Abstract
In order to define the effects of chronic renal failure (CRF) in the progress of gingival inflammation, we studied 6 patients (4 male, 2 female) with CRF who were on chronic hemodialysis for 4.25 (range 1–15) years. Six healthy individuals, age and sex matched were used as controls. The protocol which we used comprised of two periods (a) a 40-day duration period of preparation and (b) a 28-day duration experimental period. During the (a) period, all subjects went through: (1) therapy of the chronic gingivitis and (2) complete control of dental plaque by oral hygiene. During the experimental period, all subjects were advised to avoid, for at least 21 days, any mechanical or chemical media of oral hygiene and went through photographing, recording of gingival index (GI), recording of plaque index (PlI), and the collection and quantification of gingival crevicular fluid (GCF). On the 21st day, root planning and polishing were performed and subjects were advised to carry out oral hygiene. On the 28th day, all previous examinations (GI, PlI, GCF) were repeated.
In both patients and controls, GI, PlI and GCF were increased on 7th, 14th and 21st day, without significant differences between the groups and returned to normal (close to zero point) on the 28th day.
There are no significant differences between patients with CRF and normal controls in the evolution of experimental gingivitis. Therefore, chronic uremia has no effect on the defense of periodontal tissue against microbial plaque.
INTRODUCTION
Periodontium (peri = around, odons-odontos = tooth) comprises of the following tissues: (a) the gingiva; (b) the periodontal ligament; (c) the root cementum and (d) the alveolar bone. Gingival and periodontal diseases are different forms of inflammatory diseases of the periodontium of varying severity. Gingivitis represents the initial early or established inflammatory lesion manifested at the gingival tissues. When the inflammation spreads to the remaining periodontal tissues with destruction of the periodontal ligament and alveolar bone, it is manifested as a form of periodontitis Citation[[1]].
Nowadays, it is known that the major and primary etiologic agents of the periodontal disease are the microorganisms of the dental plaque. Indirect-local agents (dental calculus, anatomic-morphologic agents, etc.) and endogenic causes that affect the defensive ability of periodontal tissue also take place Citation[2-5]. Among the endogenic causes are the diabetes mellitus, the granulocytopenia, the circular neutropenia, the Papillon-Lefevre syndrome, the Down syndrome, the autoimmune deficiency syndrome (AIDS), genetic and other hormone disorders, leukemias, autoimmune diseases (i.e. systemic scleroderma), several medications, non-healthy food and several other pathologic conditions Citation[6-13].)
According to the literature, periodontal disease is common in patients with chronic renal failure (CRF) Citation[14-24]. However, it is not known whether the high frequency of periodontal disease in this population is related to host alterations due to CRF or to the carelessness of oral hygiene. In the present study we investigated the influence of CRF in the development of experimental chronic gingivitis.
MATERIAL AND METHODS
We studied 6 patients (4 men and 2 women) with CRF due to chronic glomerulonephritis who were under chronic hemodialysis for a period of 4.25 (1–15) years and 6 healthy controls (3 men and 3 female). The age of controls was comparable to this of patients (34.33 ± 7.34 and 27.88 ± 9.9, respectively).
The selection of patients was based on the homogeneity of the severity of pre-existing gingivitis. We aimed at the absence of destroyed periodontal tissues in the selected subjects (depth of sacs: 1.96 ± 0.35 in patients, 1.96 ± 0.24 in controls; loss of adhesion: 0.21 ± 0.20 in patients, 0.22 ± 0.28 in controls, p > 0.05).
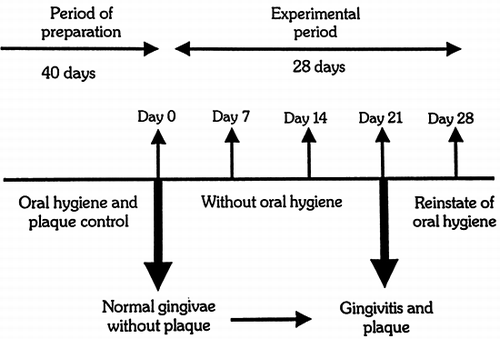
We used the LOE's model of experimental gingivitis Citation[[25]]. This model comprises of a 40-day period of preparation followed by a 28-day experimental period (). During the period of preparation, all subjects went through: (a) therapy of chronic gum inflammation and (b) complete control of plaque and teeth polishing every seven days, while they were given instructions on oral hygiene. With these actions, we ascertained that at the end of this period, an oral condition in all subjects that gingival index (GI) according to Löe-Silness Citation[[26]], plaque index (PlI) according to Silness and Löe Citation[[27]] and gingival crevicular fluid (GCF) tended to zero. The gingival inflammation is estimated by GI, while PlI is used to estimate the amount of dental plaque on the surface of the teeth Citation[26-27].
GCF is the serosity exudate in the area of gingival crevice, which exists in less quantity in normal gums and increases in inflamed gums. To collect the gingival fluid, we used strips of absorbent paper with the special technique of Löe and Holm-Pederson, as modified by Mamm Citation[28-29], as following. We placed the strips at the entrance of gingival crevice at a central point in the 6 front teeth of the upper jaw and left them for 5 minutes. To estimate the quantity of the gingival fluid, the 6 strips were put in a plate and dried in an oven (66°C for 15 minutes), then dipped in alcoholic dilution of ninhydrine 0.2% and were re-dried. The part of every strip that was moistened with gingival fluid was now coloured and measured in a stereoscopic microscope Olympus in magnification × 40.
In the beginning of the experimental period, patients and controls were advised to refrain from any mechanical or chemical media use for cleansing plaque for 21 days and keep their diet stable. Over the same period and every 7 days, they went through: photographing, indexing of GI, indexing of PlI and an estimation of the quantity of GCF. On the 21st day, after all previous indexing, they went through scaling and teeth polishing and were advised to carry out oral hygiene again. Seven days later (28th day), all previous indexing was repeated.
RESULTS
The results of the progress of the three indexes for both groups (patients and controls) at different times are shown in Tables
Table 1. Progress of Gingival Index (GI) in experimental gingivitis
Table 2. Progress of Plaque Index (PLI) in experimental gingivitis
Table 3. Comparison of Gingival Crevicular Fluid (GCF) production between patients and controls groups
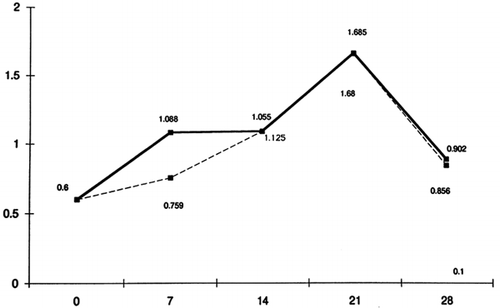
More specifically, in , the mean grades of GI are shown on the respective days (0, 7, 14, 21, 28) in patients and controls. It is shown that on the 1st day, the mean grade of GI in both groups tends to zero, while on the 7th day, the 14th and the 21st day, the mean grades rise. From the 21st to the 28th day, which is the period that oral hygiene is carried out again, the mean grade is lower in both groups and returns to about its initial levels.
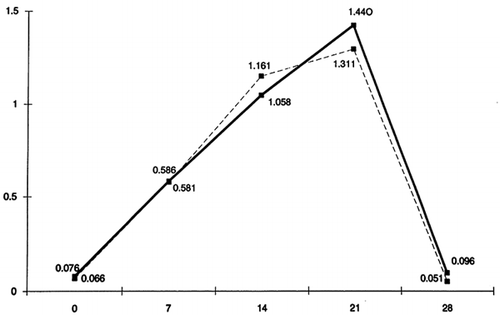
The mean grades of PlI on the respective days (0, 7, 14, 21, 28) in patients and controls are shown in . It is observed that on the 1st day, the mean grade of the index in both groups tends to zero, while on the 7th day, the 14th day and the 21st day, the mean grades of the index rise. On the 28th day, the grades of the index return to about its initial levels.
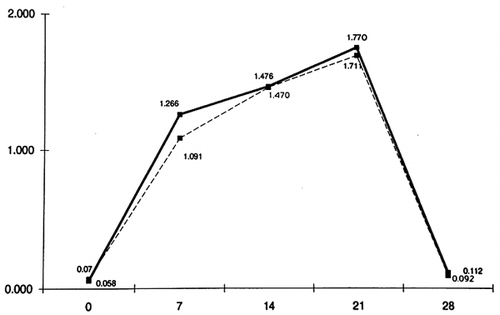
The mean grades of GCF quantity collected on the days 0, 7, 14, 21 and 28 from patients and controls are shown in . A rise in the grades is observed in both groups as the days pass by, and returns to about the initial levels on the 28th day.
The progress of GI, PlI and GCF quantity is shown in .
Due to the small number of subjects (6 subjects in every group), statistical analysis was based on non-parametric statistic methods, with the use of Ranks. That is, the initial counts were substituted with respective Ranks.
The first step in analysing was the estimation of Spearman's correlation coefficient for every pair of variables. The values of correlation coefficients are shown in . It is obvious from the analysis that for p<0.01, the variables of the table do not correlate among them.
Table 4. Rank correlation among GI, PLI and GCF
Based on the previous, for every index, we come to a model of analysis of variance by two agents with an observation in every cell. These two agents are: (a) the agent “DAY OF EXAMINATION” with five levels (0, 7, 14, 21 and 28) and (b) the agent “SUBJECTS” with two levels (P and C), while our model is: D1 = m + SUBJECTS + DAY OF EXAMINATION + E Citation[[1]].
In the previous model, m = real (unknown to us) the mean value of the specific index D in the population (in this study population is meant by the number of the subjects without distinction between patients and controls) and E = random error.
The analysis of the model Citation[[1]] for the three indexes D1 (GI), D2 (PlI) and D3 (GCF) is also shown in Tables correspondingly and that the three indexes do not present a statistically significant difference between patients and controls in days 0,7,14,21 and 28. However, there are statistically significant differences in the values among the days of subsequent evaluations for all indexes at the level of p<0.0001.
Table 5. Analysis of variance for Ranks of GI
Table 6. Analysis of variance for Ranks of P1I
Table 7. Analysis of variance for Ranks of GCF
Therefore, based on these tables we can conclude that:
there are no statistically significant differences between patients and controls for all three indexes
there are statistically significant differences among the days of subsequent evaluations for all three indexes.
DISCUSSION
We selected subjects (patients and controls) that did not present destroyed periodontal tissues, since our aim was to study the effect of plaque on the inflammatory reaction of the tissues only, which is gingivitis. Furthermore, in order to ensure similar conditions, we aimed at the same severity of pre-existing gingivitis on the selected subjects.
Following this process and methodology, we tried to evaluate the effect of the endogenic agent, and more specifically CRF, in the defensive ability of gingival tissues against the hazardous factor, which is plaque.
From the results of this experimental study, we can conclude that there are no statistically significant differences between patients and controls in the whole progress of experimental gingivitis. On the 1st day of the experiment, in both patients and controls, the GI, the PlI and the counted quantity of GCF tend to zero, while on the 7th, the 14th and the 21st day, the above values rise without a significant difference between the two groups of subjects. On the 28th day and after having carried out oral hygiene for 7 days, the indexes recur at about the initial levels in both groups. Therefore, we can support the notion that chronic uremia does not affect the defense of gingival tissues against plaque and that gingivitis has the same progress in patients as it has in controls as well.
To the best of our knowledge, no other study uses any similar experiment in uremic patients. There are, however, studies reporting on the periodontal condition of patients with CRF, patients under hemodialysis and transplanted.
In a study performed in 1992 by Rahman et al. Citation[[14]], the periodontal condition of 54 transplant patients and 52 patients with CRF under hemodialysis was compared with that of healthy controls. It was found that, both groups of patients had significantly higher plaque index than controls. There was not any significant difference however, between hemodialysis patients and controls in periodontal index and the pocket depths, and also no significant differences among the three groups were found considering the bleeding index and gingival index as well. This study confirms our conclusions, that chronic uremia does not affect the defense of periodontal tissues against plaque. The higher plaque index, which is present in hemodialysis patients in the study of Rahman et al. Citation[[14]], is possibly due to the negligence of the uremic patients with oral hygiene. Galili et al. Citation[[30]] came to the same conclusion, when studied the moods of hemodialysis patients for oral hygiene. In this study, 25 patients and 25 controls were studied and it was found that the patients had significantly a lower mood for oral care than that of controls and less interest for oral hygiene as well. In another study, Wolff et al. Citation[[31]] evaluated the oral condition of 30 children with CRF and compared their conclusions with normal children. It was found that patients presented poor oral hygiene and gingival condition. In the same period, Tollfsen and Johansen Citation[[16]] studied 65 patients (under hemodialysis and transplant) aged 17 to 68 years old. They observed that patients under hemodialysis had significantly more plaque than the transplant ones and the mean gingival index was significantly higher in the hemodialysis patients.
On the contrary, in another study involving 52 children with CRF which was compared with 52 normal ones Citation[[17]], it was ascertained that PlI was similar in both groups, GI lower in the group of patients and tartar index higher in CRF group.
Recently, we studied 51 hemodialysis patients, aged 16–74. It was observed that GI for the ages of 35–64 years was significantly higher in patients than in normal patients, while no difference was observed for the ages above 65 years, where both groups had higher index (unpublished data). The oral hygiene index was significantly higher in patients aged 35–45 years, while there was no statistically significant difference in patients aged above 55 years. It was also found that the majority of hemodialysis patients do not brush their teeth often, while a large number of patients visited a dentist only under acute pain.
Therefore, the observed in several studies worsening of periodontal tissue condition is due to negligence of oral hygiene (high PlI, etc.) rather than of chronic uremia, since patients meet controls in oral hygiene conditions and start from almost no gingival inflammation, they develop gingivitis without significant differences between the two groups.
ACKNOWLEDGMENT
We wish to express our thanks to the statistician Constantine Karakostas, Associate Professor of the University of Ioannina, Greece.
REFERENCES
- Taichman N, Lindhe J. Pathogenesis of plaque associated periodontal disease. Textbook of Clinical Periodontology, J Lindhe. 2nd Ed., Munksgaard. 1989; 153–190
- Suomi J D, Greene J C, Vermillion J R, Doyle J J, Leatherwood E C. The effect of controlled disease in adults: Results after third and final year. J Periodontol 1971; 42: 152–160
- Ramgjord S P, Knowles J W, Nissle R R, et al. Longitudinal study of periodontal therapy. J Periodontol 1973; 44: 66–77
- Lindhe J, Hamp S E, Löe H. Plaque induced periodontal disease in beagle dogs. A 4-year clinical, roentgenographical and histometric study. J Periodontol Res 1975; 10: 243–255
- Carlsson J. Microbiology Of Plaque-associated Periodontal Disease. Textbook of Clinical Periodontology, J Lindhe. 2nd, Munksgaard. 1989; 129–149
- Page R C, Schroeder H E. Pathogenesis of chronic inflammatory periodontal disease. Lab Invest 1976; 33: 235–249
- Pindborg J J, Holmstrup P. Necrotizing gingivitis related to human immuno deficiency virus (HIV) infection. Afr Dent J 1987; 1: 5–8
- Faulconbridge A R, Bradshaw W CL, Jenkins P A, Baum J B. The dental status of a group of diabetic children. Br Dent J 1981; 151: 253–255
- Pankhurst G L, Waite I M, Hicks K A, Allen Y, Harkness R D. The influence of oral contraceptives therapy on the periodontium – duration of drug therapy. J Periodontol 1981; 52: 617–620
- Pindborg J. Mannifestation of systemic disorders in the periodontium. Textbook of Clinical Periodontology, J Lindhe. 2nd, Munksgaard. 1989; 282–296
- Green L W, Tryon W W, Marks B, Huryn J. Periodontal disease as a function of life events stress. J Stress 1986; 12: 32–46
- Seymour L A, Heasman P A. Drugs and the periodontium. J Clin Periodontol 1988; 15: 1–6
- Comboli M, Mitsis F. General or endogenic factor. Periodontology, F Mitsis. Litsas Med. Pubs, AthensGreece 1990; 131–154
- Rahman M M, Caglayan F, Rahman B. Periodontal health parameters in patients with chronic renal failure and renal transplants receiving immunosuppressive therapy. J Nihon University School of Medicine 1992; 34: 265–272
- Yamalik N, Delibasi L, Guelay H, Caglayan F, Haberal M, Caglayan G. The histological investigation of gingiva from patients with chronic renal failure, renal transplants and periodontitis' hight and electron microscopic study. J Periodontol 1991; 62: 737–744
- Tollefsen T, Johansen J R. Periodontal status in patients before and after renal allotransplantation. J Periodont Res 1985; 20: 227–236
- Jaff C E, Chantler C, Carter E J. Dental findings in chronic renal failure. Br Dent J 1986; 160: 18–20
- Regolati B. Ammonia and urea in oral pathophysiology - Aliterature review. Helv Odont Acta 1971; 15(Suppl. VII)139–146
- Culter L S. Evaluation and management ofthe dental patient with renal failure, renal transplant or on renal dialysis. J Conn State Dent Assoc 1988; 62: 194–198
- Rivas M A, Carl W, Albert D J. Chronic hemodialysis and dental disease. Proc Clin Dial Transplant Forum 1972; 2: 63–66
- Nylud S, Oatis G W, Jr. Oral physiology in end-stage renal disease. US Navy Med 1984; 75: 22–25
- Vlachou A, Kakali A, Oulis KI. Dental problems and treatment of children under chronic periodic hemodialysis. J Paed Dent Med (Greek) 1992; 6(2)61–67
- Clark D B. Dental findings in patients with chronic renal failure. Can Dent Assoc J 1987; 53: 781–785
- Eigner T L, Jasta J KT, Benett W M. Achieving oral health in patients with renal failure and renal transplants. JADA 1986; 113: 612–616
- Löe H, Theilade E, Broglum-Jensen S. Experimental gingivitis in man. J Periodont 1965; 36: 177–187
- Löe H, Silness J. Periodontal disease in pregnancy. Acta Odontol Scand 1963; 21: 533–551
- Silness P, Löe H. Periodontal disease in pregnancy. Acta Odont Scand 1964; 22: 121–135
- Holm-Pedersen P, Löe H. Flow of gingival exudate as related to menstruation and pregnancy. J Periodont Res 1967; 2: 13–20
- Egelberg J, Attstrom R. Comparison between orifice and intra crevicular methods of sampling gingival fluid. J Periodont Res 1973; 8: 384–388
- Galili D, Kaufman E, Leviner E, Lowental U. The attitude of chronic hemodialysis patients toward dental treatment. Oral Surg, Oral Med, Oral Pathol 1983; 56: 602–604
- Wolff A, Stark H, Sarnat H, Binderman I, Eisenstein B, Drukker A. The dental status of children with chronic renal failure. Int J Pediatr Nephrol 1985; 6: 127–132