Abstract
An accelerated loss of lung function is one of the defining characteristics of chronic obstructive pulmonary disease (COPD). To date, the only successful intervention shown to conclusively attenuate the loss of lung function over time is smoking cessation. Pharmacological interventions including inhaled corticosteroids and ipratropium bromide have not altered the rate of decline of lung function. Tiotropium is an inhaled anticholinergic that provides 24‐hour bronchodilation with once‐daily dosing due to prolonged muscarinic M3 receptor blockade. Controlled clinical trials have suggested sustained efficacy for periods of up to one year. We therefore initiated a four‐year, controlled clinical trial (UPLIFT, Understanding the Potential Long‐Term Impacts on Function with Tiotropium) in patients with COPD to evaluate the long‐term effects of tiotropium on the rate of decline in lung function and health status as well as the frequency of exacerbations. The design of such large, long‐term clinical trials presents unique methodological challenges including the definition of endpoints, the quality and variability of spirometric measurements and premature patient discontinuations from the trial. The present manuscript outlines the rationale for the UPLIFT study, and reviews the study design and the steps taken to address methodological challenges experienced in other long term studies. Careful design and implementation of the UPLIFT trial is anticipated to yield high quality results that will help in increasing our understanding of the long term natural history of COPD in a global population as well as to elucidate the role that tiotropium can play in affecting the course of this debilitating disease.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a progressive disease leading to enormous societal costs Citation[[1]]Citation[[2]]. The disease is predicted to be the third leading cause of death worldwide by the year 2020 Citation[[1]]. The most important factor predisposing to COPD is inhalation of tobacco smoke, although 10 to 15% of COPD patients do not have a history of tobacco use. The disease often begins insidiously and progresses gradually over many years. Physiologically, COPD is characterized by airflow obstruction that is not fully reversible, increased airway resistance and hyperinflation. The spirometric hallmark of COPD is an accelerated loss of the forced expiratory volume in one second (FEV1) with age. The afflicted individuals experience the insidious development of breathlessness with activity such that disability eventually ensues. In these patients, the disease causes impaired health status, periodic exacerbations, psychosocial disorders and often premature mortality.
The annual rate of decline in FEV1 has been used as a surrogate measure for studying the natural history of COPD. There is a natural loss of FEV1 in all individuals that begins after achieving a peak at approximately 25 years of age. The normal rate of loss is between 20 and 50 mL per year Citation[[3]]Citation[[4]]. Those patients with COPD who continue to smoke may have annual losses of FEV1 that vary between 40 and 80 mL per year Citation[[3]]Citation[[4]]. However, there is significant variability among the various published reports on the rate of decline Citation[[5]]. Smoking cessation slows the rate of loss of lung function but the impairment does not resolve Citation[[6]]. Inflammation can be observed in the lungs of patients with COPD who have stopped smoking Citation[[7]]. Although the clinical course and the rate of decline of lung function are somewhat variable, there are correlations between FEV1 and each of the following outcomes: mortality, health status, breathlessness and severe exacerbations Citation[[8]]Citation[[9]]Citation[[10]]Citation[[11]]. The advantage of studying FEV1 serially over time is documented objectivity, reproducibility, and validity of the measurement Citation[[12]]. The disadvantage of FEV1 is that the correlation to clinical outcomes such as dyspnea, health status and exercise capacity, albeit present, is often weak Citation[[9]]Citation[[10]]Citation[[11]].
Tiotropium (Boehringer Ingelheim, Ridgefield, CT, USA) is an inhaled anticholinergic that has its effects through prolonged M3 receptor blockade. The dissociation half‐life from the M3 receptor is approximately 35 hours and is responsible for the physiological property of sustained bronchodilation over 24 hours with once‐daily dosing Citation[[13]]. One‐year and six‐month studies with tiotropium have repeatedly shown that tiotropium can improve lung function, breathlessness and health status, and reduce exacerbations Citation[[14]]Citation[[15]]Citation[[16]]. In a post‐hoc analysis of the one-year placebo‐controlled studies, the annual decline in morning pre‐dose FEV1 after treatment with tiotropium was substantially less than the decline observed with placebo (− 0.01 vs. − 0.04 L from day 8 until day 344, p < 0.05), raising the possibility of an impact of long‐term treatment with tiotropium on the annual rate of loss of lung function Citation[[17]]. Additional clinical studies have demonstrated that tiotropium reduces hyperinflation and improves breathlessness on activity as well as prolonging exercise endurance Citation[[18]]. These observations have led to further hypotheses regarding outcomes when treatment continues beyond one year. We have therefore designed the four‐year UPLIFT (Understanding the Potential Long‐term Impacts on Function with Tiotropium) study to evaluate the influence of tiotropium on the natural history of COPD. Previous studies evaluating the efficacy of pharmacological interventions in reducing the rate of lung function decline in COPD illustrate the methodological challenges of such studies Citation[[19]]. These challenges include defining outcome measures, limiting variability in spirometry and managing premature discontinuations from the trial. This manuscript will describe the rationale for studying the potential effects of tiotropium on the natural history of COPD and the methodology implemented to achieve that goal.
Materials and Methods
Objectives
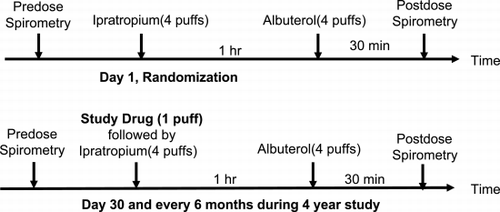
The primary objective of this trial is to determine whether treatment with tiotropium (18 mcg) inhalation capsule via the HandiHaler® device reduces the rate of decline of FEV1 over time in patients with COPD. As pharmacodynamic steady state occurs after several days of dosing and pharmacokinetic steady state occurs following several weeks of dosing, the baseline value for the calculation of the rate of decline in FEV1 over time has been set at Day 30. The co‐primary endpoints are 1) the yearly rate of decline in the morning pre‐dose FEV1 from Day 30 (steady state) until completion of double‐blind treatment, and 2) the yearly rate of decline in post‐bronchodilator FEV1 from Day 30 (steady state) until completion of double‐blind treatment. To insure maximal bronchodilation, the post‐bronchodilator FEV1 is measured following sequentially timed administration of ipratropium bromide and albuterol ().
Figure 1. Timing of administration of study drug, ipratropium bromide and albuterol for evaluation of pre and post bronchodilator responses following randomization.
Secondary objectives include the evaluation of the mean yearly rate of decline in pre‐ and post‐bronchodilator FEV1, forced vital capacity (FVC) and slow vital capacity (SVC) from Day 1 until completion of the trial (30 days after withdrawal from study drug) and the mean yearly rate of decline in pre‐ and post‐bronchodilator FVC and SVC from Day 30 until completion of double‐blind treatment. In addition, the rate of decline in health‐related quality of life as measured by the St. George's Respiratory Questionnaire, exacerbations of COPD and associated hospitalizations, and mortality (respiratory and all‐cause) will be examined.
Study Design
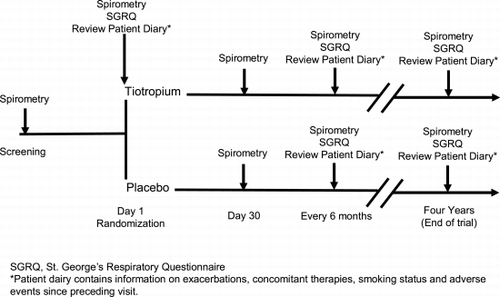
This is a four‐year, multicenter, multinational, double‐blind, randomized, placebo‐controlled, parallel‐group clinical trial involving approximately 6,000 patients with COPD (). Thirty‐seven countries are participating, including approximately 450 investigational sites. After obtaining written informed consent, study personnel advise active smokers to discontinue smoking and these patients are offered a smoking cessation program (e.g., counseling sessions, patient education and supportive literature). The first session (Visit 1) is followed by a contact at two weeks (via phone or visit) and a final session at or before randomization (Visit 2). Pharmacological intervention for smoking cessation is permitted. Patients may decline participation in the smoking cessation program. Following this initial screening period, qualifying patients are randomized to tiotropium or placebo (Visit 2). Patients are seen after one month on treatment (Visit 3), at three months (Visit 4) and then every three months until study drug termination (four years). At study drug termination, all patients will receive open‐label ipratropium for 30 days. The final visit will occur 30 days post treatment. Self‐reported smoking status is recorded at each visit.
An independent Data and Safety Monitoring Board (DSMB) will monitor the accruing safety data annually. The DSMB is composed of independent pulmonologists, a cardiologist, and a biostatistician.
Population
The inclusion criteria include a clinical diagnosis of COPD Citation[[20]], age ≥ 40 years, smoking history of ≥ 10 pack‐years, a maximal post‐bronchodilator FEV1 ≤ 70% of predicted [European Community for Coal and Steel criteria Citation[[21]]] and an FEV1 ≤ 70% of FVC, and the ability to perform satisfactory spirometry. Patients are excluded if they had a respiratory infection or an exacerbation of COPD in the four weeks prior to screening, a history of asthma or pulmonary resection, used supplemental oxygen > 12 hours per day, or had a significant disease other than COPD which, in the opinion of the investigator, may influence the results of the study or the patient's ability to participate in the study. The presence or absence of reversibility to the bronchodilator was not an entry criterion. Patients are permitted to continue using all previously prescribed respiratory medications other than inhaled anticholinergics, provided the prescriptions had not changed in the six weeks prior to randomization. There are no restrictions for medications prescribed for treatment of exacerbations.
Procedures
Spirometry
FEV1, FVC and SVC measurements are obtained using calibrated spirometers, commencing in the morning at approximately the same time of day at all visits throughout the study. With the exception of the first test set, which is only performed post bronchodilator, spirometry is performed pre‐ and post‐ bronchodilator at all visit dates. After pre‐bronchodilator spirometry is performed, four inhalations of ipratropium bromide (80 mcg via metered dose inhaler) are administered, followed by four inhalations of albuterol (400 mcg via metered dose inhaler) 60 minutes later. The post‐bronchodilator spirometry is performed 90 minutes after ipratropium bromide inhalation (30 minutes after albuterol inhalation). After randomization, study drug is administered immediately prior to the ipratropium bromide. SVC by slow exhalation maneuver is performed first, followed by the FVC maneuver. Both maneuvers are performed in triplicate, although up to five forced expiratory maneuvers are obtained in an effort to achieve three acceptable efforts. The highest acceptable FEV1 and the highest FVC each obtained on any of three blows (even if not from the same curve) meeting the American Thoracic Society criteria constitute the data for that test set Citation[[12]]. The highest SVC value was recorded for the test set.
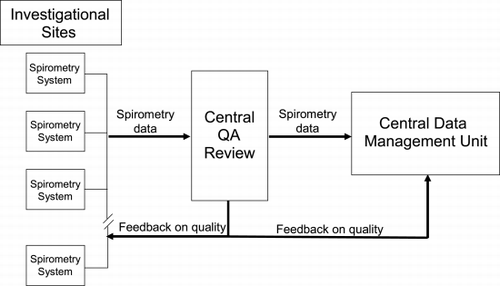
To standardize spirometry, all sites were provided with identical spirometry systems (KoKo Spirometer, Quantum Research, Inc., Louisville, CO, USA) with customized, study‐specific software. All study staff responsible for performing pulmonary function testing received identical, detailed training at the investigators meetings. All technicians were required to demonstrate proficiency in the use of the equipment and the ability to perform technically acceptable pulmonary function tests (by ATS criteria) prior to performing testing on study patients. After each test is performed, the spirometry software provides immediate feedback to the technician indicating whether the effort meets ATS acceptability and reproducibility standards. All efforts are stored electronically. After completion of testing, the study staff electronically transmits the spirometric measurements for centralized quality assurance review (Quantum Research, Inc., Louisville, CO, USA). Feedback on the quality of the measurements is then provided to the investigational site and to Boehringer Ingelheim for central data management. The process is outlined in .
Health Status
Health status is assessed with the St. George's Respiratory Questionnaire (SGRQ) Citation[[22]]. Each patient completes the SGRQ prior to spirometry at the randomization visit and then every six months until completion of the double‐blind treatment period. As part of the UPLIFT trial, the SGRQ is being administered in 27 different languages to help ensure consistent understanding of the questions across study centers located in multiple countries. The SGRQ versions have been validated in all 27 languages.
Exacerbations of COPD
To estimate and compare treatment effects on exacerbations across treatment arms, detailed information on exacerbations of COPD and associated hospitalizations is collected. An exacerbation is defined as an increase or new onset of more than one of the following respiratory symptoms (cough, sputum, sputum purulence, wheezing, dyspnea) with a duration of three or more days requiring treatment with an antibiotic and/or systemic (oral, intramuscular or intravenous) steroid. The duration of symptoms is required to be a minimum of three days including any days for which treatment has been administered. Exacerbations are categorized as mild, moderate and severe according to the following definitions:
a) Mild—treated at home without seeing a health care provider.
b) Moderate—visit with health care provider (e.g., home visit, visit to an outpatient facility or an emergency department—but not requiring admission to hospital).
c) Severe—hospitalization (an emergency department stay > 24 hours is considered a hospitalization).
A specific exacerbation case record form has been designed to capture all aspects of the exacerbation definition.
Data Analysis
The primary endpoints will be compared between the two treatment groups by performing random coefficient regression analysis Citation[[23]]. All randomized patients with at least three spirometry measurements from at least three visits after randomization will be included in this analysis. The effects of patient's baseline FEV1, age, gender, and smoking status on the yearly rate of FEV1 decline will be investigated. The secondary spirometric analyses and the rate of decline in SGRQ total score will be analyzed using the same approach. The number of exacerbations, associated hospitalizations, exacerbation and hospitalization days per patient, as recorded by the investigator, will be normalized by extent of exposure and compared between the two treatment groups using Wilcoxon (Mann‐Whitney) rank sum test. The time to the first exacerbation and associated hospitalization will be compared across treatment groups using log‐rank test.
The sample size calculation for the study was based on an assumed standard deviation for the rate of decline per year in a four‐year study of 90 mL. Therefore, to detect a difference of 15 mL in the rate of decline between tiotropium and placebo at 5% level of significance and 90% power, a random sample of 758 patients per group is needed. For the primary analysis, patients need to have at least three observations from Day 30. Assuming that as many as 35% of these patients will discontinue the study early without adequate data (at least three visits with spirometry measurements after randomization), it was calculated that 1,166 patients per treatment group would be required. Of patients recruited in previous tiotropium trials Citation[[14]]Citation[[15]]Citation[[16]], approximately 33% of patients were severe according to American Thoracic Society criteria (FEV1 < 35% predicted normal) Citation[[20]], approximately 40% were current smokers and approximately 67% were receiving inhaled corticosteroids. Based on the numbers of patients in sub‐groups of these previous trials, to be able to conduct relevant sub‐group analyses with adequate power (1,166 patients per treatment group), the sample size needed for the study was determined to be 2,916 per treatment group or 5,821 total.
Patient Recruitment and Continued Participation
An international patient recruitment and retention committee planned a strategy to ethically optimize the time required to randomize patients who meet eligibility criteria for the UPLIFT trial and maintain patient participation for the duration of the trial. Strategies were developed to assist patients, investigational sites and the internal staff monitoring the trial. Specific tactics were developed for patients, sites and internal staff to provide assistance in participation, provide updated information about the trial, provide information about COPD and to help maintain enthusiasm regarding participation over four years.
Discussion
Several studies have attempted to evaluate whether pharmacological interventions can alter the accelerated loss of lung function observed in patients with COPD Citation[[6]]Citation[[24]]Citation[[25]]Citation[[26]]. Each of these studies defined the rate of decline of FEV1 as its primary outcome. The nature of COPD is generally one of progressive deterioration with smoking cessation being the only known intervention to ameliorate the expected decline in lung function Citation[[6]]. With the exception of supplemental oxygen in hypoxemic patients, to date, pharmacological therapy of COPD has provided palliation of symptoms without definitive evidence of disease modification Citation[[27]]. Long‐term studies present unique challenges and opportunities in furthering our understanding of COPD. In addition, the methodological issues in designing such trials lead clinicians and investigators to generate additional hypotheses and novel approaches to study COPD. Data from the one‐year tiotropium clinical trials have led to the hypothesis that the agent may modify the natural course of the disease and the experience from those trials has also assisted in improving the design of such long‐term trials.
Tiotropium has consistently demonstrated improvements in lung function, dyspnea, health‐related quality of life and exacerbations in trials of up to one year in duration Citation[[14]]Citation[[15]]Citation[[16]]. A post‐hoc analysis of one‐year placebo‐controlled trials showed a statistically significant slowing in the rate of decline in trough FEV1 (12 mL/year in the tiotropium group vs. 58 mL/year in the placebo group) Citation[[17]]. However, while there was a similar trend in the rate of loss of FEV1 in the value obtained three hours post‐dose, the difference from placebo was smaller in magnitude and not statistically significant. Improvements in dyspnea (as measured by the transition dyspnea index) appear to be maintained over one year treatment Citation[[14]]Citation[[15]]. This was also true for health‐status [as measured by the St. George's Respiratory Questionnaire (SGRQ)] Citation[[14]]Citation[[15]]. In addition, exacerbations of COPD and associated hospitalizations were reduced Citation[[14]]Citation[[15]]. Although controversial, recent data from a relatively small sample suggested that the frequency of exacerbations may have a negative impact on long‐term changes in FEV1 Citation[[7]]Citation[[28]]. Finally, there was an association of SGRQ, FEV1 and COPD related hospitalizations to mortality Citation[[8]]Citation[[29]]Citation[[30]]. Therefore a hypothesis has been generated that, based on one‐year trial data, tiotropium may have long‐term impacts on the course of COPD.
There have been four large‐scale, long‐term trials evaluating the effects of pharmacological interventions on the rate of decline in lung function in patients with COPD. The Lung Health Study was the first large scale prospective evaluation of a pharmacological intervention on the rate of decline of FEV1 Citation[[6]]. Although the response to ipratropium was maintained over time, ipratropium was not shown to influence the rate of decline of lung function. It should be noted that ipratropium was prescribed three times daily and was self‐administered on average less than twice daily Citation[[31]]. Therefore, it is likely that there were significant periods of time each day during which the effect of inhaled anticholinergics was diminished considerably. Thus, the effect of continuous exposure to a sufficiently potent and long‐lasting inhaled anticholinergic on long‐term outcomes in COPD remains unknown and is the object of investigation in the UPLIFT trial. Inhaled corticosteroids are some of the most the most extensively studied agents with regard to potential effects on lung function over time. ISOLDE evaluated fluticasone Citation[[24]], EUROSCOP evaluated budesonide Citation[[25]], and Lung Health Study II evaluated triamcinolone Citation[[26]]. The period of study in each was approximately three years. All of these studies failed to demonstrate an influence of pharmacological interventions on the rate of decline in lung function, as determined by the post‐albuterol FEV1. Although the results of a recent meta‐analysis of long‐term placebo‐controlled studies of inhaled corticosteroids in COPD by Sutherland et al. Citation[[32]] suggested that treatment with inhaled corticosteroids resulted in a modestly significant overall reduction in FEV1 decline, these results were not confirmed by another meta‐analysis of randomized controlled trials of inhaled corticosteroids by Highland et al. Citation[[33]]. The observed discrepancy in the meta‐analyses serves to highlight the need to rely on appropriately powered clinical trials of sufficient duration to answer the primary question under study.
Long‐term clinical trials in COPD pose several methodological challenges. The most critical of these are determination of the primary outcome variable, control of the variability of spirometry, and reduction and management of premature discontinuations. As with other long‐term COPD trials, the FEV1 has been chosen as the primary outcome variable in the UPLIFT trial. Although there are advantages and disadvantages to this approach, FEV1 has been, and likely will continue to be, a commonly understood and readily measurable objective measure of lung function. The question arises as to whether FEV1 should be studied prior to or after bronchodilator administration. It seems intuitively obvious that conclusions drawn from long‐term changes in lung function can be interpreted as a surrogate for structural changes in the airways if the influence of bronchomotor tone has been removed. However, none of the studies to date have addressed this in the trial design. If FEV1 is studied prior to bronchodilator inhalation, the influence of bronchomotor tone remains. In contrast, post‐bronchodilator evaluation performed only after the inhalation of an inhaled beta‐agonist, may not completely remove the influence of cholinergic tone on FEV1. None of the previous studies have performed evaluations after maximal bronchodilation from inhalation of both a beta‐agonist and an anticholinergic. In the UPLIFT trial, post‐bronchodilator spirometry is performed after administration of presumably maximum doses of both short‐acting beta‐adrenergic and anticholinergic inhaled bronchodilators. Furthermore, the timing of spirometry has purposely been designed to coincide with the approximate peak action of both agents. This will provide a unique opportunity to define the rate of decline of FEV1 in patients with COPD minimizing the influence of the bronchomotor tone.
One could argue that tiotropium should be totally washed out before drawing conclusions regarding true long‐term changes in lung function. Due to the long elimination half life of tiotropium (5 to 6 days), a complete wash‐out would require approximately three to four weeks (i.e. five to six elimination half lives). This does not seem practical for re‐testing over a four year study. Nevertheless, the trough value (i.e. the morning pre‐tiotropium measurement) indicates the lowest point of removal of tiotropium and is reasonable to study. The UPLIFT study has specified co‐primary endpoints of trough and peak bronchodilator FEV1. In addition, a true “wash out” evaluation 30 days after patients have discontinued tiotropium has been requested from participants. However, given the chronic nature of COPD, patients may continue to take tiotropium for the remainder of their life. The question of where their lung function stands three weeks post removal of drug may be interesting to observe but unrepresentative of what truly happens during the course of long‐term maintenance COPD treatment.
Inherent in multicenter trials, particularly multinational trials, is the need to acquire data in a uniform and valid manner. In a trial evaluating the rate of decline in lung function, minimizing inter‐ and intra‐individual variability in spirometric measurements is critical, as the differences in spirometric measurements over time are expected to be small. For example, the difference between the rate of decline in lung function in the active treatment group compared with placebo in both the Lung Health Study II and ISOLDE studies was less than 10 mL/year Citation[[24]]Citation[[26]]. Previous trials have instituted various methods to decrease variability in spirometry. In the Lung Health Study, all centers were provided with identical spirometers, with study‐specific software, and central quality assurance review with feedback to the study centers Citation[[34]]. These techniques were associated with a relatively low degree of variability in spirometry. It should be noted, however, that the Lung Health Study was conducted in 10 centers, all within the United States. The UPLIFT trial has ambitiously sought to be globally inclusive, involving over 450 study centers in 37 countries on 6 continents. In recognition of the critical importance of minimizing variability of spirometry, an extensive global program was devised to ensure the quality of spirometry across all study centers. All sites have been provided with the same equipment and training along with standardized quality review of the pulmonary function tests across all study centers. The advancement of technology has permitted real‐time evaluation of outputs and continuous feedback and support to site technicians. Furthermore, it is relevant to consider timing of spirometric measurements, particularly in long‐term trials evaluating potentially small differences in pulmonary function. Although classically described in asthma, a diurnal variability of FEV1 occurs in patients with COPD as well; therefore, the measurement should be obtained at the same time of day Citation[[35]]. This is particularly important with regard to the baseline value. In UPLIFT, the protocol indicates the timing of measurement around a fairly narrow window, which is reinforced by the customized spirometry software.
Premature patient withdrawals occur in virtually every clinical trial and bear consideration in trial design and analysis. This is particularly true in long‐term trials as discontinuation rates increase with study duration. Discontinuations are usually highest in the treatment arm that has the least effective medication (usually the placebo control presuming that the active control has a favorable benefit/risk ratio). The placebo arm discontinuation rate in ISOLDE and EUROSCOP was 30% and 53%, respectively Citation[[24]]Citation[[25]]. Discontinuation rates in one‐year studies of long‐acting beta‐agonist/inhaled steroid combinations have ranged from 38% to 44% in the control arm Citation[[36]]Citation[[37]]. Discontinuation rates are not necessarily completely at random Citation[[38]]. This may call for more sophisticated modes of statistical analysis than the commonly used mixed effect models. However, reducing the number of “preventable” premature discontinuations should be addressed.
We estimated a 35% discontinuation rate in UPLIFT and do not anticipate rates as high as other trials given that all patients are permitted to use all respiratory medications other than inhaled anticholinergics. The study has been adequately powered to enable evaluation of the co‐primary outcomes with the anticipated discontinuation rate of approximately 35%.
A differentially higher discontinuation rate may occur in the placebo arm and this could lead to an underestimation of the true treatment effect. A four‐year strategic plan to encourage continued patient participation in the trial has been developed and implemented. This plan focuses on maintaining interest in the trial at all levels of participation, including monitoring staff, site personnel, investigators and patients. Studies have shown that physician involvement in trials and communication with patients are important factors in maintaining patient participation in clinical trials Citation[[39]].
As previously stated, spirometry is at best a surrogate for what is important to clinicians and patients. Health status has been shown to decline over time in patients with COPD. This has been demonstrated in the ISOLDE trial in which the use of SGRQ showed that fluticasone, administered at high doses (1,000 mcg per day), slowed the rate of loss of health status over time Citation[[24]]. Previous tiotropium trials have shown improvements in health status that remain above baseline one year following treatment Citation[[14]]Citation[[15]]. The present study will use the SGRQ to evaluate whether such patterns can be maintained for multiple years.
Exacerbations have received much attention but the definitions of exacerbations differ somewhat from study to study. The most functionally rigorous definitions include a change in symptoms, an intervention and a minimum duration. In this regard, the definition used in the tiotropium program seems to be robust. Data are being collected prospectively in a separate case record form. Such details are often missing from published reports where exacerbations are secondary outcomes.
In summary, the UPLIFT trial represents a unique opportunity to study the natural history of COPD in a global population of patients with COPD and to prospectively explore the effects of the once‐daily inhaled anticholinergic tiotropium on long‐term outcomes, including spirometry, health status, exacerbations and respiratory mortality. The UPLIFT trial will accumulate a wealth of data in which interactions of spirometry, health status and exacerbations can be explored. At the time of this publication, recruitment of approximately 6,000 patients has been completed. Results are anticipated in 2008.
Acknowledgment
We acknowledge Terry Keyser for her help in the preparation of this manuscript.
References
- Mannino D M, Homa D M, Akinbami L J, Ford E S, Redd S C. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. Surveillance Summaries, August 2, 2002. MMWR, 2002; Vol. 51: 1–16
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Workshop Report. National Heart Lung and Blood Institute, Bethesda April, 2001, Update of the management sections, GOLD website (www.goldcopd.com), update July 2003
- Tashkin D P, Detels R, Simmons M, Liu H, Coulson A H, Sayre J, Rokaw S. The UCLA population studies of chronic obstructive respiratory disease: XI. Impact of air pollution and smoking on annual change in forced expiratory volume in one second. Am J Respir Crit Care Med 1994; 149: 1209–1217, [PUBMED], [INFOTRIEVE], [CSA]
- Lange P, Groth S, Nyboe G J, Mortensen J, Appleyard M, Jensen G, Schnohr P. Effects of smoking and changes in smoking habits on the decline of FEV1. Eur Respir J 1989; 2: 811–816, [PUBMED], [INFOTRIEVE]
- Xu X, Weiss S T, Rijcken B, Schouten J P. Smoking, changes in smoking habits, and rate of decline in FEV1: new insight into gender differences. Eur Respir J 1994; 7: 1056–1061, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Anthonisen N R, Connett J E, Kiley J P, Altose M D, Bailey W C, Buist A S, Conway W A, Jr, Enright P L, Kanner R E, O'Hara P, Owens G R, Scanlon P D, Tashkin D P, Wise for the Lung Health Study Group R A. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1, the Lung Health Study. JAMA 1994; 272: 1497–1505, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kanner R E, Anthonisen N R, Connett J E, for the Lung Health Study Research Group. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex‐smokers with mild chronic obstructive pulmonary disease, results from the Lung Health Study. Am J Respir Crit Care Med 2000; 164: 358–364, [CSA]
- Traver G A, Cline M G, Burrows B. Predictors of mortality in chronic obstructive pulmonary disease—a 15 year follow‐up study. Am Rev Respir Dis 1979; 119: 895–902, [PUBMED], [INFOTRIEVE]
- Spencer S, Calverley P MA, Burge P S, Jones on behalf of the ISOLDE Study Group P W. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 122–128, [PUBMED], [INFOTRIEVE], [CSA]
- Witek T J, Mahler D A. Meaningful effect size and patterns of response of the transition dyspnea index. J Clin Epidemiol 2003; 56: 248–255, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Garcia‐Aymerich J, Monso E, Marrades R M, Escarrabill J, Felez M A, Sunyer J, Anto and the EFRAM Investigators J M. Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbations, EFRAM study. Am J Respir Crit Care Med 2001; 164: 1002–1007, [CSA]
- American Thoracic Society. Standardization of spirometry—1994 update. Am J Respir Crit Care Med 1995; 152: 1107–1136, [CSA]
- Disse B, Speck G A, Rominger K L, Witek T J, Jr, Hammer R. Tiotropium (Spiriva™): mechanistical considerations and clinical profile in obstructive lung disease. Life Sci 1999; 64: 457–464, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Casaburi R, Mahler D A, Jones P A, Wanner A, San Pedro G, ZuWallack R L, Menjoge S S, Serby C W, Witek T J. A long‐term evaluation of once‐daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19: 217–224, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Vincken W, van Noord J A, Greefhorst A PM, Bantje Th A, Kesten S, Korducki L, Cornielissen P JG. Improved health outcomes in patients with COPD during 1 year's treatment with tiotropium. Eur Respir J 2002; 19: 209–216, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003; 58: 399–404, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Anzueto A, Menjoge S S, Kesten S. Changes in FEV1 overtime in one year clinical trials of tiotropium in COPD. Am J Respir Crit Care Med 2001; 163: A280
- O'Donnell D, Fluge T, Gerken F, Hamilton A, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnea and exercise tolerance in patients with COPD. Eur Respir J 2004, In press
- Connett J E, Kusek J W, Bailey W C, O'Hara P, Wu M. Design of the Lung Health Study: a randomized clinical trial of early intervention for chronic obstructive pulmonary disease. Control Clin Trials 1993; 14: 3S–19S, [PUBMED], [INFOTRIEVE], [CROSSREF]
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis 1995; 152: 77–120
- Quanjer P H. Standardised lung function testing of the European community for coal and steel. Bull Euro Physiopathol Respir 1983; 19: 7–28
- Jones P W, Quirk F H, Baveystock C M, Littlejohns P. A self‐complete measure of health status for chronic airflow limitation: the St. George's respiratory questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327, [PUBMED], [INFOTRIEVE]
- Laid N M, Ware J H. Random‐effects models for longitudinal data. Biometrics 1982; 38: 963–974
- Burge P S, Calverley P MA, Jones P W, Spencer S, Anderson J A, Maslen on behalf of the ISOLDE study investigators T K. Randomised, double blind placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000; 320: 1297–1303, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Pauwels R A, Lofdahl C G, Laitinen L A, Schouten J P, Postma D S, Pride N B, Ohlsson S V, for the European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. Long‐term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking, European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med 1999; 340: 1948–1953, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000; 343: 1902–1909, [CROSSREF], [CSA]
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980; 93: 391–398
- Donaldson G C, Seemungal T AR, Bhowmik A, Wedzicha J A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57: 847–852, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Domingo‐Salveny A, Lamarca R, Ferrer M, Garcia‐Aymerich J, Alonso J, Fekez M, Khalaf A, Marrades R M, Monso E, Sera‐Batlles J, Anto J. Health‐related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166: 680–685, [CROSSREF], [CSA]
- Almagro P, Calbo E, Ochoa de Echaguen A, Barreiro B, Quintana S, Heredia J L, Garau J. Mortality after hospitalization for COPD. Chest 2002; 121: 1441–1448, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Rand C S, Wise R A, Nides M, Simmons M S, Bleecker E R, Kusch J W, Li V C, Tashkin D P. Inhaler adherence in a clinical trial: comparison of self‐report and canister weighing to the nebulizer chronolog. Am Rev Respir Dis 1992; 146: 1550–1564
- Sutherland E R, Allmers H, Ayas N T, Venn A J, Martin R J. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta‐analysis. Thorax 2003; 58: 937–941, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Highland K B, Strange C, Heffner J E. Long‐term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease. Ann Intern Med 2003; 138: 969–973, [PUBMED], [INFOTRIEVE]
- Enright P L, Hohnson L R, Connett J E, Voelker H, Buist A S. Spirometry in the Lung Health Study, methods and quality control. Am Rev Respir Dis 1991; 143: 1215–1223, [PUBMED], [INFOTRIEVE]
- Calverley P MA, Lee A, Towse L, van Noord J, Witek T J, Kesten S. Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax 2003; 58: 855–860, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden for the TRISTAN Study Group C. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003; 361: 449–456, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, Peterson S, Olsson H. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J 2003; 21: 74–81, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Little R JA, Rubin D B. Statistical Analysis with Missing Data. Wiley, New York 1987
- Shumaker S A, Dugan E, Bowen D J. Enhancing adherence in randomized contolled clinical trials. Control Clin Trials 2000; 21: 226–232, [CROSSREF]