Abstract
Secondary hyperparathyroidism is one of the most common complications of chronic renal failure (CRF). Its pathogenesis is multifactorial and still not completely understood. Pathological mechanism of hypocalcemia, hyperphosphatemia and calcitriol deficiency are basic characteristics of CRF and main reason for morphological changes in parathyroid glands and hyperparathyroidism (HP). We present a case of a female patient born in 1975. At the age of 10, a urinary infection was diagnosed for the first time and treated. Six years later, as nausea and vomiting started, CRF based on bilateral reflux was diagnosed and the patient was included in the hemodialysis treatment. The patient was again examined in 1997, when biochemical parameters, including the level of parathyroid hormone, ultrasonography of the neck, scintigraphy of the skeleton and densitometry revealed secondary HP. Parathyreoidectomy was perfomed in 1998. During the follow up period, a tumefaction on a ramus mandibulae dex. was noticed, which was cytologically diagnosed as osteitis fibrosa, “brown tumor”, a rare complication of the secondary HP. Surgery was performed and PHD was granuloma gigantocelulare. Prevention and therapy of secondary HP is a problem that demands early actions to avoid possible complications.
INTRODUCTION
Secondary hyperparathyroidism is only one among many complications of the renal insufficiency. Its pathogenesis is multifactorial and still not completely understood Citation[[1]]. Chronic renal failure (CRF) is characterized by parathyroid gland hyperplasia, which can increase up to 15 times from its normal size Citation[[2]].
The increased excretion of PTH is a basic factor causing parathyroid gland hyperplasia and secondary hyperparathyroidism associated with bone deformity.
CASE REPORT
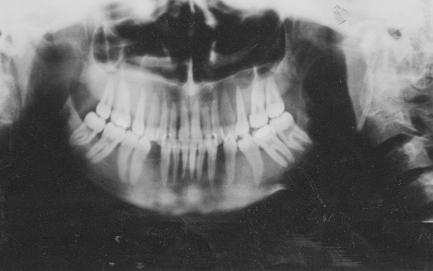
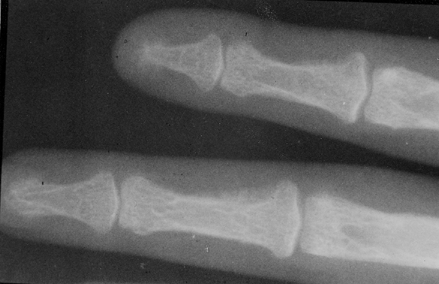
A 23-year-old female patient was admitted to our Hospital in 1997, in disturbed general condition and terminal stage of renal failure. The patient has been undergoing an intermittent chronic hemodialysis for six years. Significantly increased alkaline phosphatase and parathormone levels indicated the development of secondary hyperparathyroidism. Left side nephrectomy was performed in order to reduce the risk of common infections and prepare the patient for cadaveric transplantation. Also, on several occasions, parathyroid sclerosing therapy was used. Mandibula X-ray finding showed destroyed ramus mandibulae with a thin layer of cortex on both bone edges. Laboratory parameters are shown in . Parathyroidectomy was also performed.
Table 1. More Important Laboratory Results
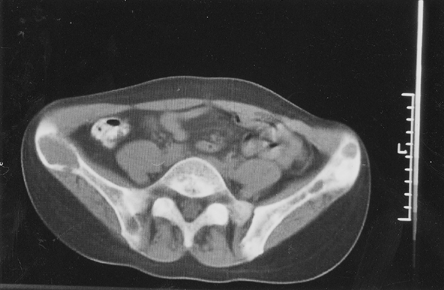
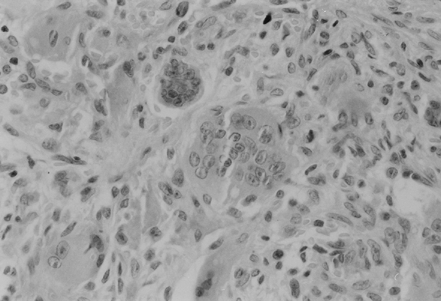
On the eighth day, swelling on the left facial side occurred, an asymmetry caused by cystic formation affecting the angulus and ramus of the left mandibula (). Cytologic punction supplied plenty of peripheral blood and a few giant multinucleated histiocytic cells (. Computed tomography (CT) of the lower jaw revealed a pathologic process affecting the whole ramus mandibula, bony tissue replaced by soft tumorous tissue, and bone substance by connective tissue. Scintigram and bone X-ray findings showed the presence of osteolytic foci resembling fibrous osteitis, and ramus mandibulae osteolytic lesion indicating the development of brown tumor. Bone parathyroid hormone matched renal osteodystrophy finding. Tumor excochleation was perfomed and bone parathyroid hormone indicated the presence of reparative gigantocellular granuloma – brown tumor (.
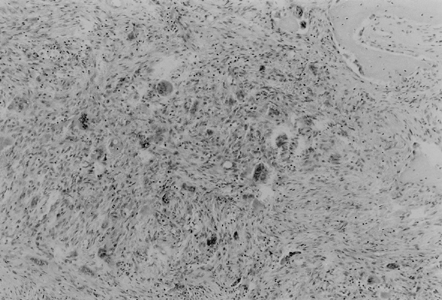
Figure 3. Trabecular bone replaced by brown tumor consisting of the fibroblastic stroma with areas of hemorrhage. Multiple giant cells scattered between the spindle cells (HE).
DISCUSSION
Parathyroid gland hyperplasia, predominantly major cells, can be present in chronic renal failure. Hypocalcemia, hyperphosphatemia, lack of calcitrol, active vitamin D metabolite, 1,25-hydroxycholecalciferol are characteristic for CRF but also basic factors of increased PTH secretion, causing morphological changes in parathyroid glands Citation[3-5]. Parathyroid gland membrane contains receptors for Ca and vitamin D Citation[6-7], while the number of receptors is significantly smaller in CRF Citation[8-10]. PTH reduces the reabsorption of the phosphorus and increases urinary excretion thus reducing the level of the serum phosphorus. Hyperphosphatemia directly reduces the ionized calcium, and PTH is a regulator of ionized Ca in the extracellular fluid Citation[[10]]. PTH changes the relation between the intracellular and extracellular calcium by changing the architecture of the cell membrane and leads to calcium sedimentation in the muscle tissue, cornea, blood vessels, heart, brain, peripheral nerves, and erythrocytes. Ca sedimentation in the miocard can cause arrhythmia and sudden death of dialysis patient. It often leads to left ventricle hypertrophy and dysfunction Citation[11-13].
PTH reduces the osmotic fragility of erythrocytes, causes bone marrow fibrosis and thus reduces the erythropoiesis Citation[14-16]. High level of PTH causes hormonal disequilibrium by stimulating the secretion of insulin, prolactin and aldosterone. It also reduces the lipolytic activity by increasing the cholesterol and triglyceride levels Citation[[17]].
In CRF, it is necessary to determine the intact PTH because of its immunoreactive fragments. Because of PTH complex activity on a whole range of organic systems, Massry set the hypothesis in 1983 that PTH could be an uremic toxin Citation[[18]]. The major effect of PTH is in changing the structure and architecture of bones by increasing the bone resorption and thus decreasing the mineral density of bones, by changing the osteoclastic activity as well as stimulating the osteocytes and causing osteolysis.
Clinical signs of secondary hyperparathyroidism are manifested through bone pain, common fractures, necrosis or calcifications of soft tissue, arthritis, periarthritis and marked pruritis. In CRF, alkaline phosphatase is significantly increased. Pathological mechanism of Ca, P, calcitrol and alkaline phosphatase lead to renal osteodystrophia with characteristic hystological signs of osteitis fibrosa cystica Citation[[12]]. Radiographical signs can be seen in 50% of dialysis patients including the supperiostal intracortical reabsorption, expressed cystic formations and osteosclerosis most commonly on femur, ulna, tibia, mandibula and distal part of clavicula. Changes on mandibula are manifested as the development of brown tumor. Brown tumor consists of giant cells and brown hemosiderin deposits Citation[[19]]. Disturbed bone structure leads to aseptic necrosis, and in some cases beta2 microglobuline sedimentation, thus increasing the bone structure changes.
In our patient PTH was 15 times greater than the normal ranges, with low calcium, high phosphorus and alkaline phosphatase levels, which all contributed to clinical picture of severe renal osteodystrophia. The loss of bone density changes the bone architecture leading to classical picture of osteopenia and osteodystrophy, common complications in patients undergoing hemodialysis.
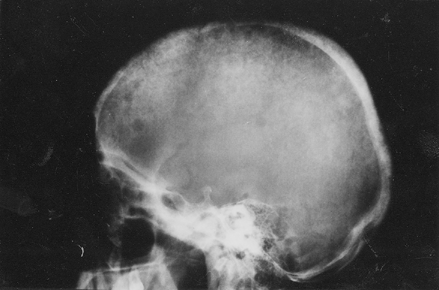
In all 27 years of work at the Department for Hemodialysis, this was the first case of secondary hyperparathyroidism combined with brown tumor. Atypical localization on the cranium base in the sphenoid sinus area can even cause a neurological deficit and develop neurological picture. Surgical procedure is the only correct therapy, and according to numerours authors, 10% of patients on hemodialysis require paratireoctomia. Postoperative complications such as disturbed homeostasis of electrolites and “hungry bone” syndrome are common.
In cases of severe ostitis fibrosa cystica, bone mineral deficiency can be significant, and after parathyroidectomy, blood calcium levels can fall to the hypocalcemic level and remain depressed for days if calcium replacement is inadequate.
Increased bone cellularity in severe osteitis fibrosa cystica involves both osteoblastic and osteoclastic cells. High levels of PTH enhance bone-blood exchange, with resorption favored over formation an abrupt decrease in PTH levels with surgery leaves bone formation favored over resorption. Calcium loss from blood is increased, and temporary hyporesponsiveness of bone to the boneresorbing actions of PTH (lowered hormone levels after parathyroid surgery in the presence of receptor downregulation) may add to the imbalance between bone resorption and bone formation. Treatment may require parenteral administration of calcium: addition of calcitrol and oral calcium supplementation may hasten the ability to withdraw parenteral calcium supplementation and/or reduce the amount needed.
REFERENCES
- Ferreira A. Biochemical markers of bone turnover in the diagnosis of renal osteodystrophy: what do we have, what do we need?. Nephrol Dial Transplant 1998; 3(13 supp)29–32
- Drüeke T B. The pathogenesis of parathyroid gland hyperplasia in chronic renal failure. Kidney Int 1995; 48: 259–272
- Geoffrey A B, Tempie E, Hulbert S, Nathan W L, Friedrich K. Association of serum phosphorus and calcium x phosphate. Product with mortallity risk in chronic hemodialysis patients; A National Study. Am J Kidney Dis 1998; 31(4)607–617
- Hruska K A. Renal osteodystrophy. Baillieres Clin Endocrinol Metab 1997; 11: 165–194
- Goodman W G, Veldhuis J D, Belin T R, Juppner H, Salusky I B. Suppressive effect of calcium on parathyroid hormone releaser in adynamic renal osteodystrophy and secondary hyperparathyroidism. Kidney Int 1997; 51: 1590–1595
- Hsu C H. Are we mismanaging calcium and phosphate metabolism in renal failure. Am J Kidney Dis 1997; 29(4)641–649
- Hory B, Drüeke T B. The parathyroid bone axis in uremia: new insights into old questions. Cur Opin Nephrol Hypertens 1997; 6: 40–48
- Nagaba Y, Heishi M, Tazawa H, Tsukamoto Y, Kobayashi Y. Vitamin D receptor gene polymorphisms affect secondary hyperparathyroidism in hemodialyzed patients. Am J Kidney Dis 1998; 32: 464–469
- Caravaca F, Cubero J, Jimenez F, Lopewz A, Aparicio M, Cid L, Pisarro J, Liso S I. Effect of the mode of calcitriol administration on PTH ionized calcium relationship in uraemic patient with secondary hyperparathyroidism. Nephrol Dial Transplant 1995; 10: 665–670
- Fournier A E, Arnaud C D, Johnson W J, Taylor W F, Goldsmith R S. Etiology of hyperparathyroidism and bone disease during chronic hemodialysis. II Factors affecting serum immunoreactive parathyroid hormone. J Clin Invest 1971; 50: 599–605
- Goldsmith R S, Furszyfer J, Johnson W J, Fournier A E, Arnaud C D. Control of secondary hyperparathyroidism during long-term hemodialysis. Am J Med 1971; 50: 692–699
- Cannella G. Left ventricular hypertrophy in the dialysed patient. What can be done about it?. Nephrol Dial Transplant 1996; 11(3)418–420
- Drüeke T B. Anomalies of phosphate and calcium metabolism in chronic renal insufficiency. Rev Prat 1998; 48(11)1207–1212
- Facchin L, Vescovo G, Levedianos G, Zannini L, Nordion M, Llorenzi S, Caturelli G, Ambrosio G B. Left ventricular morphology and diastolic function in uremia echocardiographic evidence of a specific cardiomyopathy. Br Heart J 1995; 74(2)174–179
- Rao D S, Shih M S, Mohini R. Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. New Engl J Med 1993; 328(3)171–175
- Argiles A, Mourad G, Lorho R, Kerr P G, Flavier J L, Canaud B, Mion C M. Medical treatment of severe hyperparathyreoidism and its influence on anaemia in end stage renal failure. Nephrol Dial Transplant 1994; 9(2)1809–1812
- Masoero G, Bruno M, Gallo L, Colaferro S, Cosseddu D, Vacha G M. Increased serum pancreatic enzymes in uremia: relation with treatment modality and pancreatic involvement. Pancreas 1996; 13(4)350–355
- Massry S G. Pathogenesis of uremic toxicity. Parathyroid hormone as a uremic toxin. Textbook of Nephrology, S G Massry, R J Glassock, M D Baltimore. Williams Wilkins. 1995; 1270–1303
- Balon B P, Kavalar R. Brown tumor in association with secondary hyperparathyroidism. Am J Nephrol 1998; 18: 460–463