Abstract
Urinary excretion of renal brush border enzymes may serve as an early marker of renal injury. However, the distinction between physiological and pathological levels remains controversial, since enzymuria is affected by physiological parameters. To clarify the influence of diuresis, we investigated the urinary excretion of alanine-aminopeptidase (AAP; EC 3.4.11.2) as function of diuretic state. 17 healthy volunteers of both sexes were subjected to protocols with sudden or prolonged water load preceded and followed by a thirst period. Urinary excretion of AAP was measured using an enzyme kinetic assay. As expected AAP excretion increased with urine flow, the increments diminished yielding an overall excretion pattern that resembled saturation kinetics. This function is described by a mathematical model. This model assumes, that AAP is released in proximal tubules at a constant rate and reabsorbed or inactivated in the distal tubule and collecting duct. Non-linear fits of the model equation to our data allowed two parameters, χ and μ, to be defined. χ describes the rate of AAP release independent of urinary flow, and μ the ratio of distal tubular reabsorption or inactivation. If a substrate is not reabsorbed at all, μ approximates zero. Since μ fitted for AAP differed significantly from zero, this indicates reabsorption or inactivation of AAP in the distal nephron. Therefore, our study supports the theory of flow-dependent reabsorption or inactivation of AAP in the distal nephron.
INTRODUCTION
Urinary enzymes are investigated with the aim of evaluating renal injury. Normal urine contains trace amounts of various proteins and enzymes, which are released from glomerular or tubular cells. In pathological situations enzyme excretion is elevated Citation[[1]]. This may be used for diagnostic purposes Citation[2-6]. Indeed, enzyme excretion increases already after light or moderate alteration of proximal tubular cells, and such changes occur much earlier than the development of other sings of renal injury Citation[[7]]. Therefore enzyme determinations may serve to detect tubular nephrotoxicity at an early stage.
Although it is generally accepted, that the level of enzymuria correlates well with the degree of renal injury, urinary enzymes do not play the same important role as serum enzymes do for clinical investigation and diagnosis. One reason for this is the very divergent reference values for physiological enzymuria that have been reported in literature Citation[8-9]. This may reflect analytical and methodological differences and also the influence of different physiological parameters Citation[[10]]. Although the importance of these parameters is accepted, it is not yet established how and to what extent they affect enzyme excretion. To clarify the influence of diuresis we investigated the urinary excretion of alanine-aminopeptidase (AAP; EC 3.4.11.2) as a function of diuretic state. A mathematical model was formulated to describe the curvilinear relationship between enzyme excretion and urinary flow.
METHODS
| Subjects:: | = | Our study was performed in 17 healthy volunteers (9 males, 8 females) aged 21 to 31 years. Exclusion criteria were a history of renal disease, hypertension, diabetes mellitus or long term treatment with any medication. All subjects were on free diet and were not taking any medication before or during the study. Selection depended on satisfactory laboratory profiles, including urine analyses. |
| Laboratory methods:: | = | Urine samples were obtained by spontaneous voiding and volume was measured at room temperature. Kidney function tests: Creatinine and urea were determined by blood and urine on an AutoAnalyzer System (Astra®, Beckman Instruments, Brea California) by means of the Jaffé rate method for creatinine and the enzymatic conductivity rate method for urea respectively. Enzyme determination: Urine samples were stored at 4°C and analyzed no later than 24 hours after voiding. Urine AAP activity was measured using an enzyme kinetic substrate assay. To 1.6mL of 0.05M (pH 8.0) tromethamine 0.2mL of 4.08 mg/mL L alanine-4-nitoranilide hydrochloride were added. After vortexing the rate of 4-nitroanilide production was determined using a spectro-photometer (Zeiss, Germany) set at 405nm and 37°C. |
| Experimental protocol:: | = | In order to achieve different degrees of diuresis, standardized oral water loading was performed. After over night fasting urine samples were first collected under antidiuretic conditions. Thereafter the volunteers ingested 20 mL/kg body weight unsweetened tea or water within 30 minutes and stopped fluid intake thereafter again. Urine was collected after spontaneous voiding in periods of 60 minutes for 3 hours previous to the water loading and in periods of 15 to 30 minutes for 4 hours during and after water diuresis. At the end of the investigation period, a blood sample was drawn for creatinine and urea determination. Two volunteers were subjected to a prolonged state of elevated water diuresis. After initial water loading they substituted the voided volume to keep water diuresis at a level above 10 mL/min for 4 hoursand urine samples were collected every 10 minutes. Thereafter they stopped fluid intake and further urine samples were collected for 4 hours in periods of 15 to 30 minutes. |
| Statistics:: | = | Statistical analyses included mean, standard deviation and variance to describe the measured data. If possible Pearson's correlation coefficient was used for urinary flow and enzyme excretion, otherwise Spearman's correlation coefficient was employed. All calculations were done with SPSS statistical software (SPSS® Inc., Chicago, IL, USA). For non-linear regression analyses a mathematical model was developed. |
RESULTS
After water loading maximal urinary flow rates were on average 12.8 mL/min (± 3.0 mL/min). As expected AAP concentration per milliliter urine decreased with raising urinary flow as a direct effect of dilution. However, AAP excretion per minute was not in inverse proportion to urinary volume. AAP excretion increased simultaneously with raising urinary output (a, b). The correlation of AAP excretion per minute and urinary flow was highly significant (p < 0.01) for 16 out of the 17 volunteers, indicating, that AAP excretion in healthy subjects of either sex varies in a complex fashion with diuresis (c, d). During maximal diuresis AAP excretion raises to values distinctly above normal levels. To exclude, that AAP excretion is caused by a medullary wash out phenomenon, water diuresis was maintained at levels above 10 mL/min for 4 hours in two subjects. During the entire time period AAP excretion remained elevated. AAP excretion and urinary flow rate increased and decreased absolute synchronously, even with minor changes (e). To eliminate the influence of the dilution, enzyme activity per mg urine creatinine wascalculated, and this ratio was used for correlation analysis versus urinary flow rate. Again, the correlation of enzyme excretion per mg creatinine versus urinary flow was highly significant for all volunteers (p < 0.05).
Figure 1. (a) and (b) Time course of urinary flow (-♦-), AAP excretion (-▪-) and urinary AAP concentration (-▴-) of two subjects. (c) and (d) Correlation between AAP excretion and urinary flow from data of a and b, respectively.
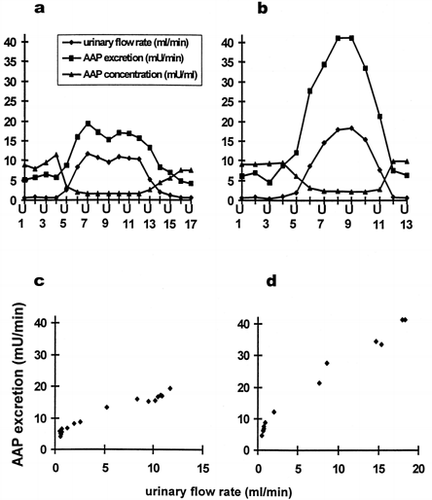
Figure 1. (e) Time course of urinary flow (-♦-), AAP excretion (-▪-) and urinary AAP concentration (-▴-) of one individual subjected to sustained water diuresis. Urine samples were collected at intervals of 10 minuteswhile elevated urinary flow was maintained by continuing water load for 4 hours. (f) Correlation between AAP excretion and urinary flow from data of e. The curved line represents the best fit of our model equation.
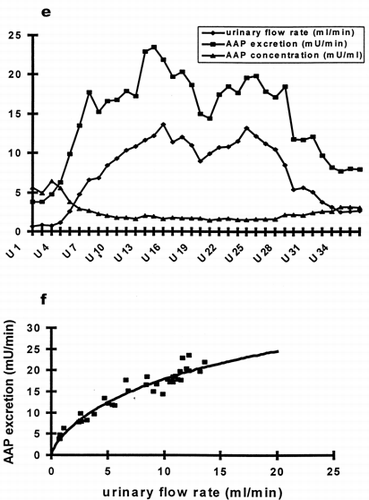
In antidiuresis a linear correlation may describe the relationship of AAP excretion versus urinary flow. With increasing urine flow, the correlation becomes curvilinear with AAP excretion approaching a maximum (, c, d, f). To describe the curvilinear relationship between AAP excretion and urine flow a mathematical model was formulated. The resulting equation wasfitted to the data (curved line, f) describing the relationship between AAP excretion and urine flow.
DISCUSSION
AAP is the main aminopeptidase in urine, which is released from the brush border membrane of the proximal tubule. It has been well established in many studies for the evaluation of nephrotoxicity Citation[[11]]. Our results indicate that apart from the direct effect of dilution, increasing urinary flow has an indirect effect on AAP elimination. Other investigators have also recognized this flow-dependence of urinary enzymes Citation[12-13]. This phenomenon was mainly attributed to an altered release of enzymes in the proximal tubule. A tubular “wash-out” of AAP with the beginning of diuresis was proposed Citation[[13]]. Since we neither found a transient “overshoot” during the onset of diuresis nor a transient “undershoot” upon return to anitidiuresis, in case of AAP excretion no evidence for such a “wash-out” phenomenon was obtained. It is reasonable, therefore, to assume that the rate of release is constant and independent of the state of diuresis/antidiuresis. AAP derives from shedding of cells or of brush border membrane components of proximal tubules probably as a result of physiological regeneration processes. To explain the flow-dependence of AAP excretion the assumption of an alteration of enzyme elimination in the distal nephron segments is reasonable. The simplest hypothesis would be that AAP is absorbed by a non-selective mechanism of constitutive endocytosis, which takes up all proteins present in the tubular fluid phase. This hypothesis would be compatible with the assumed concentration dependence of AAP extraction. Alternatively one could hypothesize that AAP was degraded and thereby inactivated by proteases in the distal nephron, and such a process would also be compatible with the assumed concentration dependence of AAP inactivation if the afinity of the presumed proteases was comparatively low.
Based on this theory we formulated a simple proximal release and distal absorption model which fitted the absorbed excretion pattern very well. For simplification the following assumptions are introduced: 1) the kidney is represented by a single nephron. 2) We assume that 80% of the glomerular filtrate (U0) is absorbed along the proximal tubule independent of proximal tubular length, luminal AAP concentration and the rate of urine flow. 3) The remaining urine (β × U0 ≈ 20%) flows into the distal part of the nephron. 4) The glomerular filtrate doesnot contain AAP, but AAP is cons tantly released into the proximal tubule. 5) In the distal tubule no further release of AAP takes place. 6) AAP is reabsorbed or inactivated in the distal tubular segments and its absorption rate rises linearly with luminal AAP concentration. So absorption can formally be described by the product of an equivalent permeability coefficient multiplied by local AAP concentration in the tubular lumen. The resulting equation for urinary AAP excretion rate (ERAAP) as function of urine flow rate (U) represents AAP release of the proximal tubule and distal absorption:Non linear fits of the model equation to our data allow 2 parameter χ and μ to be defined. μ is the product of a “transport” coefficient of the distal nephron for AAP and the surface area of this nephron segment. Therefore it characterizes the examined substrate. For substances that are completely reabsorbed like water or alcohol, it tends to be infinity. If a substrate is not reabsorbed at all like inulin, μ approaches zero Citation[[14]]. To test this hypothesis we fitted μ for creatinine, even though our equation assumes constant tubular release instead of glomerular filtration. As expected the fitted values were about zero (−0.35 ± 0.61). For AAP the mean fitted value for μ was 16.35 (6.91–26.43), indicating that there are reabsorption or inactivation processes for AAP existent in the distal tubule. χ represents the ratio of AAP excretion rate and urinary flow at the proximal tubule. For each healthy individual this is constant and determined by kidney mass and volume. We found a significant correlation of χ and body mass index. Individual differences should be relatively small (in our study between 0.13 and 2.05). It is not influenced by diuresis. We expect a significant increase of χ after tubular injury, yielding more reliable information on incipient tubular damage.
Eventually further studies on controls and patients may result in the set-up of some kind of nomogramm for AAP excretion versus urinary flow.
REFERENCES
- Scherberich J E. Urinary proteins of tubular origin: basic immunochemical and clinical aspects. Am J Nephrol 1990; 10(Suppl 1)43–51
- Mattenheimer H. Enzymes in renal diseases. Ann Clin Lab Sci 1977; 7: 422–432
- Jung K, Diego J, Strobelt V, Scholz D, Schreiber G. Diagnostic significance of some urinary enzymes for detecting acute rejection crises in renal-transplant recipients. Clin Chem 1986; 32: 1807–1811
- Jung K, Schulze B D, Sydow K. Diagnostic significance of different urinary enzymes in patients suffering from chronic renal diseases. Clin Chim Acta 1987; 168: 287–295
- Porter G A, Norton T L, Legg V. Using urinary biomarkers to evaluate renal effects of a Cox-NSAID in volunteers. Ren Fail 1999; 21: 311–317
- Lybarger J A, Lichtveld M Y, Amler R W. Biomedical testing of the kidney for persons exposed to hazardous substances in the environment. Ren Fail 1999; 21: 263–274
- Jung K, Pergande M, Graubaum H J, Fels L M, Endl U, Stolte H. Urinary proteins and enzymes as early indicators of renal dysfunction in chronic exposure to cadmium. Clin Chem 1993; 39: 757–765
- Feldmann D, Flandrois C, Jardel T, Phan T, Aymard F. Circadian variations and reference intervalsfor some enzymes in urine of healthy children. Clin Chem 1989; 35: 864–867
- Maruhn D, Fuchs I, Mues G, Bock K D. Normal limitsof urinary excretion of eleven enzymes. Clin Chem 1976; 22: 1567–1574
- Jung K. Urinary enzymes and low molecular weight proteins as markers of tubular dysfunction. Kidney Int 1994; 46(Suppl 47)S29–S33
- Mattenheimer H, Jung K, Grötsch H. Alanine Aminopeptidase. Urinary enzymes in clinical and experimental medicine, K Jung, H Mattenheimer, U Burchardt. Springer-Verlag, Berlin, Heidelberg, New York 1992; 99–105
- Jung K, Schulze G, Reinholdt C. Different diuresis-dependent excretions of urinary enzymes. Clin Chem 1986; 32: 529–532
- Burchardt U, Hempel A, Höpfner J B, Hempel R D. Alaninaminopeptidaseund Kreatininausscheidung unter verschiedenen Diuresezuständen beim Menschen. Z Gesamte Inn Med 1976; 31: 550–552
- Tang-Liu D D, Tozer T N, Riegelman S. Dependence of renal clearance on urine flow: A mathematical model and its application. J Pharm Sci 1983; 72: 154–158