Abstract
Introduction: The objective of this study was to evaluate the effects of dopexamine on renal function in 4 groups of patients either with or without renal dysfunction. Transient renal dysfunction is often not clinically relevant in patients with normal renal function, but it is an important clinical factor in patients with pre-existing renal failure. Dopexamine (DX) is a commonly used catecholamine which probably exerts a selective effect at the splanchnic bed. Material and Methods: 24 patients with normal renal function and 24 patients with impaired renal function (creatinine in serum ≥ 1.5 mg/dL) were each randomly allocated to 2 groups. Group 1 (control) without renal dysfunction and group 3 (control/dysfunction) with renal dysfunction were considered as control groups, while the patients in DX and DX/dysfunction groups received 1 μg/kg/min dopexamine until the end of surgery. Kidney function was investigated using standard parameters and by investigating specific proteins and enzymes. Results: All patients showed pathologic excretions of the investigated parameters during cardiopulmonary bypass (CPB) with no differences between the study groups. The distal tubule, the lysosomal regions, Henle's loop and the glomerular tuft were all damaged. Heart rate and cardiac index increased significantly in the DX-groups, first until the end of surgery, second until the start of ECC. Conclusion: Dopexamine at a dose of 1 μg/kg/min had no influence on renal function and protein excretion and cannot be regarded as a kidney function protecting substance.
INTRODUCTION
Transient renal dysfunctions often occur after cardiac surgery, but fortunately most are associated with no clinical consequences like dialytic therapies. Extracorporeal circulation (ECC) can be regarded almost as a standard model for inducing transient damage to the kidneys, a model which has been described in various studies Citation[1-2]. Even though the cause of damage has not yet been completely resolved, trauma to blood cells induced by the pumping system, hypothermia, and non-pulsatile blood flow during ECC are all suspected as being responsible for inducing pathological levels of kidney enzymes and protein excretion Citation[[3]].
Dopexamine is a commonly used catecholamine which acts in the first line by stimulating dopaminergic and β2-adrenoreceptors. It also probably exerts a selective vasodilating effect at the splanchnic bed and the kidney Citation[4-5].
The spectrum of parameters investigated by us included glomerular filtration rate (GFR, creatinine clearance, CCrea), angiotensinase A (ATA) as a specific parameter for glomerular structure, and N-acetyl-β-D-glucosaminidase (NAG) as a specific lysosomal marker. Tubular reabsorption was investigated by measuring the low-molecular-weight protein α1-microglobulin. Tamm-Horsfall-protein is a marker of loop of Henle integrity and the high-molecular-weight immunoglobulin G is a marker for glomerular integrity.
Even though some studies have proven that in addition to increasing renal plasma flow and GFR, dopexamine can also reduce the incidences of ARF and mortality Citation[[6]], no studies have been carried out yet on the renal-protective activity of dopexamine in coronary artery bypass grafting (CABG) patients. This study was designed to show the possible effects of dopexamine in patients with renal dysfunction.
MATERIALS AND METHODS
After approval by the ethical committee, and acquisition of written informed consent, 48 male patients were studied. Before surgery they were randomly allocated into 2 study groups with normal renal function (control and DX), and 2 groups with preexisting renal failure (control/dysfunction and DX/dysfunction, creatinine in serum ≥ 1.5 mg/dL. Dopexamine was commenced after induction at a dose of 1 mg/kg/min until the end of surgery.
The criteria for entering the study were a planned aorto-coronary bypass operation as well as the absence of hepatic dysfunction. Contrast media given during the previous 2 weeks was an exclusion criterium because of pathologic excretions of renal proteins.
All patients were premedicated using 2 mg flunitrazepam and 5 to 10 mg morphine orally. Induction and maintenance of anesthesia was comparable for all patients concerning only the weight-corrected doses of sufentanil, midazolam and pancuronium bromide for optimized standardization. All patients were mechanically ventilated during the operation period with an inspired oxygen concentration of 1.0 a normal I:E ratio, and a PEEP lower than 8 cm H2O. All patients were monitored using a radial arterial, a pulmonary arterial and a urinary catheter in situ. Intraoperative fluid replacement was performed using 20 mL/kg Ringers's solution.
A membrane oxygenator (Sorin 41, Turin, Italy) was installed for ECC with a nonpulsatile flow of 2.4 L/min/m2 and reduced flow of 2.0 L/min/m2 during hypothermia. Electrolyte solutions and albumin were used to prime the oxygenator circuit and moderate hypothermia (lowest rectal temperature during ECC was 32.7 ± 2.3°C) was applied. Anticoagulation was achieved using an initial 300 U/kg dose of sodium heparin before cannulation of the vena cava and was monitored by measuring the activated clotting time (ACT). None of the patients received aprotinin during the intraoperative or postoperative course. Venous blood was drained into the extracorporeal circuit via a two-stage cannula. A hemofiltration technique (HF-80, Fresenius, Bad Homburg, Germany) was used 15 min after the onset of ECC after need to concentrate the circulating blood. At the end of ECC, plasma heparin was neutralized using protamine (1 mg of protamine per 100 units of heparin). Mean perfusion pressure was between 40 and 60 mm Hg.
Hemodynamic parameters such as arterial pressure (AP), central venous pressure (CVP), pulmonary arterial pressure (PAP), pulmonary capillary wedge pressure (PCWP), cardiac output (CO) and urinary output were determined at all measurement points.
Blood and urine samples were collected preoperative, after induction, after start of ECC, after ECC, at the end of surgery, and at the 1st postoperative day. Arterial blood samples were taken for analysis of sodium, potassium and creatinine. Urine samples were taken to analyze osmolality, angiotensinase A (ATA), N-acetyl-β-D-glucosaminidase (NAG), α1-microglobulin (α1- Mg), Tamm-Horsfall-protein (THp) and immunoglobulin G (IgG). Urine collection periods for the measurement of creatinine clearance and excretion of THp were 24 hours.
The concentrations of proteins detected in urine were related to the excreted amount of creatinine (per g of creatinine) for comparing different rates of diuresis and for comparisons with published data.
The urine samples were centrifuged for 10 min at 3000 rpm in a Hettich rotixa/KS centrifuge. Depending on the parameter being investigated, samples were separated into aliquots and then either frozen at −20°C (for NAG) or stored at 4°C for a maximum of 7 days (for ATA, α1- mg, THp, IgG).
Angiotensinase A (ATA)
ATA as a specific parameter of the glomerular tuft was taken from urine and analyzed photometrically. Briefly, L-α-glutamyl-4-nitroanilide is cleaved hydrolytically by angiotensinase A to 4-nitroaniline and glutamic acid before photometric measurement of nitroaniline at 405 nm Citation[[7]].
N-Acetyl-β-D-glucosaminidase (NAG)
N-Acetyl-β-D-glucosaminidase (NAG) is a sensitive marker of lysosomal tubular damage Citation[[8]]. Briefly, 3-cresolsulfonphthaleinyl-N-acetyl-β-D-glucosaminide is hydrolyzed by N-acetyl-β-D-glucosaminidase (NAG) so that 3-cresolsulfonphthalein is released, a compound which can be measured photometrically at 580 nm. Sodium bicarbonate was added to stop the reaction Citation[[9]].
α-1- Microglobulin (α-1- MG), Tamm–Horsfall-Protein (THp), and Immunoglobulin G (IgG)
α1-Microglobulin is a specific parameter for indicating distal kidney tubular damage Citation[[10]] while normal levels of Tamm-Horsfall-protein level and immunoglobulin G are markers for undisturbed function of either the thick ascending limb of Henle's loop Citation[[11]] or the glomerula, respectively. α1- MG is measured in urine and serum by immunenephelometry (Behring-Nephelometer-Analyzer, Marburg), techniques described elsewhere in detail Citation[[12]]: the proteins form immunecomplexes with their corresponding antiserums, and concentration is determined photometrically. ELISA was used for measuring THp in urine (Elias, Freiburg), whereas immunenephelometry was used for determining the IgG concentration in serum and urine (according to 12).
Creatinine (Serum and Urine) and Urine Osmolality
Creatinine in serum and urine was measured using an autoanalyzer (type 30 R, WAKO Chemicals, Neuss). Creatinine clearance was calculated using a standard formula Citation[[13]]. Osmolality was measured by freezing point depression (Digital-Osmometer, Vogel, Giessen, Germany).
Statistics
Data are expressed as means ± standard deviations. Inter-group comparisons of patient characteristics, hemodynamic data etc. were performed using one-way analysis of variance. For inter- and intra-group comparisons of laboratory data, two-way repeated measures analysis of variance and post-hoc tests were used. A probability value less than 0.05 was considered significant.
RESULTS
All patients investigated were transferred successfully to outside hospitals for further treatment within 10 days after surgery without any clinical signs of or renal failure or the need for dialytic therapy. The 4 groups were comparable regarding biometric data, preoperatively at coronary angiography investigated hemodynamic data (EF, LVDEP), accompanying medication (with antidiabetic medication only in control/dysfunction and DX/dysfunction groups), the duration of surgery and extracorporeal circulation (ECC), and the retransfused blood volume ().
Table 1. Patients' Biometric Data, Preoperative Hemodynamics, Medication, Duration of Surgery, ECC, and Retransfused Blood Volume
After induction of anesthesia, the MAP increased in both control groups (control and control/dysfunction) by 8% and in both DX groups (Citation[[2]] and Citation[[4]]) by 7%. After ECC, the average MAP had decreased by 15% in all groups and returned to baseline by the end of surgery. Heart rate decreased in all groups after induction of anesthesia until ECC was started. At the end of ECC, significant differences became evident between patients without DX and those treated with DX (control: 50 ± 16 beats per minute, DX: 77 ± 13 bpm, p = 0.002; control/dysfunction: 58 ± 12 bpm; DX/dysfunction: 79 ± 14 bpm; p<0.01). These differences remained until surgery was finished (p = 0.001 and p = 0.05). CVP did not change after induction of anesthesia, neither over time nor amongst the groups; the same applied to the pulmonary arterial pressure (PAP) and pulmonary capillary wedge pressure (PCWP).
Cardiac index (CI) increased significantly in the DX-groups until the start of ECC by approximately 40% (differences between the control groups (Citation[[1]] and Citation[[3]]) and their respective DX groups (Citation[[2]] and Citation[[4]]) were both p = 0.001). It then decreased to baseline after ECC and increased significantly at the end of surgery by 25 % (respective differences for groups 1 and 3 versus groups 2 and 4 were both p = 0.002). The total peripheral resistance and the pulmonary resistance decreased until the start of ECC and at that time showed significant differences compared to the groups without DX-treatment (SVR: p = 0.01; PVR: p = 0.048). ST-changes were seen in some patients, but no medical interventions were necessary.
Urine excretion increased in DX groups (Citation[[2]] and Citation[[4]]) until the end of surgery and decreased to baseline by the 1st postoperative day. Creatinine clearance did not change over time or between the groups. The colloidal osmotic pressure in serum increased steadily until the 1st postoperative day in all groups; there was no difference between the groups. No differences were detected in urine osmolality either over time or between the groups ().
Table 2. Changes in Urine Volume, Urine Osmolality, and Creatinine Clearance
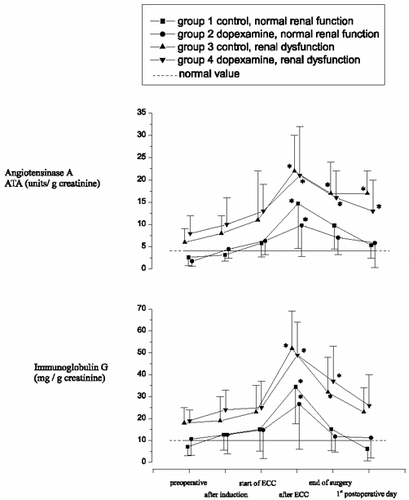
THp excretion decreased on the day of surgery in all groups and reached baseline values by the 1st postoperative day in both groups without renal dysfunction ().
Figure 2. Changes in excretions of N-acetyl-β-D-glucosaminidase, α1-microglobulin, and Tamm-Horsfall-protein in urine.
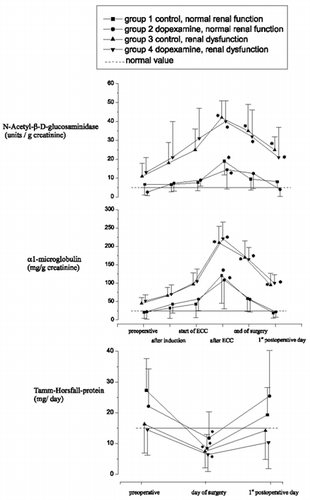
The excretion of N-Acetyl-β-D-glucosaminidase increased until the end of ECC before it returned to normal levels by the 1st postoperative day for groups 1 and 2 (). For groups 3 and 4, the changes were somewhat larger (and significantly so), and although by the first postoperative day the return was not complete, excretion levels appeared to be on their way back to normal. DX treatment did not exert any effect whether dysfunction was present or not. Of all the investigated proteins and enzymes, excretion of α1-microglobulin was increased the most during ECC. It was also the parameter that showed the largest response differences between the control and dysfunction groups ().
Immunoglobulin G significantly increased during ECC and reached baseline levels by the first postoperative day in the control group. An almost identical pattern was seen with DX treatment (). As with NAG, the changes were similar but more exaggerated (i.e. significantly larger) in patients with dysfunction, whether DX was present or not. Return was also incomplete by the 1st postoperative day in patients with dysfunction, although levels appeared to be on their way to normalization. Serum immunoglobulin G concentrations remained at normal levels over the entire study period for all groups (data not presented).
Patterns of angiotensinase A were also similar to those of NAG and immunoglobulin G, except that after the levels increased during ECC, baseline values were not achieved by the 1st postoperative day even in the absence of dysfunction (). Once again, increases during ECC were more prominent in the presence of dysfunction, and dopexamine did not alter the response whether dysfunction was present or not.
DISCUSSION
Open heart surgery with extracorporeal circulation (ECC) causes transient damage to the kidneys, an effect which has often been described using only insensitive parameters in various studies Citation[[1]], Citation[14-15]. Preexisting renal dysfunction is accompanied by extended stays in the ICU and hospital. For this relevant clinical problem a prophylactic substance for preventing renal dysfunction is clearly needed.
α1-microglobulin (α1- MG) and Tamm-Horsfall-protein (THp) were measured to reveal structural alterations in non-glomerular regions. Our results indicated localized damage during CPB in all groups, whether initial dysfunction was present or not. This was reflected as a decreased capacity to reabsorb low molecular weight proteins such as α1- MG and a decreased secretion rate of THp as a protein of the thick ascending loop of Henle (TALH). α1- MG is one of the best markers for disturbed tubular function. Increasing excretion rates of this very sensitive marker are often seen before reductions in serum creatinine and generalized proteinuria are observed Citation[[16]]. The increased urinary excretion of low-molecular weight proteins suggests a progressive loss of tubular function on the one hand, while the changes in THp secretion seem to indicate that the whole of the nephron then becomes involved.
N-acetyl-β-D-glucosaminidase (NAG) is a specific lysosomal marker and is present in large amounts in renal tubules. Increased excretion rates are seen in patients treated with specific tubolotoxic agents such as aminoglycosides. NAG is in fact one of the most sensitive parameters for indicating renal damage Citation[17-19]. The fact that this parameter seemed to be more sensitive towards ECC induced renal damage than the other parameters may indicate that the ECC had preferentially affected the tubules (). Furthermore, changes in NAG were much greater in the presence of preexisting renal dysfunction than they were in its absence.
The activity of angiotensinase A (ATA), a glycoprotein of the glomerular tuft and of the proximal tubule microvilli, was analyzed as an indicator primarily of glomerular damage. Our results indicated clear increases in this parameter during ECC which recovered again after the procedure, albeit incompletely. The large extent of this response probably reflected shedding of the ATA from the glomeruli into the urinary filtrate, although one cannot rule out that some of this response arose from ATA shedded from the proximal tubules. The extent of the response to ECC was the same whether dysfunction was present or not (unlike the situation with NAG).
The passage of macromolecules across the glomerular filtration barrier depends upon the transglomerular pressure gradient as well as the size and charge of the circulating molecules. Alterations in the size and charge selectivity of the glomerular filter have been inferred from measurements made on albumin and immunoglobulin G secretion in the early stages of diabetic renal disease Citation[[20]]. The changes in immunoglobulin G we observed suggest also that glomerular function was compromised with preexisting renal damage, and that ECC exacerbated this problem.
Damage to cell structures particularly in the tubules and the loop of Henle should be prevented by an increase in splanchnic bed and renal plasma flow because of a direct action on the renal vascular and tubular DA-1-receptors Citation[[21]].
This was the effect we were actually hoping for when the 1 μg/kg/min dopexamine was applied to our patients. Dopexamine-dihydrochloride (DX) is a synthetic catecholamine with a structure similar to dopamine. It exerts a positive inotropic effect by indirectly stimulating β1 and directly stimulating β2-adrenoreceptors. It also induces peripheral vasodilatation by acting at β2 and dopamine receptors Citation[[22]]. In this way it improves kidney and splanchnic perfusion directly and in so doing acts as a diuretic Citation[[23]]. DX increases the effective renal plasma flow less than dopamine without changing renal vascular resistance, and also increases glomerular filtration rate Citation[[24]]. Our results, however, showed in general that dopexamine at the dose chosen was incapable of significantly altering any of the effects exerted by the ECC whether kidney dysfunction was already present or not. Compared to dopamine, DX acts more weakly at the DA-1-receptor (dopamine three times higher), but it is 60 times more effective at the β2-adrenoreceptor Citation[[22]], Citation[[25]]. Given these facts, it was not unreasonable to expect that dopexamine might have acted in a renal-protective manner via a hemodynamic mechanism or direct action on β2-adrenoreceptors Citation[[26]].
A number of studies have described increased hemodynamic parameters following dopexamine at doses between 1 and 10 μg/kg/min Citation[27-28]. A significant decrease in peripheral resistance and significant increases in cardiac output and heart rate were already apparent at 1 μg/kg/min, i.e. the dose used by us Citation[[27]]. These data are consistent with ours since a decrease in peripheral resistance and an increase in cardiac index before ECC was detected in both the DX and DX/dysfunction groups: this observation also confirms the cardiovascular efficacy of the DX used in this study. The heart rate significantly increased after ECC and developed into tachycardia in some patients before it abated at the end of the DX application. Because of the limited ST-changes and the spontaneous remission of tachycardia after the end of dopexamine infusion, no interventions were necessary. Animal models have shown that DX exerts a renal-protective effect in dogs undergoing hemorrhagic shock Citation[[21]]. Measurements of various renal parameters such as GFR, urine, and sodium excretion showed that an organ recovery occurred in the DX-treated group, an effect which could be specifically inhibited by a DA-1 antagonist. These data suggest that the renal vasodilating, natriuretic and diuretic effects of DX as well as its ability to promote renal recovery are mediated by a direct effect on kidney blood vessels mediated via tubular DA-1 receptors Citation[[21]]. Patients with kidney dysfunction suffer from a vessel induced restriction of renal function. If such vessels were not able to react, the dysfunctioning kidneys could not be treated effectively by DX. This conclusion is supported by the fact that a lower increase of ATA was seen during CPB in the DX-group.
In summary, dopexamine at a dose of 1 μg/kg/min conferred no protection on the kidneys in open heart surgery patients with or without kidney dysfunction. Our results could not demonstrate the reduced damage and faster recovery of renal structures seen in animal models. Dopexamine at the dose applied only brought about increases in cardiac index, heart rate, and diuresis. According to our results, application of dopexamine to open heart surgery patients with preoperatively normal or slightly subnormal renal function does not seem to convey any advantages towards the kidneys and is therefore unnecessary.
REFERENCES
- Dehne M G, Boldt J, Heise D, et al. Tamm-Horsfall protein, alpha-1- and beta-2-microglobulin as kidney function markers in heart surgery. Anaesthesist 1995; 44: 545–551
- Sakakibara Y, Fukuda I, Koishizawa T, et al. Early detection and differential diagnosis of renal insufficiency following cardiac surgery–diagnostic value of free water clearance and fractional excretion of sodium. Nippon Kyobu Geka Gakkai Zasshi 1984; 32: 2060–2066
- Corwin H L, Sprague S M, Delaria G A, et al. Acute renal failure associated with cardiac operations. A case-control study. J Thorac Cardiovasc Surg 1989; 98: 1107–1112
- Fitton A, Benfield P. Dopexamine hydrochloride. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in acute cardiac insufficiency [published erratum appears in Drugs 1990 Aug;40(2):following Table of Contents]. Drugs 1990; 39: 308–330
- Jamison M, Widerhorn J, Weber L, et al. Central and renal hemodynamic effects of a new agonist at peripheral dopamine- and beta-2 adrenoreceptors (dopexamine) in patients with heart failure. Am Heart J 1989; 117: 607–614
- Garre D N Gomez, Farre A Lopez, Eleno N, et al. Comparative effects of dopexamine and dopamine on glycerol-induced acute renal failure in rats. Ren Fail 1996; 18: 59–68
- Scherberich J E. Aminopeptidase A (Angiotensinase A). Urinary Enzymes in Clinincal and Experimental Medicine, K Jung, H Mattenheimer, U Burchardt. Springer, Berlin 1992; 116–124
- McAuley F T, Simpson J G, Thomson A W, et al. The predictive value of enzymuria in cyclosporin A-induced renal toxicity in the rat. Toxicol Lett 1986; 32: 163–169
- Simane Z J. N-Acetyl-b-D-Glucosaminidase. Urinary Enzymes in Clinical and Experimental Medicine, K Jung, H Mattenheimer, U Burchardt. Springer, Berlin 1992; 118–124
- Itoh Y, Enomoto H, Takagi K, et al. Clinical usefulness of serum alpha 1-microglobulin as a sensitive indicator for renal insufficiency [letter]. Nephron 1983; 33: 69–70
- Kumar S, Muchmore A. Tamm-Horsfall protein-uromodulin (1950–1990). Kid Int 1990; 37: 1395–1401
- Dati F, Lammers M. Immunochemical Methods for Determination of Urinary Proteins in Kidney Disease. JFCC 1989; 1: 68–77
- Sharif M N, Kaushal R D, Iyer P, et al. Diltiazem potentiates angiotensin II-mediated renal prostacyclin synthesis. J Cardiovasc Pharmacol 1992; 20: 638–642
- Llopart T, Lombardi R, Forselledo M, Andrade R. Acute renal failure in open heart surgery. Ren Fail 1997; 19(2)319–323
- Sural S, Sharma R K, Singhal M, et al. Etilolgy, prognosis, and outcome of post-operative acute renal failure. Ren Fail 2000; 1: 87–97
- Itoh Y, Kawai T. Human alpha 1-microglobulin: its measurement and clinical significance. J Clin Lab Anal 1990; 4: 376–384
- Donadio C, Puccini R, Lucchesi A, et al. Urinary excretion of proteins and tubular enzymes in renal transplant patients. Ren Fail 1998; 20: 707–715
- Wedeen R P, Udasin I, Fiedler N, et al. Urinary biomarkers as indicators of renal disease. Ren Fail 1999; 21: 241–249
- Kumle B, Boldt J, Piper S, et al. The influence of different intravascular volume replacement regimens on renal function in the elderly. Anesth Analg 1999; 89: 1124–1130
- Viberti G. Prognostic significance of microalbuminuria. Am J Hypertens 1994; 7: 69S–72S
- Chintala M S, Lokhandwala M F, Jandhyala B S. Protective effects of dopexamine hydrochloride in renal failure after acute haemorrhage in anaesthetized dogs. J Auton Pharmacol 1990; 10(Suppl 1)95–102
- Smith G W, O'Connor S E. An introduction to the pharmacologic properties of Dopacard (dopexamine hydrochloride). Am J Cardiol 1988; 62: 9C–17C
- Brown R A, Dixon J, Farmer J B, et al. Dopexamine: a novel agonist at peripheral dopamine receptors and beta 2-adrenoceptors. Br J Pharmacol 1985; 85: 599–608
- Olsen N V, Lund J, Jensen P F, et al. Dopamine, dobutamine, and dopexamine. A comparison of renal effects in unanesthetized human volunteers. Anesthesiology 1993; 79: 685–694
- Olsen N V, Hansen J M, Ladefoged S D, et al. Renal tubular reabsorption of sodium and water during infusion of low-dose dopamine in normal man. Clin Sci Colch 1990; 78: 503–507
- MacGregor D A, Prielipp R C, Butterworth J F, et al. Relative efficacy and potency of beta-adrenoceptor agonists for generating cAMP in human lymphocytes. Chest 1996; 109: 194–200
- van der Starre P J, Rosseel P M. Dopexamine hydrochloride after coronary artery bypass grafting. Am J Cardiol 1988; 62: 78C–82C
- Jamison M, Widerhorn J, Weber L, et al. Central and renal hemodynamic effects of a new agonist at peripheral dopamine- and beta-2 adrenoreceptors (dopexamine) in patients with heart failure. Am Heart J 1989; 117: 607–614