Abstract
Limitation of physical activity occupies a central role in the symptom complex of patients with chronic obstructive pulmonary disease (COPD), and improvement in exercise capacity is a key outcome of response to COPD therapy. Maximum exercise capacity testing facilitates assessment of physiologic mechanisms of exercise and allows quantitation of the degree of limitation. This manuscript utilizes published data from the National Emphysema Treatment Trial to investigate the minimal clinically important difference (MCID) in maximum exercise capacity in patients with severe emphysema. Distribution- and opinion-based methods were used to estimate MCID. Expert clinician opinion yielded a value of 10 Watts as the MCID for change in maximum exercise capacity. Baseline standard deviation and error data yielded a one-half standard deviation-based estimate of 10.5 Watts and a standard error-based estimate of 4.2 Watts. In subjects randomized to medical therapy, the mean ( ± SD) 24-month change in maximum exercise capacity following medical therapy was − 9.2 ± 1.2 Watts, whereas among those randomized to lung volume reduction surgery, mean 24-month change in maximum exercise capacity was 1.7 ± 17.7 Watts, with a mean difference between the groups of 10.9 Watts. The observed difference in maximum exercise capacity after 24 months between subjects randomized to medical versus surgical therapy conforms to both opinion- and distribution-based estimates of MCID. Further investigation is needed to develop and validate estimates of MCID for maximum exercise capacity and other key clinical outcomes in COPD.
Introduction
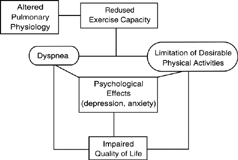
Limitation of physical activity occupies a central role in the symptom complex of patients with chronic obstructive pulmonary disease (COPD). Limitation of performance of daily activities is most often caused by shortness of breath due to pulmonary physiologic abnormalities associated with airflow limitation in COPD. Reduced exercise capacity is associated with patients' inability to fully participate in daily pursuits, including vocational, recreational and social activities. In patients with severe disease basic activities of daily living are frequently impaired Citation[[1]]. Reduction in activity also adversely impacts other factors of importance to patients including health-related quality of life. A conceptual relationship between reduced exercise capacity and the other outcomes of importance in COPD are embodied in .
Improvement in the capacity for physical activity has been recognized as a key outcome of response to therapies for COPD and the 6-minute walk is the most commonly employed measure of physical activity Citation[[2]]. Clinical trials in patients with COPD frequently assess the impact of the intervention on patient-centered outcomes such as dyspnea and quality of life. However, as shown in , these patient-centered outcomes are “downstream” from reduced exercise capacity. Thus, direct measurement of the capacity for physical performance provides an objective basis for dyspnea and health status outcomes.
There are four general methods of assessing the amount of physical activity that can be performed by patients with COPD: 1) questionnaires asking subjects what activities they can perform and the amount of limitation or symptomsassociated with activities, such as a) the activity questions and subscales of some quality of life measures Citation[[1]]Citation[[3]], and b) dyspnea questionnaires Citation[[4]]; 2) walking tests assessing the ability of subjects to perform a maximum walk, such as the 6-minute walk and shuttle walk tests Citation[[2]]; 3) exercise endurance tests, such as a stationary cycle or treadmill steady state exercise test to assess the maximum duration of exercise Citation[[5]]; and 4) maximum exercise tests—formal laboratory tests assessing the capacity for maximum activity, such as the maximum incremental cycle exercise test. Each of these methods has been employed to assess outcomes in COPD. Other articles in this journal review the use of steady state exercise tests and walking tests to assess physical capacity for activity in patients with COPD. This article addresses maximum exercise testing and focuses on incremental tests performed on a stationary cycle ergometer.
Maximum exercise testing has several key conceptual and practical advantages as a tool to measure outcomes in COPD (). Maximum exercise testing provides objective information about capacity for activity of an individual. The most widely used indices are maximum work (a measure of external work such as watts on a cycle ergometer) and maximum oxygen consumption (a measure of the work performed by the exercising muscles). Normative values for incremental cycle ergometry are based on age and sex and the reference values have recently been reviewed Citation[[6]].
Table 1. Advantages of Maximum Exercise as an Outcome in COPD.
Maximum exercise testing is performed in a controlled laboratory setting. It is a routine test in many pulmonary function laboratories, the exercise testing equipment is commercially available, and a recent statement from the American Thoracic Society and American College of Chest Physicians provides an overview of methodologies Citation[[6]]. Protocols for test performance can be customized to meet the needs of the therapy under study and testing can be applied in a standardized manner to large numbers of subjects. Defined test protocols allow subsequent investigations to reproduce the test methods. As a measure of impairment, maximum exercise can provide a basis for determination of disability Citation[[7]]. Maximum cardiopulmonary exercise tests provide the additive benefit of assessing the physiologic mechanisms of exercise limitation. Lastly, maximum exercise tests may be necessary for reasons other than to assess treatment outcomes, such as preoperative assessment for lung surgery, to screen for cardiac disease, and to assess need for oxygen therapy.
On the other hand, there are disadvantages of employing maximum exercise tests as an outcome in COPD (). The test requires a maximum effort on the part of the patient, and patients may be reluctant to perform the maximum effort required because of uncomfortable dyspnea or effort. However, test reproducibility, as noted later, appears to be high Citation[[8]]. Physical activity is limited by multiple physiologic mechanisms as well as the markedly unpleasant symptom of dyspnea. summarizes the physiologic mechanisms that may be associated with reduced exercise capacity in COPD. Maximum exercise tests provide the additional benefit of assessing the physiologic limitations to exercise and the physiologic basis of therapeutic interventions.
Table 2. Disadvantages of Maximum Exercise as an Outcome in COPD.
Table 3. Physiologic Mechanisms That May Be Associated with Reduced Exercise Capacity in COPD.
Because of the requisite effort on the part of the investigative team and patient, time, and expense for formal cardiopulmonary exercise testing to assess maximum exercise capacity, this outcome is not commonly assessed in clinical trials. Nevertheless, the investigators in a large trial of lung volume reduction surgery chose exercise as a co-primaryoutcome and used maximum exercise capacity as the measure of exercise Citation[[9]]. Therefore, this manuscript employs the published results of this multi-center clinical trial of lung volume reduction surgery sponsored by the National Institutes of Health to investigate the minimal clinically important difference in maximum exercise in patients with severe emphysema Citation[[10]].
Methods
Human Subjects
Data were obtained from published reports from the National Emphysema Treatment Trial (NETT), a randomized trial comparing lung volume reduction surgery with medical therapy for severe emphysema Citation[9-11]. In this study, 1218 subjects with severe emphysema underwent pulmonary rehabilitation and then were randomly assigned to undergo lung volume reduction surgery or to receive continued medical therapy. We excluded subjects at increased risk of death following lung volume reduction surgery Citation[[11]] and applied estimates of minimal clinically important difference to published data from all remaining subjects. As data for the entire study cohort were not reported, estimates were applied to pre-randomization exercise tests from surgical and medical therapy groups separately. Complete methods have been described previously Citation[[9]]. All subjects provided written informed consent.
Maximum Exercise Protocol
Maximum exercise capacity was determined by means of cycle ergometry with 5 or 10 Watt incremental increase per minute, initiated after 3 minutes of unloaded pedaling (0 Watts). Subjects unable to complete 3 minutes of unloaded pedaling were excluded from randomization. All subjects inspired 30% oxygen during maximum exercise testing Citation[[10]].
Analysis
Minimal clinically important difference was estimated by distribution-based estimates including one-half standard deviation Citation[[12]] [standard deviation × 0.5], effect size Citation[[13]] [mean change/baseline standard deviation], standard error of measurement Citation[14&15], and minimal detectable change Citation[[16]] [1.96 × 2 × standard error of measurement], as well as survey of expert clinician perspective Citation[[17]] as an opinion-based estimate. Minimal clinically important difference was determined in the entire study cohort, as well as in subsets of responders and non-responders Citation[[10]] as reported by the NETT investigators (see later).
Test–retest reliability was determined using data reported by Cox and colleagues, who in 1989 performed maximum exercise capacity testing on 2 consecutive days in 11 subjects with moderate chronic obstructive pulmonary disease and asthma (mean ( ± SEM) FEV1% predicted 62.1 ± 5.1) Citation[[18]]. Test–retest reliability from patients with severe airflow limitation (similar to the severity of obstruction in the NETT cohort) have not been published.
Change in exercise capacity in response to intervention, either medical therapy or lung volume reduction surgery, was determined by subtracting the value for maximum exercise capacity achieved at the post-intervention visit (which occurred 24 months post-randomization) from that achieved at the post-rehabilitation/pre-randomization visit Citation[[9]].
To determine expert clinician opinion, NETT investigators were queried by electronic mail to ascertain the minimal clinically significant difference in maximum exercise capacity. To evaluate specific responses based on disease and intervention, investigators were asked to define the minimal clinically important difference in maximum exercise for patients with severe emphysema undergoing lung volume reduction surgery.
Results
Baseline Characteristics and Distribution-Based Estimates
Subject characteristics are shown in . Subjects demonstrated evidence of severe chronic obstructive pulmonary disease Citation[[19]] with mean FEV1 < 30% of predicted. Baseline maximum exercise capacity was evaluated at enrollment into the study, prior to pulmonary rehabilitation, and at the time of randomization to medical therapy or lung volume reduction surgery. Mean baseline maximum exercise capacity was 38.7 ± 21.1 Watts ( ± standard deviation) in subjects randomized to surgery and 39.4 ± 22.2 Watts in subjects randomized to medical therapy. Standard error of themean was 0.9 Watts in both groups. These baseline standard deviation and standard error data were used to calculate various minimal clinically important difference estimates, yielding a one-half standard deviation-based estimate of 10.6 Watts in subjects randomized to surgery and 11.1 Watts in subjects randomized to surgical therapy. Other estimates are reported in .
Table 4. Subject Characteristics.
Table 5. Maximum Incremental Cycle Ergometry Exercise MCID Summary.
Test–Retest Reliability
Based on the data of Cox and colleagues Citation[[18]], test-retest reliability coefficient was determined to be 0.96, with a 95% confidence interval between 0.89 and 1.0.
Opinion-Based Estimates of Minimal Clinically-Important Difference
Consensus response from NETT investigators was that a change of 10 Watts be considered the minimal clinically-important difference in maximum exercise capacity.
Application of Distribution-Based Estimates to NETT Cohort
In subjects who were randomized to medical therapy (n = 610), the mean (± SD) change in maximum exercise capacity after 24 months of medical therapy was − 9.2 ± 13.3 Watts, whereas among those randomized to lung volume reduction surgery (n = 608), mean change in exercise capacity at 24 months was + 1.7 ± 13.3 Watts. This resulted in a mean difference between the groups of 10.9 Watts. Minimal clinically important difference estimates for the comparison of medical versus surgical therapy in all subjects are reported in .
Discussion
This report provides insights in determining the minimal clinically important difference for maximum workload during incremental cardiopulmonary exercise testing (a physiologically based outcome measure of exercise) in patients with COPD. Using available published data from a large, multi-center trial of lung volume reduction surgery, estimates of MCID based upon various methods of estimation are summarized in .
The distribution-based estimates of MCID for maximum exercise workload yielded a range of values, from 0.9 watts using the standard error of measurement to 10.5 watts based upon the half standard deviation estimate. While these statistical approaches only provide estimates of the minimal clinically important difference, an opinion-based method based on expert clinician interview provided a value of 10 Watts, which falls at the top end of range of the distribution-based estimates. Interestingly, this opinion-based estimate conforms to the 10.9 Watt change in maximum work observed over the 24-month follow-up period in NETT Citation[[10]].
The opinion-based estimate of MCID should be viewed in light of a surgical intervention, lung volume reduction surgery. Physician-investigators were asked to provide an MCID specifically with respect to the surgical intervention. Although not polled as to their rationale for choosing a 10 watt MCID, physicians likely considered the benefits in terms of the risks, including possible death, of this major surgical intervention. The investigators did not know there was a mortality benefit in selected subjects at the time they provided an estimate of MCID, but they did know the overall short-term surgical mortality was below the 8% they had previously designed into the study as a stopping rule for the trial. The investigators were also aware of the short-term and long-term complications of LVRS, based upon their personal experience during the trial. It is also unclear what weight, if any, the physicians placed upon the limited exercise capacity of the subjects prior to the surgical intervention. Given the mean baseline maximum workload of 36 watts, the 10 watt MCID chosen by the investigators represented a 28% increase following surgery. We suspect that these same physicians might choose a lower MCID if the question were posed in light of a less invasive and less risky therapy such as pulmonary rehabilitation or a commonly used medication like bronchodilators.
There are several factors potentially limiting the widespread application of the MCID estimates determined herein to all COPD patients. First, the patients enrolled in NETT had more severe disease than typically seen in other research cohorts. Patients in NETT had very severe airflow limitation with a mean FEV1 of approximately 27% predicted (). By contrast, the majority of published studies of pharmacologic therapies such as bronchodilators and inhaled steroids for patients with COPD report mean FEV1 values of between 35% and 40% of predicted Citation[20-23]. Second, determination of maximum workload is not typically performed as a component of routine clinical evaluation of patients with COPD, and the clinical applicability and importance of a 10 Watt change in maximum exercise capacity requires clinical validation.
The determination of MCID for maximum exercise workload as outlined here highlights the ability to use data from large multi-center clinical trials to address this important issue. The NETT has additional outcome measures including healthstatus and dyspnea. Further investigations of data from NETT are planned to develop an anchor-based estimate of MCID for maximum exercise, and to use the methodology described above to determine MCID for other outcomes in COPD.
Acknowledgment
The authors wish to recognize Kathleen W. Wyrwich, Ph.D. for her discussions regarding statistical methodology.
REFERENCES
- Jones P W. St. George's Respiratory Questionnaire: MCID. J COPD 2005, this issue.
- Brown C D, Wise R A. Minimal clinically important differences in the six minute walk test and the incremental shuttle walking test. J COPD 2005, this issue.
- Schunemann H J, Puhan M, Goldstein R, Jaeschke R, Guyatt G H. Measurement properties and interpretability of the Chronic Respiratory Disease Questionnaire (CRQ). J COPD 2005, this issue.
- Ries A L. Minimally clinically important difference for the UCSD shortness of breath questionnaire, Borg Scale, and Visual Analog Scale. J COPD 2005, this issue.
- Casaburi R. Factors determining constant work rate exercise tolerance in COPD and their role in dictating the minimal clinically important difference in response to interventions. J COPD 2005, this issue.
- ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167:211–277. [CSA], [CROSSREF]
- Cotes J E. Rating respiratory disability: a report on behalf of a working group of the European society for clinical respiratory physiology. Eur Respir J 1990; 3:1074–1077. [PUBMED], [INFOTRIEVE]
- Cox N M, Hendrik J CM, Binkhorst R A, Folgering H TM, van Herwaarden C L. Reproducibility of incremental maximum cycle ergometer tests in patients with mild to moderate obstructive lung disease. Lung 1989; 167:129–133. [PUBMED], [INFOTRIEVE]
- Rationale and design of the National Emphysema Treatment Trial (NETT): a prospective randomized trial of lung volume reduction surgery. J Thorac Cardiovasc Surg 1999; 118:518–528.
- Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood D E. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001; 345:1075–1083. [CSA], [CROSSREF]
- Norman G R, Sloan J A, Wyrwich K W. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003; 41:582–592. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kazis L E, Anderson J J, Meenan R F. Effect sizes for interpreting changes in health status. Med Care 1989; 27:S178–S189. [PUBMED], [INFOTRIEVE]
- Wyrwich K W, Nienaber N A, Tierney W M, Wolinsky F D. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care 1999; 37:469–478. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Wyrwich K W, Tierney W M, Wolinsky F D. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999; 52:861–873. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wells G, Beaton D, Shea B, Boers M, Simon L, Strand V, Brooks P, Tugwell P. Minimal clinically important differences: review of methods. J Rheumatol 2001; 28:406–412. [PUBMED], [INFOTRIEVE]
- Guyatt G H, Osoba D, Wu A W, Wyrwich K W, Norman G R. Methods to explain the clinical significance of health status measures. Mayo Clin Proc 2002; 77:371–383. [PUBMED], [INFOTRIEVE], [CSA]
- Cox N J, Hendriks J C, Binkhorst R A, Folgering H T, van Herwaarden C L. Reproducibility of incremental maximum cycle ergometer tests in patients with mild to moderate obstructive lung diseases. Lung 1989; 167:129–133. [PUBMED], [INFOTRIEVE]
- Fabbri L M, Hurd S S. Global strategy for the diagnosis, management and prevention of COPD: 2003 update. Eur Respir J 2003; 22:1–2. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Calverley P, Pauwels R A, Vestbo J, Jones P W, Pride N B, Gulsvik A, Anderson J A, Maden C. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003; 361:449–456. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Casaburi R, Mahler D, Jones P W, Wanner A, Pedro G S, ZuWallack R L, Menjoge S S, Serby C W. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19:217–224. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Hanania N A, Darken P, Horstman D, Reisner C, Lee B, Davis S, Shah T. The efficacy and safety of fluticasone propionate/salmeterol combined in the Diskus inhaler for the treatment of COPD. Chest 2003; 124:834–843. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Burge P S, Calverley P MA, Spencer J S, Anderson J A, Maslen T K. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000; 320:1297–1303. [PUBMED], [INFOTRIEVE], [CROSSREF]