Abstract
Exacerbations often complicate the progressive course of chronic obstructive pulmonary disease (COPD), mainly due to infectious agents. The precise role of bacterial infections in the course and the pathogenesis of COPD has been a source of controversy for decades. Also viruses and other non-infectious causes of exacerbation play a relevant role and also contribute to persisting airway inflammation. Usually, the etiologic identification of the infective causes of COPD require considerable time and costs. The development of more rapid, reliable, and widely applicable methods to promptly define the etiology of COPD exacerbations should represent a relevant issue in devising earlier and more specific strategies for their effective therapeutic control. Aim: of the study was to assess the predictive role of some pro-inflammatory cytokines measured in spontaneous bronchial secretions in discriminating the main infectious causes of COPD exacerbations. Methods: 124 subjects with moderate COPD (51–79 y; mean basal FEV1 = 49.6% pred. ± 4.6 sd; FEV1 reversibility + 3.9% from baseline ± 4.8 sd after salbutamol 200 mcg) were studied during acute exacerbation. Respiratory viruses were isolated from bronchial secretions in 21 cases; common bacteria (CFU ≥ 106/ml) in 28 cases; Pseudomonas Aeruginosa (Ps.Ae.; CFU ≥ 106/ml) in 20 cases. The cytokines IL1β, IL8, and TNFα (pg/ml; Immulite; Diagnostic Product Corp, Los Angeles, CA, USA), and neutrophils (% total count) were measured in bronchial secretions of all patients. Statistics: a two-stage logistic model was chosen for discriminating the different causes of COPD exacerbations (such as: non-infectious, or viral, bacterial, or due to Ps.Ae.). Results: At the first decisional step, the two-stage logistic model proved that TNFα levels in bronchial secretions recognise clearly patients belonging to the Ps.Ae. group from those of all othergroups (Area under ROC curve = 0.96; 95% CI = 0.91–0.99), and that, at the second decisional step, IL8 + IL1β levels discriminate patients with bacterial causes (such as all bacteria) from the non-infected ones and from those with a viral cause of exacerbation (Area under ROC curve = 0.87; 95% CI = 0.77–0.94). Neutrophil percent count did not support any contribution in discriminating the different subgroups of COPD subjects. Conclusions: when exacerbated, COPD subjects express different patterns of pro-inflammatory mediators in bronchial secretions, which appear modulated according to the etiological cause of the exacerbation. In particular, TNFα concentration per se enables recognition of COPD exacerbations due to Ps.Ae., while IL8 + IL1β levels prove helpful in discriminating those to common bacteria from those to viral agents and to non-infectious causes. When present data are further confirmed, the use of a decisional rule based on cytokine measurements might be regarded as a helpful predictive tool. As measures of pro-inflammatory cytokines are low-cost, simple, and faster to perform, they could support rapid clinical decision making at the bedside regarding therapeutic strategy for COPD exacerbations, in particular when they are needed for severe COPD patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease state characterized by airflow limitation that is not fully reversible, and progressive lung function decline Citation[[1]], actually representing the fourth leading cause of mortality world wide Citation[[2]]. COPD is associated with intermittent exacerbations characterised by acute deterioration in symptoms, lung function, and quality of life Citation[3&4].
Exacerbations have major effects on health status; they are associated with considerable morbidity and mortality Citation[[5]], and often lead to hospital admissions, that represent a major component of the socio-economic burden related to COPD Citation[6-8].
The etiology of acute exacerbations of chronic bronchitis (AECB) is complex, including mucous plugging and regional atelectasis, inhalation of environmental irritants, discontinuation of medications, deviation from diet, viral infections, atypical bacteria, and common bacterial infections Citation[[9]].
Even though the precise role of bacterial infection in the course and the pathogenesis of COPD has been a source of controversy for decades, bacteria have a crucial role in the pathogenesis of COPD exacerbations Citation[10-12]: bacteria may be isolated during periods of quiescence, but quantitative cultures have demonstrated an increase of some pathogens during an acute exacerbation Citation[[13]]. Moreover, bacteria can cause direct epithelial damage, and bacterial endotoxin has been shown to increase epithelial expression of some pro-inflammatory cytokines in vitro, providing potential mechanisms to up-regulate inflammation in cultured human bronchial epithelial cells Citation[[14]].
Airway bacterial load has been shown to correlate directly with markers of neutrophilic inflammation, irrespective of the pathogen isolated Citation[[15]]. Similarly, the presence of potentially pathogenic microbes isolated from bronchoalveolar lavage fluid is strongly associated with increased neutrophils and concentrations of TNF-α Citation[[16]].
Viral infections are also important triggers for COPD exacerbations, which are frequently triggered by upper respiratory tract infections. These infections are more common in the winter months when there are more respiratory viruses present in the community Citation[[17]]; also rhinoviruses have been considered an important etiologic factor in COPD exacerbations in a recent study Citation[[18]].
In patients with COPD, lower respiratory tract infections of viral origin may also cause direct damage to the airway epithelium resulting in loss of ciliated epithelium, increased mucus production, and increased plasma exudation Citation[[19]]. The role of atypical organisms (Mycoplasma Pneumoniae and Chlamydia pneumoniae, intracellular bacteria that share some of the characteristics of viruses) in exacerbations of COPD is still controversial. The mechanism by which they can cause exacerbation is likely to be similar to that of viral infections, and the investigations into the role of these organisms have depended largely on serological diagnosis Citation[20&21].
Routine procedures to obtain an effective bacteriological assessment usually require several hours (or a few days) and therapeutic decisions can be sometimes dangerously delayed or over-estimated if empiric choices are the first therapeutic approach. The development of more reliable, rapid, and widely applicable methods to timely define the etiology of COPD exacerbations should represent a relevant issue in devising earlier and more specific strategies for their effective prevention and treatment Citation[[22]].
Aim of the study was to assess the predictive role of some pro-inflammatory cytokines measured in spontaneous bronchial secretions in discriminating the main infectious causes of COPD exacerbation.
Material and Methods
Study Population
Adult patients with moderate COPD suffering from an acute exacerbation of their condition were eligible for enrolment. The severity of the disease was defined according to the criteria of the GOLD guidelines Citation[[1]]. Demographics and baseline clinical characteristics are summarized in .
Table 1. Demographics and Clinical Characteristics of Patients Sampled.
One hundred and twenty-four moderate COPD patients with mild-to-moderate respiratory exacerbations entered the study and were categorized according to the criteria by Anthonisenet al. Citation[[23]]. Type I exacerbation: characterized by increased breathlessness, increased sputum volume, and new or increased sputum purulence; type II: the presence of any two of these symptoms; type III: the presence of any one of the symptoms, together with at least one additional feature, including sore throat or nasal discharge within the last five days, increased cough, presence of fever (oral temperature ≥ 38°C).
Exclusion criteria were: atopic condition; bronchial asthma; bronctiectasis (checked by CXR and CT); pregnancy or lactation; chest X-ray suggestive of pneumonia; recent diagnosis or unresolved lung malignancy; evidence of significant immunosuppression; evidence of significant liver impairment; renal insufficiency; other life-threatening illness; previous use of antibiotics in the 4 weeks prior to the enrollment.
Adjunctive medications including inhaled steroids and bronchodilators, mucolytics, and oral theophylline were permitted according to usual practice.
Bacteriological Assessments
When admitted, a spontaneous sputum sample was obtained from each patient for culture within 2 hours. Bacterial identification was performed by standard techniques. Bacterial titres were measured by counting the number of colonies in the dilution plates and multiplying the count by the appropriate dilution factor. Bacterial count was expressed in CFU/ml (Colony Forming Units)/ml, and only a CFU/ml titres = 106 was considered of causative value for exacerbation. None of the patients included in the present study (who were periodically controlled) had a CFU value > 104/ml outside the exacerbation period. The standard methods used for sputum cultures were: the COS + Optochina/Haem/ CAN/ CPS ID 2 at 37°C in CO2 for 24–48 hours for gram+ agents, and the McConkey at 37°C in aerobiosys for 24–48 h for gram− agents, by Biomerieux, Paris, France.
Diagnosis of Atypical Organisms
A blood sample was taken from every patient for the diagnosis of Mycoplasma pneumoniae and Chlamidia pneumoniae by enzyme immunoassay (EIA) kit (Adaltis Spa, Casalecchio di Reno, Italia) for the determination of antibodies in human serum.
The detection of antibodies is based on the principle of enzyme-linked immunosorbent assay (ELISA). The purified, homogeneous antigen is fixed to each well of the micro-plate. Any specific antibodies present in the patient’s serum are bound during the first incubation. After removing unbound material by washing, the presence of specific antibodies is detected by using anti-human IgG or IgM or IgA peroxidase-linked conjugate during the second incubation. Excess conjugate is then removed and substrate is added, resulting in the development of a blue color. The enzyme reaction is terminated by the addition of a stop solution. The intensity of a yellow color thus developed is proportional to the concentration of antibodies in the sample. The infection was considered active when it was possible to detect IgA and IgM antibodies.
Indirect immunofluorescence was used for the diagnosis of Chlamydia pneumoniae. The serum from the patients is incubated with bacterial antigen (Euroimmun, Lübeck, Germany). The antibodies present in the patient’s serum are bound during the incubation and they react after with anti-human antibodies conjugate with fluorescine and are recognized by fluorescence microscopy.
The specificity was of 94% for Mycoplasmapn. and of 95% for Chlamydia pn. respectively, and sensitivity was 98% for both.
Detection of Respiratory Viruses
The PathoDx Respiratory Virus Panel (RVP) kit (Diagnostic Products Corporation, Los Angeles, CA, USA) consists of a direct immunofluorescence test for the qualitative detection of the 7 most common respiratory viruses (Respiratory Syncytial Virus – RSV; virus Influenza A; virus Influenza B; Parainfluenza viruses 1, 2, and 3, and Adenoviruses) in prepared patient specimens, such as bronchial secretions. The PathoDx RVP consists of one screening reagent containing monoclonal antibodies to each respiratory virus and seven virus-specific monoclonal antibody reagents. All reagents contain monoclonal antibody labelled with fluorescin. Acetone-fixed cells from either patient’s specimens or cell culture are stained with the RVP screening reagent and the negative control reagent. The screening reagents contain fluorescin-labelled monoclonal antibodies for each virus. They will react specifically to any of the viral antigens, if present in the cell. The negative control reagent contains fluorescin-labeled murine antibodies that do not react with the viral agents. Under fluorescence microscopy, the viral antigens recognised by the monoclonal antibodies will show a characteristic apple-green fluorescence, while uninfected cells will counterstain red with Evan blue. To identify which of the seven respiratory viruses is reactive with the screening reagent, acetone-fixed cell preparations are stained with each of the seven virus-specific reagents, washed free of unbound antibody, mounted with buffered glycerol, and observed under fluorescence microscopy. The responsible viruses will show characteristic apple-green fluorescencewith uninfected cells counterstaining in red. The PathoDx RVP can be used to provide rapid and accurate identification of viral respiratory pathogens by direct detection of prepared patient specimens or in confirmation of cell culture isolates Citation[[24]]. Sensitivity and specificity of the method for diagnosing respiratory viruses vary from 100% to 98.6%, respectively Citation[[25]].
Sputum Processing and Counting
Following the bacteriological procedures, the remainder of the sputum sample was filled into a 1-ml Eppendorf tube, weighted, and mixed with an equal volume of 0.1% dithiothreitol (Calbiochem; Schwalbach, Germany) in phosphate-buffered saline solution. Sputum was gently vortexed and placed into a water bath at 37°C for 15 minutes to allow homogenisation of the sample. Sample was then centrifuged (2800 revolutions per minute for 10 minutes); the supernatant was aspirated and re-centrifuged (3000 revolutions per minute for 5 minutes; 1500 G force generated by Cytocentrifuge Sukura Finetek, Torrance, CA, USA) to completely remove cellular components, and immediately frozen at − 70°C. Sputum cells were counted after cytospin preparation and staining with Hemacolor staining to assess the quality of the sample. Only supernatant of sputum samples with a squamous cell contamination of < 20% was used for further analysis. Neutrophil count was expressed in % total cell count.
IL-1β, IL-8, and TNFα Assay
The concentration of IL1β, IL-8, and TNFα in pooled secretion supernatant was measured by an automated immuno-analyser (Immulite; Diagnostic Product Corp., Los Angeles, CA, USA), which represents a new approach for the fully automated determinations of these cytokines. For a single determination, a total volume of 0.05 ml secretion volume + 0.1 ml void volume is required. The technique is based on a solid phase (bead) with two sites for the chemiluminescent enzyme immunometric assay. The analysis is performed within 60 to 90 minutes, with a good stability of the calibration curve for 15 days Citation[[25]].
Statistical Analysis
The dependent variable “Causes of COPD exacerbations” has been encoded as follows: 1) Non-infected, 2) Viruses+, 3) Common Bacteria+, and 4)Pseudomonas aerations+. We tried to model and predict this variable by means of a set of discriminant statistical models and four explanatory variables (TNFα, IL1β, IL8, and Neutrophil count). Confidence intervals were estimated by the bootstrap bias corrected (BC) method taking 20,000 bootstrap replications Citation[[26]].
The ability of the model to predict the dependent variable, that is the agreement between true and predicted classes, was measured by the Cohen Kappa statistic. The main advantage of this statistic is that it adjusts the agreement rate for the agreement due to chance Citation[[27]]. The estimated values of Kappa were interpreted according to the standard benchmarks proposed by Landis and Koch: Kappa < 0 indicates no agreement; 0–0.19 poor agreement; 0.20–0.39 fair agreement; 0.40–0.59 moderate agreement; 0.60–0.79 good agreement; 0.80–1.00 excellent agreement Citation[[28]].
The predictive performance of the proposed discriminant model was assessed on in-sample and out-of-sample data. Inthe former case, we used the whole sample to estimate the model and to test its predictive power. In the second case, the sample was split into a learning set and a testing test: the first set of data was used to estimate the parameters, while the second one to measure the generalization properties of the model. The rate between the size of the learning and the testing set was 3:1. It is worth noting that out-of-sample confidence intervals for Kappa are always wider than in-sample ones, because the latter does not take into account the generalization error of the model.
In addition to global performance, the predictive power of the model for the single classes was also measured by estimating the Kappa statistics (k1 + 2, k3, and k4) of the three 2 × 2 matrices obtained collapsing two rows and two columns of the confusion matrix. Classification trees where pruned at the best level of pruning. This level was estimated minimizing a 10-fold cross-validation estimation of the misclassification costs Citation[[29]].
Data management, Cohen’s Kappa, bias-corrected (BC) bootstrap confidence intervals, logistic and multinomial logit model estimation were undertaken by Stata 8.0 Citation[[30]]. Linear, quadratic, and mahalanobis discriminant analysis, classification and regression trees (CART) were estimated by Matlab 6.5 with the Statistics toolbox Citation[[31]].
Results
The detection of respiratory viruses and the bacteriological cultures were negative in 55 cases (Anthonisen type 1: n = 10; type 2: n = 25, and type 3: n = 20), and these exacerbations were considered of non-infectious cause.
Direct immunofluorescence tests for respiratory viruses were positive in 21 cases (Anthonisen type 1: n = 5; type 2: n = 12, and type 3: n = 4) such as: RSV n = 6; Virus Influenza A n = 4; Virus Influenza B n = 4; Virus Parainfluenza n = 2; Adenoviruses n = 5, while bacteriological cultures were negative for pathogens.
Bacteriological cultures for common bacteria were positive in 28 cases (Anthonisen type 1: n = 18; type 2 n = 3, and type 3: n = 7) including: Streptococcus pneumoniae n = 8; Haemophilus influenza n = 6; Moraxella catarrhalis n = 6; Haemophilus parainfluenzae n = 4; Klebsiella pneumoniae n = 2; Staphylococcus aureus n = 2, and negative by direct immunofluorescence tests for respiratory viruses.
In further 20 cases (Anthonisen type 1: n = 10; type 2:n = 7, and type 3: n = 3), strains of Pseudomonas aeruginosa were isolated, with negative direct immunofluorescence tests for respiratory viruses. Their bacterial load (defined by the CFU/ml value) was 106 in 13 cases, and 107 in the remaining 7 Pseudomonas ae. infected.
In 122/124 acutely exacerbated patients, enzyme immunoassay and indirect immunofluorescence for Mycoplasma pneumoniae and Chlamydia pneumoniae did not suggest any causative role of these agents in the current exacerbation.
Exacerbated subjects with mixed microbes (i.e., the presence of more than one bacterial agent, or of bacteria + virus) were not included in the present study in order to firstly investigate and assess the role of “pure conditions” in affecting cytokine production.
Exploratory and Univariate Analysis
Mean values (± sd) of the four independent variables (IL1β, IL-8, and TNFα and neutrophil count) are reported in for each subgroup of exacerbated subjects. gives further details about the distributions of the variables inside the four subgroups.
Table 2. Mean Levels of IL1β, IL-8, And TNFα in Bronchial Secretions, Together with % Neutrophil Count Assessed in Bronchial Secretions (Interquartile Range in Parentheses) According to the Pathogen Type.
Figure 1. Statistical distribution (box and whisker plot) of TNFα, IL1β, IL-8 levels and of neutrophil % count in bronchial secretions according to the four causes for COPD exacerbations: 1) Non-infected, 2) Virus +, 3) Common bacteria, and 4) Pseudomonas aeruginosa +. The central box represents the values from the lower to upper quartile (25 to 75 percentile). The middle line inside the box represents the median value. The dashed lines (whiskers) extend from the minimum to the maximum values, excluding outliers that are displayed as separate points. An outlier is defined a value that is smaller than the lower quartile minus 1.5 times the interquartile range, or larger than the upper quartile plus 1.5 times the interquartile range. These values are plotted with a + marker.
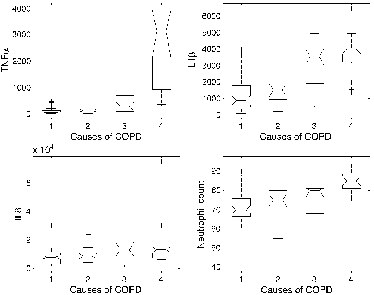
In order to explore the association between IL1β, IL-8, TNFα, and neutrophil count and the causes of COPD exacerbations, four univariate multinomial logit models were estimated. reports the coefficients of these models, together with their 95% confidence intervals and the corresponding p-values.
Table 3. Figures Report the Coefficients of the Multinomial Logit Model.
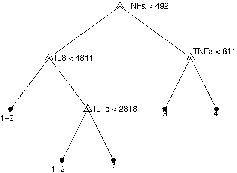
Going further into the analysis of the relationship existing between dependent and independent variables, the classification tree (CART) was also estimated and reported in . TNFα appeared to be the most influential variable in the classification scheme. Moreover, the tree indicated a threshold value close to 492 pg/ml. Adopting the simple rule TNFα ≥ 492 pg/ml for classifying “Pseudomonas aeruginosa+ ” subjects, the confusion matrix reported in is obtained. The estimated in-sample Kappa statistic was 0.68 (95% CI = 0.52–0.85).
Table 4. Confusion Matrix of the Simple Classification Rule TNFα ≥492.
Figure 2. Pruned classification tree model. Numbers near the final nodes indicate the causes of COPD exacerbations predicted by the model: (1 + 2) Non-infected and Virus +ve; (3) Common bacteria +ve and (4) Pseudomonas aeruginosa +ve. The values reported in the figure (such as: IL8 < 4811, TNFα < 811, and IL1b < 2818) indicate the classification threshold values (in pg/ml) for group 1 + 2; 3, and 4, respectively. The predictive performances of this model were compared to those of all other classification models considered in the present study (see ).
Predictive Model
The proposed “two-stage” predictive model is shown in . At the first level, using a logistic model with the TNFα variable only (threshold value for the output probability thr1 = 0.15), the model discriminates subjects with Pseudomonas aeruginosa from the remaining ones (area under ROC curve = 0.96, 95% CI = 0.91–0.99).
Figure 3. The proposed is a “two stage” model. At the first level, using a logistic model with the TNFα variable only (threshold value for the output probability thr1 = 0.15), the model discriminates subjects with pseudomonas aeruginosa from the remaining ones (odds ration for standardized TNFα OR = 4.58, 95% Cl 2.56–8.21, area under ROC curve = 0.96, 95% Cl = 0.91–0.99). At the second level, another logistic model with IL1b and IL-8 (threshold value for the output probability thr2 = 0.23) discriminates subjects exacerbated by common bacteria from the non-infected and the virus-infected ones (odds ratio for standardized IL-8 OR = 3.72, 95% Cl 1.85–7.47, area under ROC curve = 0.87, 95% Cl = 0.77–0.94).
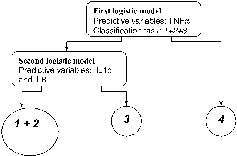
At the second level, another logistic model with IL-1β and IL-8 (threshold value for the output probability thr2 = 0.23) discriminates subjects exacerbated by common bacteria from the non-infected and the virus-infected ones (area under ROC curve = 0.87, 95%CI = 0.77–0.94).
The confusion matrix of the whole model is given in . The estimated in-sample Kappa was 0.62 (95%CI = 0.45–0.75). The out-of-sample Kappa was 0.59 (95%CI = 0.22–0.88). gives a detailed description of the predictive performances of the model. The generalization properties of this model were also compared with those of five other discriminating models: multinomial logit model, linear, quadratic, mahalanobis discriminant analysis, and CART. Results are summarized in .
Table 5. Confusion Matrix of the “Two-Stage” Logistic Model.
Table 6. The Predictive Performances of the Proposed Two Stage Logistic Model and the Comparison with Other Discriminant Models.
Discussion
Factors determining susceptibility to exacerbations are strictly related to the pathogenic determinants of COPD, such as a reduced mucociliary clearance and an increased mucus production, which can facilitate mucosal invasion of infectious agents and their adherence to the bronchial structures Citation[[32]], together with a relevant increase of local inflammatory processes.
Recurrence of exacerbation of symptoms characterizes the natural history of COPD with varying frequency: these episodes lead to a progressive increase in medical care, hospitalization, and to a corresponding drop in quality of life Citation[[7]]. Even though infections are regarded as crucial events in the course and the pathogenesis of COPD, the precise role of different infectious agents has been a source of controversy for decades.
The role of inflammatory mediators is even less clear, both in the stable condition and during exacerbations, with the changes in pro-inflammatory cytokines during exacerbations still a hotly debated topic. While a relationship was found between IL-8 and IL-6 concentrations in induced sputum and the frequency of exacerbations Citation[[33]], further studies did not confirm this relationship when other confounding factors were taken into account (such as: smoking habit, severity of airway obstruction, presence of bronchiectasis, steroid treatment) Citation[34&35].
Neutrophilic inflammatory changes are typical in COPD, and neutrophils are recruited from the circulation in response to chemoattractant mediators, particularly LTB4 and IL-8, with the latter being the most expressed cytokine in most severe episodes of COPD exacerbations Citation[[36]]. Also TNFα tends to increase in spontaneous sputum during exacerbations, and its higher concentrations may facilitate the expression of adhesion molecules, with the consequent cell migration and neutrophil activation Citation[[12]]Citation[[37]].
At present, the precise relationship between the different infectious agents involved in COPD exacerbations and the pro-inflammatory cytokine response is still unclear, even though bacterial load was found directly related with markers of neutrophilic inflammation in a study carried out on subjects with chronic bronchitis irrespective of the pathogen isolated Citation[[15]]. Few studies have investigated the cytokine response during COPD exacerbations, and they were mainly animal models, in vitro studies on human tissues or on very few human subjects in experimental conditions, and were not pathogen specific.
In clinics, the causative role of bacterial agents in COPD exacerbations is difficult to define as is that of respiratory viruses in causing inflammatory changes and/or promoting secondary bacterial infections. Furthermore, the evidence for the viral etiology of exacerbation is usually indirect, and usually depending on serological assessment and seroconversion.
Moreover, also non-infectious factors (such as: air pollution, ozone, sulphur dioxide, diesel particulate, nitrogen oxide, etc.) can work as confounding factors by stimulating the production of pro-inflammatory cytokines directly, and facilitating a significant neutrophilic inflammation.
In bacterial exacerbations, airway inflammation has been recently presumed to be characterized by greater neutrophilic inflammation, particularly in the presence of high bacterial load Citation[[15]]: the effect of the different species of bacterial pathogens still remains to be clarified even though the persistence of Hemophilus influenzae in lower airways is regarded as able to stimulate airway epithelium to produce pro-inflammatory cytokines, particularly those producing neutrophil chemotaxis. On the other hand, the enhancement of epithelial cell permeability, the increased expression of intracellular adhesion molecules, and the increase in IL-6, IL-8, and TNFα release were demonstrated in in vitro studies carried out on cultures of human bronchial epithelial cells Citation[[38]]. Similarly, the presence of potentially pathogenic microbes isolated from bronchoalveolar lavage fluid in stable COPD has been shown to be strongly associated with increased neutrophils and concentrations of TNF-α in a clinical model Citation[[16]], suggesting that the presence of bacteria in the lower airways of stable COPD subjects is not innocuous.
Data of the present study demonstrate that, when exacerbated, COPD subjects are characterised by different patterns of pro-inflammatory cytokines in bronchial secretions according to the different infectious cause of the exacerbation. The univariate analysis of and showed that only the distribution of TNFα in the “Pseudomonas aeruginosa+ ” group clearly differentiated those from the remaining groups. This is clearly confirmed by the CART estimation: the variable at the top of the tree (and therefore the most discriminating one) is TNFα (). Moreover, the simple rule TNFα ≥ 492 pg/ml suggested by the CART model has a strong capability for discriminating the “Pseudomonas aeruginosa+ ” group (Tables and ).
Preliminary analysis also showed that neutrophil percent count did not support any contribution in discriminating the different subgroups of COPD subjects and that not one of the variables (singularly or jointly) considered could discriminate between non-infected and virus-infected subjects. This is the reason why these two groups were collapsed into a single one. Unfortunately, the absolute neutrophil count was not calculated in the present paper, and hence we cannot comment regarding the absolute neutrophil changes during the different causes of exacerbations.
Despite IL8 and neutrophil percent differential counts not appearing to be affected by DTT use Citation[39&40], a reduction in TNFα absolute concentration has been described in sputum processed with DTT Citation[40&41]. In our study, great differences in TNFα concentrations were measured depending on the different cause of exacerbation even though all sputum samples were processed according to the same protocol (reducing the likelihood of a systematic error). In particular, the power of the two-stage logistic model is evident by the highly differentiated TNFα concentrations in different causes of exacerbation (such as quite low in viral and non-infectious exacerbations, and quite high in exacerbations due to Pseudomonas ae.).
Multi-nomial logit models () and CART model also suggested that IL-1β and IL-8 could contribute in discriminating common bacteria-infected subjects. Therefore, the exploratory and univariate analysis supported and supplied some useful information for the construction of the “two-stage” predictive model shown in . When compared to all other possible classification models, the “two stage logistic” model provides the best performance for the true classification of the sample units in the different groups: Ktot = 0.68 represents the higher value obtained.
Results reported in Tables and lead to the following conclusions: 1) the “two-stage” logistic model showed a substantial agreement between true and predicted categories; 2) each model considered here showed a moderate or fair agreement between true and predicted “common bacteria+” categories (see K3 estimates in ); 3) the discriminating capability of the “two-stage” logistic model l is approximately equal or superior to any other classification models taken into account.
In other words, the proposed model proved to be a simple, easy to interpret, and helpful tool for discriminating the main causes of COPD exacerbation by using some pro-inflammatory cytokines measured in spontaneous bronchial secretions. Of course, given the limited size of our sample, no definitive conclusions can be drawn and further analyses are needed.
Among the bacterial species isolated, only Pseudomonas ae. (the most dangerous pathogen for severe COPD patients) manifested a well-defined TNFα production, while all other pathogens did not show individually any peculiar cytokine pattern in bronchial secretions. This is partially in agreement with other studies Citation[15&16], where correlation between markers of neutrophil inflammation and bacterial infection was described as absolutely independent of the pathogen isolated in the airways.
Similarly to other studies, also in our experience neutrophil percent count did not contribute to clarify per se the etiologic cause of exacerbation: its poor sensitivity is likely due to the high bacterial load required for inclusion (such as CFU = 106/ml) . Cases with lower bacterial loads in bronchial secretions were excluded from the study in order to reduce their possible role as confounding factors in the logistic model.
Unlike other studies concerning the effect of airway infection on pro-inflammatory markers, in the present study the role or respiratory viruses per se was also investigated, and their cytokine production appeared indistinguishable from that of non-infectious causes of acute exacerbations. Even though further studies are needed to confirm present data, it represents an interesting finding that deserves further attention.
In the present study HRV was not detected. On the other hand, the aim of the present study was not that of calculating the prevalence of exacerbations due to viral, common bacteria, and to Pseudomonas ae. The particular aim of the study was to assess the discriminating value of cytokine response during COPD exacerbations of different causes (to viruses, common bacteria, and Pseudomonas ae., or non-infective). Actually, non-infective and viral exacerbations are combined in the same class (1 + 2) because these two conditions proved indistinguishable from each other in terms of cytokine response, while class 3 and class 4 were very well discriminated. We are aware that the non-detection of HRV may represent a limit for the present study (we are, in fact, testing its detection by means of much more specific and sensitive genetic probes), particularly with regard to precise identification of the viral epidemiology of COPD exacerbations. The HRV non-detection, however, does not affect the value of the logistic regression model as presented here because the non-infective and viral exacerbations, such as class (1 + 2), are absolutely indistinguishable in terms of cytokines production. In other words, only the percentage of non-infective causes would change (such as decrease) in favor of the viral causes of exacerbation, but nothing would change at all in general terms, with regard to the proportion of exacerbations due to common bacteria and Pseudomonas ae.
At present, data from true clinical models carried out on adequate numbers of patient samples are lacking. To our knowledge, the present study represents a pivotal attempt to support a probabilistic model for discriminating in clinics the main COPD exacerbation causes in respect to the pathogen isolated.
Further studies are in progress to investigate more extensively any other correlation between some particular clinical conditions (such as: smoking history, duration of the disease, duration of some chronic therapeutic treatments, severity of exacerbation, etc.) and the expression of these pro-inflammatory cytokines in bronchial secretions.
In conclusion, when acutely exacerbated, COPD subjects display characteristically different patterns of pro-inflammatory cytokines in bronchial secretions, according to the etiological cause of exacerbation: the assessment of these cytokines in sputum can then be presumed to possibly be of some utility in the clinical management of COPD patients.
As TNFα, IL8, and IL-1β measures are not costly or time-consuming procedures to perform, when present data further confirmed, the use of a decisional rule based on these pro-inflammatory indices might be regarded as a promising diagnostic tool in addition to usual bacteriological assessment, and particularly when decisions for a specific treatment of a COPD exacerbation (i.e., the most convenient choice of antibiotic) is needed at the bedside in short order due to the severity of the patient’s clinical conditions.
REFERENCES
- Pawels R A, Buist A S, Calverley P M, Janekins C R, Hurd S S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163:1256–1276. [CSA]
- Ilurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest 2000; 117:1S–4S. [CROSSREF]
- Sethi S. Infectious etiology of acute exacerbations of chronic bronchitis. Chest 2000; 117(5 suppl 2):380–385S. [CROSSREF]
- Seemungal T A, Donaldson G C, Paul E A, Bestall J C, Jeffriess D J, Wedzicha J A. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1418–1422. [PUBMED], [INFOTRIEVE], [CSA]
- Groenewegen K H, Schols A MWJ, Wouters E FM. Mortality and mortality-related factors after hospitalization for acute exacerbations of COPD. Chest 2003; 124:459–467. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Monso E, Rossel A, Bonet G, Manterola J, Cardona P J, Ruiz J, Morera J. Risk factors for lower airway bacterial colonization in chronic bronchitis. Eur Respir J 1999; 13:338–342. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Dal Negro R W, Rossi A, Cerveri I. The burden of COPD in Italy: results from the confronting COPD survey. Respir Med 2003; 97(suppl C):S43–S50. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Miravitlles M, Murio C, Guerrero T, Gisbert R. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest 2002; 121:1449–1455. [PUBMED], [INFOTRIEVE], [CROSSREF]
- White A J, Gompertz S, Stocley R A. Chronic obstructive pulmonary disease: the aetiology of exacerbations of chronic obstructive polmonary disease. Thorax 2003; 58:73–80. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Wilson R, Grossman R. Introduction: the role of bacteria infection in chronich bronchitis. Semin Respir Infect 2000; 15:1–6. [PUBMED], [INFOTRIEVE], [CSA]
- Sethi S. Management of acute exacerbations of chronic bronchitis. Infect Dis Clin Pract 1998; 7:S300–S308.
- Sethi S, Muscarella K, Evans N, Klingman K L, Grant B J, Murphy T F. Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest 2000; 118:1557–1565. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Hirschmann J V. Do bacteria cause exacerbation of COPD? Chest 2000; 118:193–203. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Khair O A, Devalia J L, Abdelaziz M M, Sapsford R J, Tarraf H, Davies R J. Effect of Haemophilus influenza endotoxin on the synthesis of IL-6, IL-8, TNF-alpha and expression of ICAM-1 in cultured human bronchial epithelial cells. Eur Resp J 1994; 7:2109–2116. [CROSSREF]
- Hill A T, Campbell E J, Hill S L, Bayley D L, Stockley R A. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med 2000; 109:288–295. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Soler N, Ewing S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J 1999; 14:1015–1022., [PUBMED], [INFOTRIEVE], [CROSSREF]
- Wedzicha J A. Exacerbations—Etiology and pathohysiologic mechanism. Chest 2002; 121:136S–141S. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Seemungal T A, Harper-Owen R, Bhowmik A, Jeffries D J, Wedzicha J A. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J 2000; 16:677–683. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Hegele R G, Hayashi S, Hogg J G, Pare P DI. Mechanisms of airway narrowing and hyperresponsiveness in viral respiratory tract infections. Am J Respir Crit Care Med 1995; 151:1659–1664. [PUBMED], [INFOTRIEVE], [CSA]
- Mogulkoc N, Karakurt S, Isalka B, Bayindir U, Celikel T, Korten V, Colpan N. Acute purulent exacerbation of chronic obstructive pulmonary disease and Chlamydia pneumoniae infection. Am J Respir Crit Care Med 1999; 160:349–353. [PUBMED], [INFOTRIEVE], [CSA]
- Verkooyen R P, Van Lent N A, Mausavi Joulandan S A, Snijder R J, van den Bosch J M, Van Helden H P, Verbrugh H A. Diagnosis of Chlamydia pneumoniae infection in patient with COPD by micro-immunofluerescence and ELISA. J Med Microbiol 1997; 46:959–964. [PUBMED], [INFOTRIEVE], [CSA]
- Sethi S, Murphy T F. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state of the art review. Clin Microbiol Rev 2001; 14(2):336–363. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Anthonisen N R, Manfreda J, Warren C P, Hershfield E S, Harding G K, Nelson L A. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196–204. [PUBMED], [INFOTRIEVE]
- Schirm J, Luijt D S, Pastoor G W, Mandema J M, Schroder F P. Rapid detenction of respiratory viruses using mixture of monoclonal antiboides on shell vial cultures. J Med Virol 1992; 38(2):147–151. [PUBMED], [INFOTRIEVE]
- Berthier F, Lambert C, Genin C, Bienvenu J. Evaluation of an automated immunoassay method for cytokin measurement using the Immulite Immunoassay system. Clin Chem Lab Med 1999; 37(5):593–599. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Efron B, Tibshirani R J. An Introduction to the Bootstrap. Chapman & Hall, 1993.
- Cohen J. A coefficient of agreement for nominal scales. Psychol Meas 1960; 20:37–46. [CSA]
- Landis J, Koch G. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PUBMED], [INFOTRIEVE]
- Breiman L, Friedman J H, Olshen R A, Stone C J. Classification and Regression Trees. Belmont: Wadsworth, 1984.
- StataCorp. Stata Statistical Software: Release 8.0. College Station, TX: Stata Corporation, 2003.
- TheMathworks. Matlab 6.5. Natick, MA: The Mathworks Inc., 2003.
- Mossberg B, Afzelius B, Camner P. Mucociliary clearance in obstructive lung diseases. Correlations to the immotile cilia syndrome. Eur Respir J 1986; 146:295–301.
- Bhowmik A, Seemungal T A, Sapsford R J, Wedzicha J A. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 2000; 55:114–120. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Hill A T, Bayley D, Campbell E J, Hill S L, Stockley R A. Airway inflammation in chronic bronchitis: the effect of smoking and alpha-1 antitrypsin deficiency. Eur Respir J 2000; 15:886–890. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Gompertz S, O'Brien C, Bayley D, Hill S L, Stokley R A. Comparison of stable state bronchial inflammation in chronic bronchitis and previously unsuspected bronchiectasis. Eur Respir J 1999; 14:165s.
- Crooks S W, Bayley D L, Hill S L, Stockley R A. Bronchial inflammation in acute bacterial exacerbations of chronic bronchitis: the role of leukotriene B4. Eur Respir J 2000; 15:274–280. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Aaron S, Angel J B, Lunau M. Granulocyte inflammation markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Repir Crit Care Med 2001; 163:349–355.
- Khair O A, Davies R J, Devalia J L. Bacterial-induced release of inflammatory mediators by bronchial epithelial cells. Eur Respir J 1996; 9:1913–1922. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Efthimiadis A, Pizzichini M M, Pizzichini E, Dolovich J, Hargreave F. Induced sputum cell and fluid-phase indices of inflammation: comparison of treatment with dithiothreitol vs phosphate-buffered saline. Eur Respir J 1997; 10:1336–1340. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Louis R, Shute J, Goldring K, Perks B, Lau L C, Radermecker M, Djukanovic R. The effect of processing on inflammatory markers in induced sputum. Eur Respir J 1999; 13(3):660–667. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Woolhouse I S, Bayley D L, Stockley R A. Effect of sputum processing with dithiothreithol on the detection of inflammatory mediators in chronic bronchitis and bronchiectasis. Thorax 2002; 57:667–671. [PUBMED], [INFOTRIEVE], [CROSSREF]