, of which 785 EUR (38%) corresponded to drug therapy.
Table 2. Characteristics of 560 Patients by Stages of COPD Severity
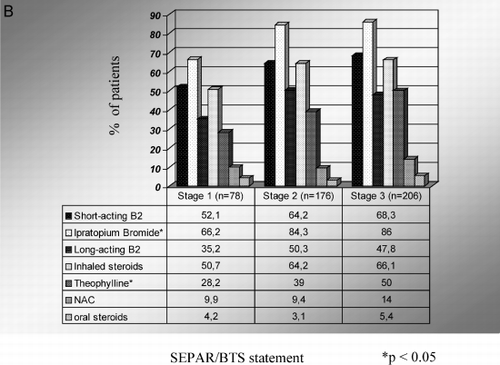
A total of 97% of patients were treated with more than one drug. Drug classes prescribed according to severity of disease are shown in . There were a few differences in pharmacological treatment according to disease severity, dyspnea score, and quality of life independent of the classification used for assessing COPD severity ().
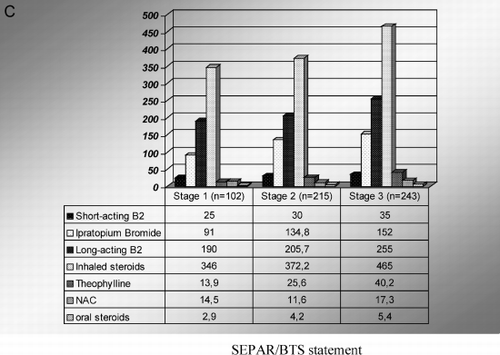
Figure 1. A) Drug classes prescribed by general practicioners according to severity of the disease. B) Drug classes prescribed by pneumologists according to severity of the disease. C) Costs of each drug class according to severity of the disease. Mean drug costs in each group (Euros/patient/year).
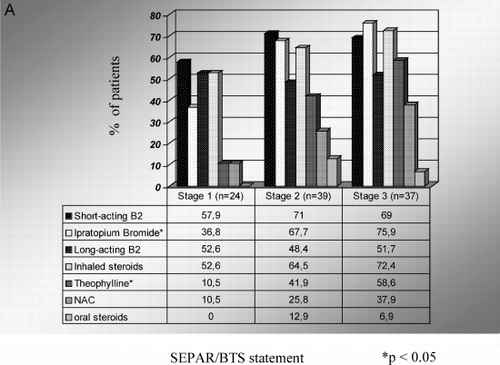
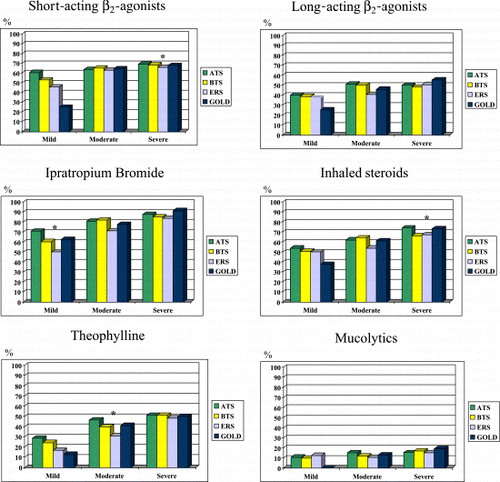
Figure 2. Distribution of drug prescription based on severity by different consensus guidelines. (Full color version available online.)
Pharmaceutical costs were not significantly associated with degree of airflow impairment (718 EUR for patients in stage I, 914 EUR in stage II, and 938 EUR in stage III). The mean number of episodes of acute exacerbation, visits to the emergency room, outpatient visits, hospitalizations, and long‐term oxygen therapy according to severity of disease are shown in . The mean costs per year for these variables was significantly higher in severe or moderate disease than in mild disease.
Table 3. Mean Number of Events and Mean Costs Per Year of Non‐Pharmaceutical On‐Pharmaceutical Direct Costs in Stable COPD According to Severity of Disease
Drug therapy for stable COPD patients accounted for 43%, 37.6%, and 28,4% of total direct costs for stage I, II, and III, respectively. Inhaled steroids and long‐acting β2‐agonists accounted for 78% of total drugs costs in stage I, 76% in stage II, and 75% in stage III. Drug therapy which was not in accordance with guidelines accounted for 78.7% of total drug costs in stage I and for 54% in stage II. Patients with severe disease were mostly treated in accordance with GOLD recommendations.
Discussion
The estimation of direct medical costs of stable COPD presented in this study is important for defining the current status of the question, comparing data with previous surveys, and assessing the economical burden of the disease, which is indispensable for the development and implementation of health policy interventions. The present results should be interpreted taking into account the sampling strategy, that is, ambulatory patients with a confirmed diagnosis of COPD visited by their general practitioners or attending control visits at outpatient clinics of respiratory diseases at secondary/tertiary care level. In this respect, our results are different than those reported from a confronting COPD survey carried out in seven areas of Spain Citation[[18]] in which a random telephone‐dialing method was used.
We found that inappropriate treatment had a main influence upon direct medical costs in patients with mild and moderate stable COPD. The effect of inadequate drug therapy on the burden of COPD may be even greater when considering the limited number of patients receiving some kind of treatment for COPD and the important proportion of patients with no previous diagnosis of the disease. In the population‐based study of Sobradillo Peña et al. Citation[[15]], only 49.3% of patients with severe COPD, 11.8% of patients with moderate COPD, and 10% of patients with mild COPD were receiving some kind of treatment; on the other hand, there was no previous diagnosis of COPD in 78.2% of cases. Underdiagnosis may contribute to the burden of COPD by limiting the number of patients who receive appropriate medication, increasing the probability of poor symptom management and the likelihood of acute exacerbations and hospitalization. In our study, the mean direct cost of pharmacological treatment for COPD was 785 EUR per patient/year. This figure can be largely reduced by effective implementation of consensus recommendations directed to improve the diagnosis and management of COPD. In addition, considering that 1.5 to 1.7 million people suffered from the disease, actual drug costs in our country would be about 293,590,000 EUR, which is a little more than 20% of that expected if all COPD patients would have been diagnosed for their disease. In fact, it has been shown that expenditures are disproportionately distributed, with 10–20% of patients accounting for about 70% of the total costs Citation[[2]]Citation[[3]].
Treatment for COPD has been generally limited to symptomatic measures. However, several studies have shown that some treatments can prevent or decrease the occurrence of acute exacerbations, and therefore could improve not only symptoms but the burden of the disease Citation[[19]]Citation[[20]]. In fact, hospitalization costs represent between 40% and 70% of total direct costs in patients with COPD Citation[[21]]Citation[[22]]. Recent guidelines recommend a stepped‐treatment approach. In patients with mild or moderate airflow impairment, measures to quit smoking and symptomatic relief with short‐acting β2‐agonists or ipratropium bromide can be adequate in a large number of cases. In COPD patients with mild or moderate severity, the usefulness of inhaled steroids or a clear advantage of long‐acting β2‐agonists over cheaper agents, such as ipratropium bromide, has not been demonstrated Citation[[23]]. However, in spite of this lack of positioning of inhaled steroids and long‐acting β2‐agonists, both agents accounted for 75% of total drug therapy costs in our patients with mild or moderate COPD. Recently, Sin et al. Citation[[24]] conducted a study to evaluate the long‐term impact of varying doses of inhaled corticosteroids on COPD mortality in patients aged ≥ 65 years recently hospitalized due to COPD. Inhaled corticosteroid therapy after discharge was associated with a 25% relative reduction in risk for all‐cause mortality. Patients on medium‐ or high‐dose therapy showed lower risks for mortality than those on low doses. On the other hand, in a double‐blind single center study (the COPE study), van der Valk et al. Citation[[25]] showed that discontinuation of fluticasone in patients with COPD was associated with a more rapid onset and higher recurrence risk of exacerbations and a significant deterioration in aspects of health‐related quality of life.
Severity of disease is associated with the incidence of exacerbations. It has been shown that in some COPD patients, treatment with inhaled steroids, N‐acetylcysteine or long‐acting β2‐agonists have a favorable effect on the incidence and severity of exacerbations. In patients with severe airflow limitation, these treatments might be cost‐effective because they could not only improve the quality of life of the patients, but also reduce the number of exacerbations. β2‐Agonists and anticholinergic agents also have a beneficial effect on dyspnea as well as on the frequency of exacerbations Citation[[21]]Citation[[22]], with a subsequent effect on the patient's quality of life. According to GOLD guidelines these drugs have a therapeutic role in patients suffering from dyspnea during daily life activities. However, there are substantial differences in the costs of short‐acting β2‐agonists, anticholinergic agents, and long‐acting β2‐agonists.
Although long‐acting β2‐agonists can improve respiratory symptoms, tolerance to efforts, and quality of life of patients with stable COPD, when the influence of new drug classes on the economic burden of COPD is considered, a comparison of treatment costs, individually, with respect to the best possible alternative therapy seems to be the most useful approach. Although in patients with COPD (FEV1≤ 65% of predicted value), it has been shown that a combination of formoterol and ipratropium is more effective that a combination of salbutamol and ipratropium Citation[[23]], long‐term results related to quality of life and frequency of exacerbation episodes are lacking. In a 12‐month, randomized, double‐blind, placebo‐controlled, parallel‐group study in 812 adults with COPD Citation[[26]], budesonide/formoterol in a single inhaler was compared with placebo. Budesonide/formoterol decreased all symptom scores and use of reliever β2‐agonists significantly and improved quality of life significantly compared to placebo. Other studies Citation[[27]]Citation[[28]], however, have reported that formoterol or salmeterol are better than ipratropium. In fact, the study of Rennard et al. Citation[[29]] showed that salmeterol and ipratropium clearly are better than placebo, but no significant differences were found between the two drugs. Therefore, long‐acting β2‐agonists can be useful for the clinical management of some patients, although at the present time a clear superiority of this class of drugs over cheaper drugs with an excellent therapeutic profile has not been proved. At the present time, inhaled steroids account for 50%, 48%, and 49% of total drug costs in mild, moderate, and severe COPD respectively, representing 21.5% and 18% of total direct costs in mild and moderate COPD despite the fact that its role in the management of these groups of patients is not established. By contrast, in patients with a FEV1 < 50% of predicted or with frequent exacerbations that require the use of antibiotics or corticosteroids, the use of inhaled steroids improves the frequency and severity of exacerbations Citation[[20]]. In patients with severe airflow impairment, inhaled steroids can improve the quality of life and, preventing or limiting exacerbations, could substantially influence the overall burden of this disease. Our study confirms that exacerbations and hospitalizations had a great impact on total direct costs in patients with severe COPD. Thus, any treatments that have a favorable effect on exacerbations would be of benefit.
A meta‐analysis of N‐acetylcysteine in COPD has reported a decrease in the number of exacerbations Citation[[30]], but the long‐term trial BONCHUS will clarify the usefulness of this drug in the treatment of COPD. Theophylline is an effective treatment for COPD. However, it has an unfavorable therapeutic index, so it is only considered as a third‐line therapy in severe COPD patients. From an economic point of view both drugs have little impact on total costs compared with inhaled steroids or long‐acting β2‐agonists.
In summary, patients with stable COPD of mild or moderate severity are commonly treated inappropriately with drug classes that are not in accordance with accepted guidelines. Implementation of these recommendations can improve the optimal management of these patients and reduce the direct costs of the disease. However, by replacing our costs figures with official costs in different countries, it is possible to compare the economic impact of COPD in different scenarios and to determine whether differences in the characteristics of the patients or in the variability of strategies for the management of COPD exert an influence on the economic burden of the disease.
Acknowledgments
We thank Marta Pulido, MD, for editing the manuscript and editorial assistance.
References
- Strassels S A, Smith D H, Sullivan S D, Mahajan P S. The costs of treating COPD in the United States. Chest 2001; 119: 344–352, [PUBMED], [INFOTRIEVE]
- Sullivan S D, Ramsey S D, Lee T A. The economic burden of COPD. Chest 2000; 117: 5s–9s, [PUBMED], [INFOTRIEVE], [CROSSREF]
- The COPD Guidelines Group of the Standards of Care Committee of the BTS. BTS guidelines for the management of chronic obstructive pulmonary disease. Thorax 1997; 52(suppl 5)S1–S28
- Siafakas N M, Vermeire P, Pride N B, Paoletti P, Gibson J, Howard P. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). A consensus statement of the European Respiratory Society. Eur Respir J 1995; 8: 1398–1420, [PUBMED], [INFOTRIEVE], [CROSSREF]
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152: S77–S120, [CSA]
- Pauwels R A, Buist A S, Calverley P MA, Jenkins C R, Hurd S S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global initiative for chronic obstructive lung disease (GOLD) workshop summary. Am J Respir Crit Care Med 2001; 163: 1256–1276, [PUBMED], [INFOTRIEVE], [CSA]
- Barberà J A, Peces‐Barba G, Agustí A GN, Izquierdo J L, Monsó E, Montemayor T, Viejo L. Guía clínica para el diagnóstico y el tratamiento de la enfermedad pulmonar obstructiva crónica. Arch Bronconeumol 2001; 37: 297–316, [CSA]
- Grupo DAFNE. Costes directos de la bronquitis crónica en atención primaria. Análisis de un estudio prospectivo. Aten Primaria 2000; 27: 388–393, [CSA]
- Ruiz I, Cía M A, Aznar M T, Company V, Orozco A. Análisis del coste farmacéutico en el GRD‐88: enfermedad pulmonar obstructiva crónica. Farm Hosp 1996; 20: 49–54
- Figueras M, Brosa M, Gisbert R. El coste de la bronquitis crónica en España. Enfoque incidencia. Rev Esp Farmacoeconomía 1999; 2: 33–43
- de Miguel Díez J, Izquierdo Alonso J L, Molina París J, Rodríguez González‐Moro J M, de Lucas Ramos P, Gaspar Alonso‐Vega G. Fiabilidad del diagnóstico de la EPOC en atención primaria y neumología en España. Factores predictivos. Arch Bronconeumol 2003; 39: 203–208, [CROSSREF], [CSA]
- Bestall J C, Paul E A, Garrod R, Garnham R, Jones P W, Wedzicha A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54: 581–586, [PUBMED], [INFOTRIEVE]
- Ferrer M, Alonso J, Prieto L, Plata V, Monso E, Marrades R, Aguar M C, Khalaf A, Anto J M. Validity and reliability of the St George's respiratory questionnaire after adaptation to a different language and culture: the Spanish example. Eur Respir J 1996; 9: 1160–1166, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Vademecum Internacional. Medicom, MadridSpain 2000
- Sobradillo Peña V, Miratvilles M, Gabriel R, Jiménez‐Ruiz C A, Villasante C, Masa J F, Viejo J L, Fernández‐Fau L. Geographical variations in prevalence and underdiagnosis of COPD. Results of the IBERPOC multicentre epidemiologic study. Chest 2000; 118: 981–989, [CROSSREF]
- Stang P, Lydick E, Silberman C, Kempel A, Keating E T. The prevalence of COPD. Using smoking rates to estimate disease frequency in the general population. Chest 2000; 117: 354s–359s, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Miravitlles M. Evaluación económica en la EPOC. Arch Bronconeumol 2001; 37(suppl. 2)38–42
- Izquierdo J L. The burden of COPD in Spain: results from the confronting COPD survey. Respir Med 2003; 97(suppl. C)S61–S69, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Burge P S, Calverley P MA, Jones P W, Spencer S, Anderson J A, Maslen T K. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease. The ISOLDE trial. BMJ 2000; 320: 1297–1303, [PUBMED], [INFOTRIEVE], [CROSSREF]
- The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000; 343: 1902–1909, [CROSSREF], [CSA]
- Niewoehner D E. Impact of drug classes used in COPD on outcome measures other than exacerbations. Eur Respir J 2002; 12: 11–12
- Wedzicha J A. Impact of drug classes used in COPD exacerbations. Eur Respir J 2002; 12: 13–14
- D'Urzo A D, De Salvo M C, Ramirez‐Rivera A, Almeida J, Sichletidis L, Rapatz G, Kottkis J. In patients with COPD, treatment with a combination of formoterol and ipratropium is more effective than a combination of salbutamol and ipratropium: a 3‐week, randomized, double‐blind, within‐patient multicenter study. Chest 2001; 119: 1347–1356, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Sin D D, Man S F. Inhaled corticosteroids and survival in chronic obstructive pulmonary disease: does the dose matter?. Eur Respir J 2003; 21: 260–266, [PUBMED], [INFOTRIEVE], [CROSSREF]
- van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. Am J Respir Crit Care Med 2002; 166: 1358–1363, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, Peterson S, Olsson H. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J 2003; 21: 74–81, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Mahler D A, Donohue J F, Barbee R A, Goldman M D, Gross N J, Wisniewski M E. Eficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999; 115: 957–965, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Dhal R, Greefhorst L, Nowak D, Nonikov V, Byrne A M, Thomson M H, Till D, Della Cioppa G. Inhaled formeterol dry powder versus ipratropium bromide in chorinc obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 778–784, [CSA]
- Rennard S I, Anderson W, ZuWallack R, Broughton J, Bailey W, Friedman M, Wiesniewski M, Rickard K. Use of long‐acting inhaled beta‐2‐adrenergic agonists, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 1087–1092, [PUBMED], [INFOTRIEVE], [CSA]
- Grandjean E M, Berthet P, Ruffmaun R, Leueuberger P. Efficacy of oral long‐term N‐acetylcysteine in chronic bronchopulmonary disease: a meta‐analysis of published double‐blind, placebo‐controlled clinical trials. Clin Ther 2000; 22: 209–221, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]