Abstract
Chromogranin A (CGA) is a low MW (49,000) acidic hydrophilic protein. It is synthesized in the chromaffm granules of the neuroendocrine cells, and has been found circulating in the blood of healthy subjects. The aim of this study was to assess the relationship between serum levels of CGA and renal function. One hundred two renal patients (45 M and 57 F; age 14–76 years, mean 52) participated in the study. Glomerular filtration rate (GFR) was measured by the bladder cumulative method, using 99mTc-DTPA as a tracer. Blood CGA was determined by RIA. Plasma creatinine, β2microglobulin (β2m) and tumor associated trypsin inhibitor (TATI) were also determined. The reduction in renal function was associated with an increase in all of the above studied parameters. In patients with advanced renal failure (GFR < 20 mL/min) CGA levels increased by 22-fold as compared to the patients with normal renal function (GFR > 100 mL/min). The other studied parameters were also increased but to a lesser degree, e.g., TATI 14-, β2m 8- and creatinine 5-fold. The results of this study demonstrate that renal handling of the CGA is similar to other low MW proteins, and it accumulates in the blood in renal failure.
INTRODUCTION
Chromogranin A (CGA) is an acidic, hydrophilic protein made up of 439 aminoacids, with a molecular weight of 49 kDa. It acts as a pro-hormone that releases biologically active peptides such as vasostatins, pancreastatin and others after proteolysis. It was discovered in tumor cells of neuroendocrine origin. A new radioimmunoassay method has been described by which CGA can be easily measured in the blood, and has been detected in high levels in patients with neuroendocrine tumors. Interestingly, basal levels of CGA in healthy subjects are more or less constant and are independent of age and sex Citation[1-3].
The kidney plays an important role in the handling of low MW proteins, i.e., < 68 kDa. The majority of the filtered low MW proteins are reabsorbed by the tubules. After reabsorption they are then further processed in the proximal tubular cells Citation[4-5]. Thus a rise in the blood levels of low MW proteins with the decline in renal function occurs Citation[6-14]. Previously, elevation in the blood levels of low MW proteins that have been regarded as tumor markers have been found in compromised renal function Citation[15-25]. It would be interesting to determine as to whether CGA levels are influenced by a compromise in the renal function.
The aim of this study was to evaluate the relationship between renal function and blood levels of CGA. We determined the glomerular filtration rate (GFR) and blood levels of CGA in renal patients. For comparison, plasma creatinine and two other low MW proteins, β2 microglobulin (β2m) and tumor associated trypsin inhibitor (TATI), were determined.
MATERIAL AND METHODS
One hundred two patients (45 males and 57 females) participated in this study. Their age ranged between 14 and 76 years, mean 52. All patients submitted for evaluation of the renal function had kidney-related diseases and did not have any signs or symptoms of neuroendocrine tumors. The informed consent was obtained from all of the subjects.
Glomerular filtration rate (GFR) was determined by means of the bladder cumulative method in which renal clearance of the tracer, i.e., 99mTc-DTPA (TCK6, CIS bio international), is measured Citation[[26]]. Briefly, this method is based upon direct measurement of the radioactivity over the bladder and in a blood sample after a single intravenous injection of a low dose (0.3 mCi) of the tracer.
For CGA determination another blood sample was collected, frozen and stored at −20°C until tested. To compare CGA with other low MW proteins employed as markers of renal function, we measured in the same blood sample β2m and TATI. Plasma creatinine was also determined.
CGA was measured by the CGA-RIA CT (CIS bio international). β2m was determined by RIA, β2-microglobuline (Immunotech); TATI by the RIA Spectria TATI Update (Orion Diagnostica). Creatinine was measured by Autoanalyzer Hitachi 717.
Statistical analysis
Statistical analysis was performed using the nonparametric Mann- Whitney test.
RESULTS
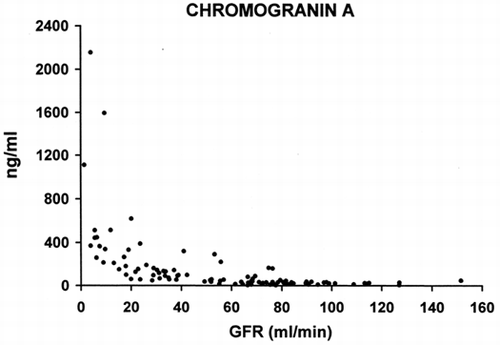
The relationship between serum values of CGA and GFR are reported in . The serum levels of CGA do not change significantly until GFR is reduced to 40 mL/min. Further reduction in GFR is accompanied by a progressive increase in serum values of CGA.
Figure 1. Relationship between serum levels of Chromogranin A (ordinate) and glomerular filtration rate (abscissa).
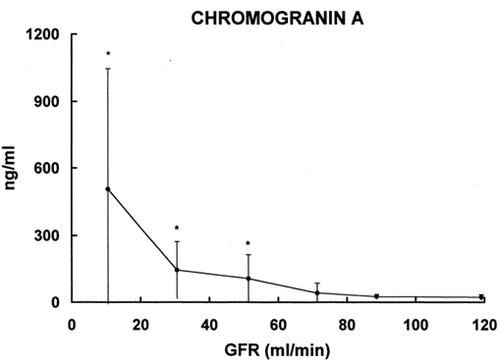
The levels of CGA in various groups with different GFR values are included in . Mean and standard deviation are given for the following groups: 0–20, 20–40, 40–60, 60–80, 80–100, and >l00 mL/min. The increase in blood level of CGA is statistically significant in the group with GFR 40- 60 mL/min. In the groups with lower GFR the increase is more evident.
Figure 2. Relationship between serum level of Chromogranin A (ordinate) and glomerular filtration rate (abscissa). The results are grouped on the basis of GFR (mean ± SD). * = p < 0.002 vs. group with GFR >100 mL/min.
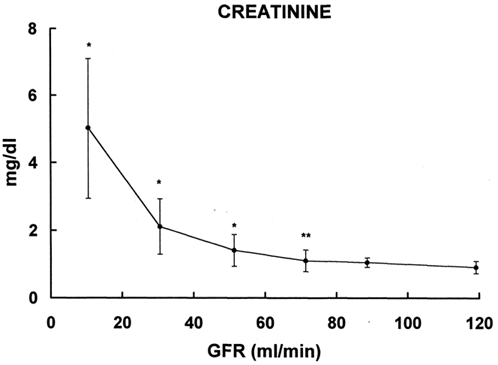
The results of plasma creatinine obtained in the same patients are reported in . The slope is similar to that of CGA. However, the increase in plasma creatinine is statistically significant in the group with GFR 80–60 mL/min.
Figure 3. Relationship between plasma levels of creatinine (ordinate) and glomerular filtration rate (abscissa). * = p < 0.05, ** = p < 0.003 vs. group with GFR > 100mL/min.
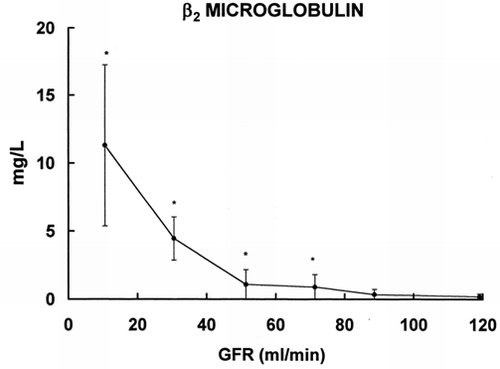
In the results of β2m, a low MW protein widely employed for the assessment of renal function are shown. The increase in β2m is statistically significant in the group with GFR 80–60 mL/min.
Figure 4. Relationship between serum levels of β2 microglobulin (ordinate) and glomerular filtration rate (abscissa). * = p < 0.005 vs. group with GFR >100mL/min.
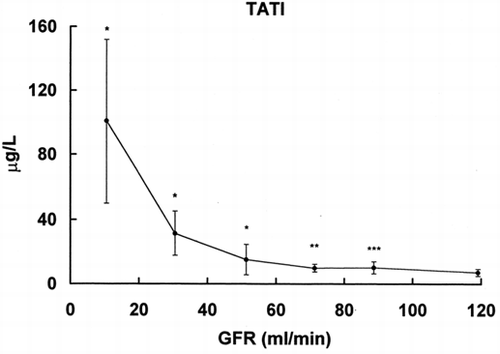
In the results of TATI, a low MW protein and a proposed marker of renal function, are given. The serum concentration of TATI increases in the group with GFR l00–80 mL/min.
Figure 5. Relationship between serum levels of TATI (ordinate) and glomerular filtration rate (abscissa). * = p < 0.001, ** = p < 0.01, *** = p > 0.02 vs. group with GFR > 100 mL/min.
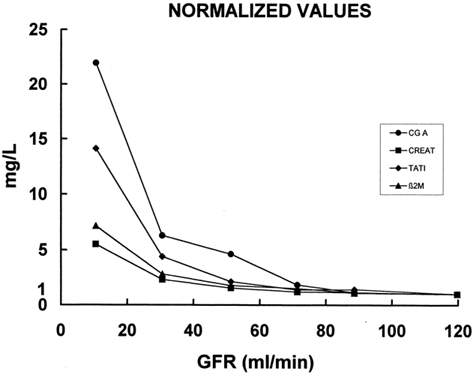
In the data of several parameters collected from various groups with different GFR are included. The results were normalized considering a value of one for patients with normal renal function, that is, GFR more than l00 mL/min. The data from other groups are expressed as a ratio of the parameters in patients with GFR > 100 mL/min. The decrease in GFR is associated with an increase in all four studied parameters. However, the degree of such an increase is variable. In fact plasma creatinine increases in patients with advanced renal failure, that is, in patients with GFR < 20 mL/min, about five fold. While the low MW proteins increase much higher than the plasma creatinine. The increase in β2m and TATI in the same patients is about eight- and fourteen-fold, respectively. CGA shows the highest increase in the ratio, that is 22-fold, between patients with renal failure and normal renal function. Of note is that in the group of patients with GFR between 60–40 the increase in the ratio of CGA is about five times. This increase is similar to that of plasma creatinine in patients with advanced renal failure (GFR > 20 mL/min).
Figure 6. Relationship between blood levels of Chromogranin A, creatinine, β2 microglobuline, TATI (ordinate) and glomerular filtration rate (abscissa). The values are normalized considering 1 the value of the patients with GFR > 100 mL/min and expressing the values of other groups as the proportional increase vs. the normal GFR group.
DISCUSSION
The results of this study indicate that CGA, like many other low MW proteins, is handled by the kidney. In fact, its relationship with GFR is very similar to that of other substances eliminated by the kidney like creatinine, film and TATI. However, serum CGA increases in renal failure more than creatinine and the other studied low MW proteins, β2m and TATI. To explain these findings one can propose the followings hypotheses. First, since the MW of CGA is the highest among the studied proteins, a reduction in its filterability in renal failure may occur. Second, CGA could play a role in uremic syndrome being its production enhanced. Whatever the cause, it should be noted that the increase in CGA in renal failure is variable among the studied patients, and in fact, the data points are scattered. Recently, a role of CGA in the modulation of PTH secretion has been described Citation[[29]]. Thus, the high levels of CGA found in some patients could be related to uremic osteodystrophy. Furthermore, an increased content of low MW proteins in the remaining kidney of uninephrectomized rats has been demonstrated Citation[30-32]. The increased kidney content of proteins is thought to be one among the several factors involved in progression of renal damage Citation[[33]]. An increase of CGA, like other low MW proteins, in tubular cells should happen during the compromise in renal function, and as a consequence it, most likely, is involved in the pathobiology and progression of renal failure.
As far as the possibility of its use as a marker of renal function, CGA seems to have no advantages with respect to the other low MW proteins. In fact, even CGA had the highest increase, the wide scattering of the data points makes it difficult as far as the interpretation of the results is concerned. TATI and β2m seem to be better than CGA as markers of renal function. In fact, despite their increase is lower than that of CGA data points are less scattered. It is of a particular note that is worth mentioning that the blood levels of TATI increase much higher in renal failure but the data points are not scattered. For these reasons TATI can be considered the best marker of renal function among those that were evaluated in this investigation Citation[[14]], Citation[27-28].
Since CGA has been proposed as a marker of neuroendocrine tumors, the renal function of the studied patients must be assessed. In fact, an increase in blood level of CGA can be due to a decrease in renal function and not to the presence of neoplasia.
In conclusion, CGA, like other low MW proteins, is handled by the kidneys. When CGA is used as a tumor marker, it is recommended that the renal function must be also concomitantly evaluated.
REFERENCES
- Baudin D, Gigliotti A, Ducreux M, Ropers J, Comoy E, Sabourin J C, Bidart J M, Cailleux A F, Bonacci R, Ruffle P, Schlumberger M. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. Br J Cancer 1998; 78(8)1102–1107
- Degorce F, Goumon Y, Jacquemart L, Vidaud C, Bellanger L, Pons-Anicet D, Seguin P, Metz-Boutigue M B, Aunis D. A new human chromogranin A (CGA) immunoradiometric assay involving monoclonal antibodies raised against the unprocessed central domain (145–245). Br J Cancer 1999; 79(1)65–71
- Taupenot L, Remacle J E, Helle K B, Aunis D, Bader M F. Recombinant human chromogranin A: expression, purification and characterization of the N-terminal derived peptides. Regul Pept 1995; 7; 56(1)71–88
- Maack T, Hyung Park C, Camargo M JF. Renal filtration, transport and metabolism of proteins. The Kidney: Physiology and Pathophysiology, D W Seldin, G Giebisch. 2nd ed., Raven Press, New York 1992; 3005–3038
- Bianchi C, Donadio C, Tramonti G, Auner I, Lorusso P, Deleide G, Lunghi F, Vannucci C, Vitali S, Ricchiuti V, Guzzardi R. High and preferential accumulation in the kidney of anionicand cationic small proteins. Contrib Nephrol 1990; 83: 39–46
- Johansson B G, Ravnskov U. The serum levels and urinary excretion of a α2M, β2M and lysozyme in renal disease. Scand J Urol Nephrol 1972; 6: 249–256
- Sturfelt G, Truedsson L, Thysell H, Bjork H. Serum level of complement factor D in systemic lupus erythematosus – an indicator of glomerular filtration rate. Acta Med Scand 1984; 216: 171–177
- Grubb A, Simonsen O, Strufelt G, Truedsson L, Thysell H. Serum concentration of cystatin C, factor D and β2M as a measure of glomerular filtration rate. Acta Med Scand 1985; 218: 499–503
- Kusano E, Suzuki M, Asano Y, Itoh Y, Takagi K, Kawai T. Human αlM and its relationship to renal function. Nephron 1985; 41: 320–324
- Acchiardo S, Kraus A P, Jennings B R. β2M levels in patients with renal insufficiency. Am J Kidney Dis 1989; 13: 70–74
- Kyhse-Andersen J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindstrom V, Grubb A. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem 1994; 40: 1921–1926
- Newman D J, Thakkar H, Edwards R G, Wilkie M, White T, Grubb A O, Price C P. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int 1995; 47: 312–318
- Schardijn G HC. Statius van Eps LW: β2M: its significance in the evaluation of renal function. Kidney Int 1987; 32: 635–641
- Tramonti G, Ferdeghini M, Donadio C, Annichiarico C, Norpoth M, Bianchi R, Bianchi C. Serum levels of tumor associated trypsin inhibitor (TATI) and glomerular filtration rate. Renal Failure 1998; 20(2)295–302
- Lasson A, Borgstrom A, Ohlsson K. Elevated pancreatic sectetory trypsin inhibitor levels during severe inflammatory disease, renal insufficiency, and after various surgical procedure. Scand J Gastroenterol 1986; 21: 1275–1280
- DeSanto N, Veneroso S, Capodicasa G, et al. Tumor markers in uremia: carcinoembryonic antigen, neuron-specific enolase, carbohydrate antigen CA-50 and α-fetoprotein. Am J Nephrol 1986; 6: 458–463
- Docci D, Pistocchi B, Turci F, et al. Serum CA 19-9 and Ca 50 antigens in hemodialysis patients. Clinical Nephrology 1987; 27: 179–181
- Zeferos N, Digenis G B, Christophoraki M, et al. Tumor markers in patients undergoing hemodialysis or kidney transplantation. Nephron 1991; 59: 618–620
- Banfi G, Murone M, Slaviero G. High concentration of tumor-associated trypsin inhibitor in hemodialyzed patients. Clin Chem 1988; 34: 174–175
- Kamata K, Uchida M, Tekeuchi Y, et al. Increased serum concentrations of pro-gastrin-releasing peptide in patients with renal dysfunction. Nephrol Dial Transplant 1996; 11: 1267–1270
- Polenakovic M, Sikole A, Dzikova B, et al. Tumor markers in maintenance hemodialysis patients with acquired renal cystic disease. JASN 1996; 7: 1340
- Nakahama H, Tanaka Y, Fujita Y, et al. CYFRA 21-1 and Pro-GRP, tumor makers of lung cancer, are elevated in chronic renal failure patients. Respirology 1998; 3: 207–210
- Cases A, Filella X, Molina R, et al. Tumor markers in chronic renal failure and hemodialysis patients. Nephron 1991; 57: 183–186
- Eskiocak S, Dortok H, Alvur M, et al. Tumor markers in chronic renal failure and hemodialysis patients. Nephrol Dial Transplant 1995; 10: 1256
- Tramonti G, Ferdeghini M, Donadio C, Norpoth M, Annichiarico C, Bianchi R, Bianchi C. Renal function and serum concentration of five tumor markers (TATI, SCC, CYFRA 21-1, TPA, and TPS) in patients without evidence of neoplasia. Cancer Detection and Prevention 2000; 24(1)86–90
- Bianchi C, Bonadio M, Donadio C, Tramonti G, Figus S. Measurement of glomerular filtration rate in man using DTPA-99mTc. Nephron 1979; 24: 174–178
- Tramonti G, Donadio C, Ferdeghini M, Annichiarico C, Norpoth M, Bianchi R, Bianchi C. Serum tumor-associated trypsin inhibitor (TATI) and renal function. Scand J Clin Lab Invest 1996; 56: 653–656
- Tramonti G, Ferdeghini M, Donadio C, Annichiarico C, Norpoth M, Bianchi R, Bianchi C. Tumor-associated trypsin inhibitor (TATI) and renal function. Kidney Int 1997; 52(suppl 63)S179–S181
- Lewin E, Nielsen P K, Olgaard K. The calcium/parathyroid hormone concept of the parathyroid glands. Curr Opin Nephrol Hypertens 1995; 4(4)324–333
- Bianchi C, Donadio C, Tramonti G, Vannucci C, Ricchiuti V, Casani A, Lorusso P, Bonino C, Seccamani F, Lunghi F. L'accumulation renale de α1M radioiodee augmente chez le rat mononephrectomise. Nephrologie 1992; 13: 221–225
- Bianchi C, Donadio C, Tramonti G, Vannucci C, Ricchiuti V, Casani A, Lucchetti A, Bonino C, Lunghi F. Increased kidney accumulation of 131I-lysozyme in the uninephrectomized rat. Contrib Nephrol 1993; 101: 85–91
- Bianchi C, Donadio C, Tramonti G, Vannucci C, Ricchiuti V, Casani A, Della Capanna S, Lorusso P, Bonino C, Lunghi F. Kidney accumulation of α2M increases in uninephrectomized rats. Abstract of the XIIth International Congress of Nephrology. June, 13–181993. JerusalemIsrael, 554
- Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int 1997; 51: 2–15