Abstract
Introduction. Beta 2 microglobulin(β2M) is filtered by the glomeruli and reabsorbed by the proximal tubularcells where it is metabolized. Its plasma concentration increases with decreasingrenal function. Aim. To compare serum creatinine(Cr) and serum β2M as markers of GFR. Patientsand Methods. In 160 adult patients, with various kidney diseasesand different GFR, serum Cr (autoanalyzer), serum β2M (RIA) and GFR (bladdercumulative method using 99mTc-DTPA as glomerulartracer) were measured in the same day. Results.A linear relationship was observed between ln GFR and both ln serum Cr (lnCr= 3.112–0.716lnGFR; r = 0.92) and ln serum β2M (lnβ2M = 4.274–0.814lnGFR; r = 0.90). With decreasing GFR the increase in serum β2M was higherthan that of serum Cr (see regression coefficients that are significantlydifferent). The normal upper limit of serum Cr corresponds to a GFR 48.1 mL/minwhile that of serum β2M to a GFR 65.0. With decreasing GFR the increaseof serum β2M occurs before than that of serum Cr. Conclusions.With declining renal function, serum β2M increases more and before thanserum Cr. Serum β2M is a good endogenous marker of GFR, better than serumCr.
INTRODUCTION
Beta 2 microglobulin (β2M) is an anionic low molecular weight proteinconsisting of a single aminoacid chain of 99 aminoacids with one disulfidebridge (MW 11,800; pI 5.8; Einstein-Stokes radius 16Å). β2M ispart of the histocompatibility leukocyte antigen complex present on cell membranesof all nucleated cells by which it is synthesized Citation[[1]].It is released into circulation, at constant rate in normal subjects, duringthe normal cell turnover Citation[2-3].
Like other low MW proteins, β2M is filtered by the glomeruli (glomerularsieving coefficient 0.94) and almost completely reabsorbed by the proximaltubular cells, where it is metabolized with no return of the filtered proteinto the circulation Citation[[4]]. Most probably, β2Mis removed from blood only by the kidney and its plasma concentration increaseswith decreasing renal function Citation[5-7].It is widely acknowledged that β2M is not influenced by factors thataffect the serum concentration of creatinine (Cr) such as muscle mass anddrugs. Therefore, β2M would be ideal as a GFR marker and indeed it hasbeen used to evaluate GFR in patients with renal disease Citation[[5]], Citation[7-9].β2M has now been abandoned as marker of GFR because its production, andtherefore its serum level, seems to be increased in some pathological conditions,particularly lymphoproliferative disorders and some inflammatory diseasesCitation[10-12].
The aim of this study was to take again into consideration β2Mas marker of GFR. For this purpose serum β2M, serum Cr and GFR (as areference parameter) were measured in a large number of patients with variouskidney diseases and different degrees of kidney damage.
PATIENTS AND METHODS
We have considered for enrollment in the study 161 (80 males and 81females) consecutive adult patients, with various renal diseases, referredto U.O. University Nephrology, Department of Internal Medicine, S. ChiaraHospital, Pisa, for determination of the GFR. Their diagnoses were as follows:24 primary glomerulonephritis, 17 hypertensive nephropathy, 15 ischemic nephropathy,10 chronic renal failure, 7 polycystic kidney disease, 6 chronic interstitialnephritis, 3 secondary glomerulonephritis, 2 IgA nephropathy, 2 SLE, 2 renallymphoma, 37 urologic nephropathy, 36 unknown.
SLE affected one of these with GFR 21 mL/min, serum Cr 2.1 mg/dL andserum β2M 9.1 mg/L. This patient's serum β2M seemed too elevatedto be explained by the reduction of GFR and this discrepancy was attributedto the autoimmune disease. The case was excluded.
The age of accepted patients (80 males and 80 females) ranged from 16to 83 years (mean 55) and their body weight from 43 to 108 kg (mean 69). Generalconditions of these patients were good. They were stable without intercurrentillness. Muscle mass and nutritional status seemed to be normal.
Their renal function ranged from normality to advanced renal failure(serum creatinine 0.4–13.3 mg/dL, mean 1.7). Most frequently used drugswere antihypertensives.
In all patients, blood for serum Cr and serum β2M determinationwas drawn on the morning of the same day of that the GFR was measured. Bloodsamples were drawn from the antecubital vein using S-monovette Sarsteadt tubes.After centrifugation at 3000 rpm for 8 min, serum was isolated and analyzedon the same day (for creatinine) or within 7 days after immediate storageat –80°C (for β2M). Serum Cr was measured by an autoanalyzertechnique on the Hitachi 912 or 917 analyzer according to the manufacturer'sprocedure. The upper limit of normal is 1.4 mg/dL for men and 1.2 mg/dL forwomen. Serum β2M was determined using a radioimmunoassay method (Immunotech).The upper limit of normal is 2.4 mg/L for both, males and females.
GFR was measured by the bladder cumulative method. This consists ofa single i.v. injection (≅0.3 mC) of glomerular tracer 99mTc-DTPA(CIS Bio International, France) followed by external counting of the radioactivityaccumulated in the bladder during 30 min. GFR has been adjusted to a bodysurface area of 1.73m2. Bladder cumulative methodis a simple and accurate measurement of GFR, which can be performed eitherwith a scintillation counter provided with a suitable collimator for the externalcounting of the bladder, as in this study, or with a gamma camera. In ourhands its mean variation coefficient was 7.2 ± 6.5 SD Citation[13-14], Citation[[14bis]].
The relationships between GFR, serum Cr and serum β2M were studiedby linear regression analysis. Comparison of regression coefficients was performedby t-test. Pearson's correlation coefficients were also calculated. A p-value < 0.05 was considered significant.
RESULTS
Our results confirm the data of the literature Citation[15-16]that sex and body weight influence serum Cr, which is higher in males thanin females and tends to increase with increasing body weight, while serumβ2M is not affected neither by sex nor by body weight.
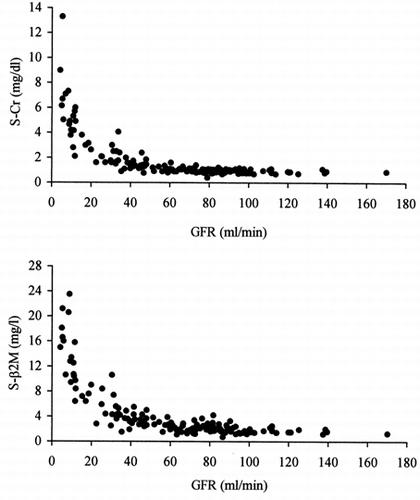
shows the observed values of serumCr and serum β2M versus GFR. Changes in the serum values of both markerswith declining GFR appear roughly similar.
Figure 1. Relationships between GFR (99mTc-DTPArenal clearance per 1.73m2), serum creatinine (top)and serum β2-microglobulin (bottom).
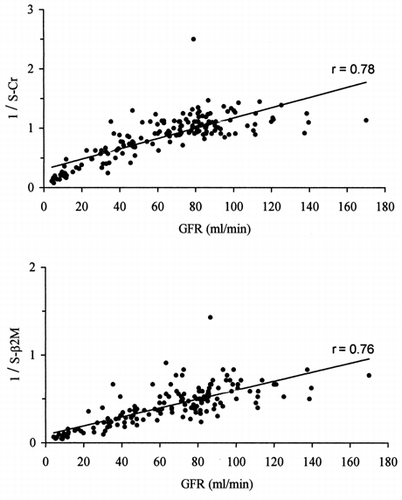
In reciprocals of serum Cr and serumβ2M values are plotted against GFR. A linear relationship with GFR seemsto be weak for both, 1/Cr (r = 0.78) and 1/β2M (r = 0.76).
Figure 2. Relationships between GFR (99mTc-DTPArenal clearance per 1.73m2) and reciprocal valuesof serum creatinine (top) and serum β2-microglobulin (bottom).
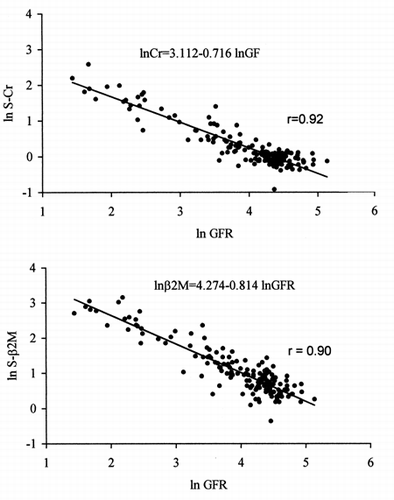
indicates that the relationshipsbetween serum Cr, serum β2M and GFR are exponential, as demonstratedby the closer linear relationship observed between ln GFR and both ln serumCr (lnCr = 3.112–0.716 lnGFR; r = 0.92) and ln serum β2M (lnβ2M= 4.274–0.814 lnGFR; r = 0.90). This means that to a constant percentdecrease in GFR corresponds a constant percent increase in serum Cr and serumβ2M. Note that with decreasing GFR, the increase in serum β2M ishigher than that of serum Cr as indicated by regression coefficients, whichare significantly different (p<0.001).
Figure 3. Relationships between ln GFR (99mTc-DTPArenal clearance per 1.73m2), ln serum creatinine(top) and ln β2-microglobulin (bottom).
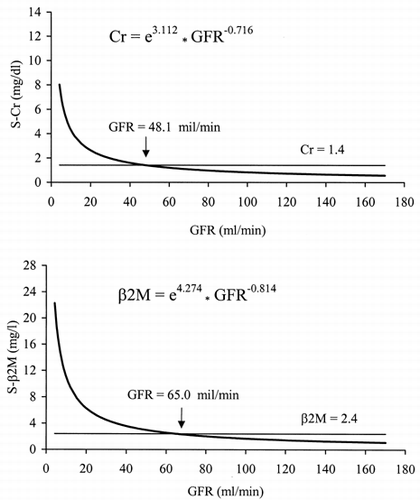
In the curves fitting the observedvalues of serum Cr and serum β2M versus GFR are plotted. The generallyaccepted normal upper limits for serum Cr (1.4 mg/dL) and for serum β2M(2.4 mg/L) are represented as horizontal lines. The normal upper limit ofserum Cr corresponds to a GFR value of 48.1 mL/min, while that of serum β2Mto a GFR value of 65.0 mL/min. Therefore, with decreasing GFR the increasein serum β2M occurs clearly before that of serum Cr.
Figure 4. Estimated lines of the relationships between GFR (99mTc-DTPArenal clearance per 1.73m2), serum creatinine (top)and serum β2-microglobulin (bottom). The horizontal lines represent theupper limits of normal for serum creatinine (1.4 mg/dL) and for β2-microglobulin(2.4 mg/L).
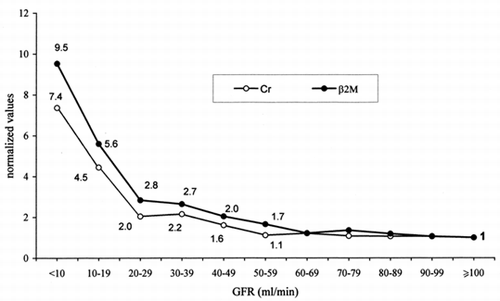
In the normalized mean values ofserum Cr and serum β2M are plotted versus GFR. The observed values havebeen assembled in groups with increasing GFR (GFR < 10, 10–19, 20–29,mL/min, and so on), assuming 1 the mean value of “normal” patients(GFR ≥ 100 mL/min). Also this approach confirms that the increase of serumβ2M with decreasing GFR occurs before and is higher than that of serumCr. Note that in the group of patients with GFR 50–59 mL/min, the serumβ2M is 1.7 times higher than that of patients with normal GFR, whilethe corresponding increase in serum Cr is 1.1 times higher.
Figure 5. Normalized mean values of serum creatinine and serum β2-microglobulinin groups of patients with increasing GFR (99mTc-DTPArenal clearance per 1.73m2). The mean value ofserum creatinine and serum β2-microglobulin in patients with GFR ≥100 mL/min is used as a reference value (starting point = 1).
DISCUSSION
In clinical practice and in some research settings, serum Cr determinationis acknowledged a mainstay in the laboratory profile of glomerular filtration.Nevertheless, on the basis of the available data, doubt as to the reliabilityof Cr as marker of GFR seems widely justified. In fact it is widely acknowledgedthat GFR may fall by more than 50% before the serum Cr value rises.
Many factors and pathological conditions may influence serum Cr. Inpatients with chronic severe renal insufficiency an increase in tubular secretionand an extrarenal elimination in creatinine occur Citation[17-19].At high serum levels, many normal plasma constituents and drugs may interferewith some laboratory methods used to measure creatinine: glucose and otherssugars, pyruvate, ketones and ketoacids, ascorbic acid, urate, bilirubin,fatty acids, cephalosporins, dopamine, others Citation[[15]], Citation[20-21].Production and elimination pathways for Cr can change in different conditions.Cr production is decreased in all pathological status accompanied by musclemass reduction, and probably in hepatic disease Citation[22-23].Urine elimination of Cr can be modified by drugs interfering with tubularsecretion, leading to an increase in Cr serum level: acetylsalicilic acid,amiloride, calcitriol, cimetidine, dapsone, pyrimethamine, spironolactone,triamterene, trimethoprim Citation[[15]], Citation[23-24].Dietary intake of creatinine with cooked meat can influence serum Cr Citation[[23]]. Furthermore, clinical studies have demonstrated thatserum Cr is inadequate as marker of GFR in patients with glomerular diseasesCitation[[19]], Citation[[25]], lupus nephritis Citation[26-27] and diabetic nephropathy Citation[[28]].
In spite of all these evidences, serum Cr continues to be a populartest of renal function, probably because of its semplicity and low cost.
Most low MW proteins (small proteins of size less than that of albumin)are mainly or exclusively eliminated from the blood by glomerular filtration.For this behavior they represent potential markers of GFR. A large numberof low MW proteins (including β2M, factor D, Cystatin C, α1-microglobulin,tumor associated trypsin inhibitor) accumulate in the plasma with decliningrenal function. In the past years they have been suggested as markers of GFRCitation[5-6], Citation[8-9], Citation[29-33].
The two low MW proteins most studied as markers of GFR are β2Mand Cystatin C. β2M was the first small protein introduced in the clinicalpractice to predict GFR Citation[5-9], Citation[[30]], Citation[34-35].Nevertheless its use has been rather limited, probably due to some technicalproblems and to the high cost, and especially because some extrarenal diseasesseem to influence serum β2M, in particular lymphoproliferative disorderand inflammatory diseases such as rheumatoid arthritis and Sjögren'ssyndrome Citation[10-12]. Inthese conditions there may be elevation of the plasma concentration, whichrepresents increased production rather than reduced clearance. Elevated serumβ2M values have been observed also in some liver diseases Citation[[36]] and in sickle cell anemia Citation[[37]].
A problem arises: are the many above cited factors able to modify laboratorydetermination of Cr or its serum levels more or less important than the conditionsaffecting β2M production?
As a matter of fact, the clinical and laboratory factors, which canmodify serum Cr are more numerous and their identification is often difficult.With regard to β2M, its production can be increased, independently fromdecrease of GFR, in a limited number of well-defined diseases that are easilydiagnosed.
The results of this study confirm that serum β2M is not influencedby sex and body weight while serum Cr is Citation[15-16],and demonstrate that, with declining renal function, serum β2M increasesmore and before than the serum Cr. As the latter, also serum β2M underevaluatesGFR decrease but definitely less than serum Cr does.
Our results are similar to those of previous studies Citation[5-6], Citation[8-9].Unlike these, the present study was performed in a large number of adult patientswith various kidney diseases and a wide range of GFR (from normality to advancedrenal failure). Furthermore, in this study GFR has been measured by renalclearance of 99mTc-DTPA and external counting ofthe bladder (bladder cumulative method) Citation[13-14], Citation[[14bis]].
In conclusion, our results together with previously reported studies(concerning the different factors which, can modify serum Cr and/or serumβ2M and their laboratory measurement) justify the statement that serumβ2M is a good endogenous marker of GFR, better than serum Cr. Thereforethe use of serum Cr as a sole filtration marker is no longer justified.
We predict that in the future the measurement of serum low MW proteinswill become more widely used in diagnostic Nephrology. In particular someof them (β2M, Cystatin C, tumor associated trypsin inhibitor or others)could be widely employed instead of or together with Cr as markers of GFR.
ACKNOWLEDGMENT
This work was supported in part by a research fund from the MinisteroUniversità e Ricerca Scientifica e Tecnologica, Italy. Ms. CristinaSnyder is gratefully acknowledged for her valuable help in the preparationof this manuscript.
REFERENCES
- Creswell P, Springer T, Strominger J L, et al. Immunological identity of the small subunit of HLA antigensand β2-microglobulin and its turnover on the cell membrane. Proc Nat Acad Sci USA 1974; 71: 2123–2207
- Berggård I, Bearn A G. Isolation and properties of low molecular weight β2-globulinoccurring in human biological fluids. J Biol Chem 1968; 243: 4095–4103
- Nakamuro K, Tanigaki N, Pressman D. Multiple common properties of human β2-microglobulin andthe common portion fragment derived from HLA antigen molecules. Proc Nat Acad Sci USA 1973; 70: 2863–2865
- Johnson V, Maak T. Renal tubular handling of proteins and peptides. Textbook of Nephrology, S G Massry, R J Glassock. 2nd ed, Williams and Wilkins, Baltimore 1989; 97–102
- Wibell L, Evrin P E, Berggård I. Serum β2-microglobulin in renal disease. Nephron 1973; 10: 320–331
- Trollfors B, Norrby R. Estimation of glomerular filtration rate by serum creatinineand serum β2-microglobulin. Nephron 1981; 28: 196–199
- Acchiardo S, Kraus A P, Jennings B R. β2-microglobulin levels in patients with renal insuficiency. Amer J Kid Dis 1989; 13: 70–74
- Viberti G C, Keen H, Mackintosh D. Beta2-microglobulinaemia: a sensitive index of diminishing renalfunction in diabetics. British Medical J 1981; 282: 95–98
- Shea P H, Maher J F, Horak E. Prediction of glomerular filtration rate by serum creatinineand β2-microglobulin. Nephron 1981; 29: 30–35
- Karlsson F A, Wibell L, Evrin P E. β2-microglobulin in clinical medicine. Scand J Clin Lab Invest 1980; 40: 27–37
- Mavligit G M, Stuckey S E, Cabanillas F F, et al. Diagnosis of leukemia or lymphoma in the central nervous systemby β2-microglobulin determination. New Eng J Med 1980; 303: 718–722
- Brenning G, Wibell L, Bergström R. Serum β2-microglobulin at remission and relapse in patientswith multiple myeloma. Eur J Clin Invest 1985; 15: 242–247
- Bianchi C. Noninvasive methods for the measurement of renal function. Renal Function Tests-Clinical Laboratory Procedures and Diagnosis, C G Duarte. Little, Brown and Company, Boston 1980; 65–84
- Bianchi C, Donadio C, Tramonti G, et al. A reappraisal of the bladder cumulative method as a reliabletechnique for the measurement of glomerular filtration rate. Renal Failure 1998; 20: 257–265
- Bianchi C, Donadio C, Tramonti G, et al. A reappraisal of the bladder cumulative method as a reliabletechnique for the measurement of glomerular filtration rate. Renal Failure 1999; 21: 235–237, ERRATUM
- Cameron J S. Renal function testing. Oxford Textbook of Clinical Nephrology, S Cameron, A M Davison, J P Grünfeld, D Kerr, E. Ritz. OxfordUniversity Press, Oxford 1992; 24–49
- Lafayette R A, Perrone R D, Levey A S. Laboratory evaluation of renal function. Diseases of the Kidney, R W Schrier, C W Gottschalk. 6th ed, Little, Brown and Company, Boston 1997; 307–354
- Jones J D, Burnett P C. Creatinine metabolism in humans with decreased renal function:creatinine deficit. Clin Chem 1974; 20: 1204–1212
- Mitch W E, Collier V U, Walser M. Creatinine metabolism in chronic renal failure. Clin Sc 1980; 58: 327–335
- Shemesh O, Golbetz H, Kriss J P, Myers B D. Limitations of creatinine as a filtration marker in glomerulopathicpatients. Kidney Int 1985; 28: 830–838
- Narayanan S, Appleton H D. Creatinine: a review. Clin Chem 1980; 26: 1119–1126
- Weber J A, van Zanten A P. Interferences in current methods for measurements of creatinine. Clin Chem 1991; 37: 695–700
- Horber F F, Scheidegger J, Frey F J. Overestimation of renal function in glucocorticosteroid treatedpatients. Eur J Clin Pharmacol 1985; 28: 537–541
- Perrone R D, Madias N E, Levey A S. Serum creatinine as an index of renal function: new insightsinto old concepts. Clin Chem 1992; 38: 1933–1953
- Kasise B L, Keane W F. Laboratory assessment of renal disease: clearance, urinalysis and renal biopsy. The Kidney, B M Brenner. 5th ed, WB Sanders Company, Philadelphia 1996; 1137–1174
- Carrie B J, Golbetz H V, Michaels A S, Myers B D. Creatinine: an inadequate filtration marker in glomerular disease. Am J Med 1980; 69: 177–182
- Petri M, Bockenstedt L, Colman J, et al. Serial assessment of glomerular filtration rate in lupus nephropathy. Kidney Int 1988; 34: 832–839
- Myers B D, Chagnac A, Golbetz H, et al. Extent of glomerular injury in active and resolving lupus nephritis:a theoretical analysis. Am J Physiol 1991; 260: F717–F727
- Viberti G C, Bilous R W, Mackintosh D, Keen H. Monitoring glomerular function in diabetic nephropathy. Am J Med 1983; 74: 256–264
- Sturfelt G, Truedsson L, Thysell H, Björck L. Serum level of complement factor D in systemic lupus erythematosus– an indicator of glomerular filtration rate. Acta Med Scand 1984; 216: 171–177
- Grubb A, Simonsen O, Sturfelt G, et al. Serum concentration of cystatin C, factor D and β2-microglobulinas a measure of glomerular filtration rate. Acta Med Scand 1985; 218: 499–503
- Kusano E, Suzuki M, Asano Y, et al. Human α1-microglobulin and its relationship to renal function. Nephron 1985; 41: 320–324
- Simonsen O, Grubb A, Thysell H. The blood serum concentration of cystatin C (γ-trace)as a measure of the glomerular filtration rate. Scand J Clin Lab Invest 1985; 45: 97–101
- Tramonti G, Ferdeghini M, Donadio C, et al. Tumor-associated trypsin inhibitor (TATI) and renal function. Kidney Int 1997; 52: S179–S181
- Schardijn G HC, Statius van Eps L W. β2-microglobulin: its significance in the evaluation ofrenal function. Kidney Intl 1987; 32: 635–641
- Filler G, Witt I, Priem F, et al. Are cystatin C and β2-microglobulin better markers thanserum creatinine for prediction of a normal glomerular filtration rate inpediatric subjects?. Clin Chem 1997; 43: 1077–1078
- Hällgren R. Serum β2-microglobulin in liver disease. Scand J Clin Lab Invest 1979; 39: 441–447
- de Jong P E, de Jong-van den Berg LTW, Sewrajsingh G S, et al. Beta-2-microglobulin in sickle cell anaemia – Evidenceof increased tubular reabsorption. Nephron 1981; 29: 138–141