Abstract
Connective tissue growth factor (CTGF), a member of the closely related CCN family of cytokines appears to be fibrotic in skin. To determine whether CTGF is implicated in diabetic glomerulosclerosis we studied cultured rat mesangial cells (MC) as well as kidney cortex and microdissected glomeruli from obese, diabetic db/db mice and their normal counterparts. Exposure of MC to rhCTGF significantly increased fibronectin and collagen type I secretion. Further, unstimulated MC expressed low levels of CTGF message and secreted minimal amounts of CTGF protein (36–38 kDa). However, exposure to TGF-β, increased glucose concentrations, or cyclic mechanical strain, all causal factors in glomerulosclerosis, markedly induced the expression of CTGF transcripts. With all but mechanical strain there was a concomitant stimulation of CTGF protein secretion. TGF-β also induced abundant quantities of a small molecular weight form of CTGF (18kDa). The induction of CTGF protein by a high glucose concentration was mediated by TGF-β, since a TGF-β neutralizing antibody blocked this stimulation. In vivo studies using quantitative RT-PCR demonstrated that while CTGF transcripts were low in the glomeruli of control mice, expression was increased 27-fold after approximately 3.5 months of diabetes. These changes occurred early in diabetic nephropathy when mesangial expansion was mild, and interstitial disease and proteinuria were absent. A substantially reduced elevation of CTGF mRNA (2-fold) observed in whole kidney cortices indicted that the primary alteration of CTGF expression was in the glomerulus. These results suggest that CTGF upregulation is an important factor in the pathogenesis of mesangial matrix accumulation in both diabetic and non-diabetic glomerulosclerosis, acting downstream of TGF-β.
INTRODUCTION
While acute imbalances in cytokine activity, most prominently TGF-β, are an important part of the initial response to renal injury and necessary for the increased collagen formation that is part of normal wound healing Citation[[1]], a sustained imbalance can lead to progressive renal sclerosis with eventual organ failure. TGF-β activity is increased in a variety of both human and experimental forms of glomerulosclerosis Citation[2-3] and selective modulation in the latter, results in corresponding alteration of extracellular matrix (ECM) accumulation Citation[4-6]. Further, the exposure of either cultured mesangial cells (MC) or glomeruli to TGF-β results in increased extracellular matrix (ECM) production Citation[7-8] mirroring the overaccumulation of mesangial matrix components that characterizes the lesion in vivo Citation[[9]].
Altered glomerular hemodynamics and a high extracellular glucose concentration appear responsible for the sustained overexpression of TGF-β. Exposure of MC to increased glucose concentrations stimulates the production and binding of TGF-β1 Citation[10-12] and ECM production Citation[[13]]. In diabetes and in the remnant kidney, the impairment of glomerular pressure autoregulation Citation[[14]] results in exposure to the large moment-to-moment variations in systemic blood pressure Citation[[15]], and due to the elasticity of the structure, produces glomerular expansion and MC mechanical strain Citation[[16]]. This mechanical stimulation increases production, release, activation and binding of TGF-β1 Citation[[12]], Citation[[17]] and increased synthesis and accumulation of mesangial matrix components, fibronectin, laminin, and collagen types I and IV Citation[[16]]. Further, the marked induction of MC collagen synthesis by high glucose levels alone, or in combination with cyclic stretching are significantly reduced by neutralization of TGF-β Citation[[11]].
Connective tissue growth factor (CTGF) is a recently identified peptide that may induce sclerosis, possibly by acting downstream of TGF-β. Human CTGF (hCTGF) was First identified as a product of human umbilical vein endothelial cells, and was shown to be chemotactic and mitogenic for fibroblasts Citation[[18]]. It is now known to be one of the secreted proteins that comprise the CCN (CTGF, Cyr-61, nov-1) family Citation[[19]]. These related proteins consist of a signal peptide and four modules, an insulin-like growth factor (IGF) binding domain, a von Willebrand factor type C repeat, a thrombospondin type 1 repeat, and a C-terminal module Citation[[19]]. The human, mouse and rat CTGFs are highly conserved proteins with as yet largely unknown biological activity Citation[[19]]. However, CTGF appears to be prosclerotic in dermal fibrosis Citation[20-21], and the transcripts have been reported to be upregulated in coronary heart disease Citation[[22]]. Further, CTGF may mediate, at least in part, the well known fibrogenic properties of TGF-β. A novel TGF-β responsive element is present in the CTGF promoter in fibroblasts Citation[[23]]. Our laboratory has investigated the possibility that CTGF is a critical mediator of ECM accumulation in glomerulosclerosis. To test this, we First examined the expression and matrix-inducing effects of CTGF in cultured MC. Second, we studied factors that might regulate CTGF expression in the kidney. Last, we examined CTGF activity in experimental diabetes and its relationship to mesangial expansion and proteinuria.
Regulation and Action of CTGF on Mesangial Cells In Vitro
We First examined the expression of CTGF mRNA in cultured rat MC and compared the results with those from whole kidney, and extra-renal tissue. A single (2.4 kb) CTGF transcript was weakly expressed in cultured MC and brain tissue, and was not detectable in cultured kidney fibroblasts Citation[[24]]. In comparison, the message appeared strongly expressed in the heart and kidney tissue. To determine whether CTGF could influence the synthesis or secretion of ECM, MC were exposed to recombinant human (rh) CTGF, and the effects compared to those produced by TGF-β or high glucose exposure. CTGF treatment produced a 45% increase in fibronectin, compared to a 23 and 30% increase with TGF-β and the high glucose respectively (). Similarly, the quantity of collagen type I was also increased by CTGF (64%) to levels equal, if not greater, than those induced by TGF-β (50%) or high glucose (22%).
Table 1. Effect of Exogenous CTGF Treatment on Mesangial Cell Secretion of Extracellular Matrix Molecules
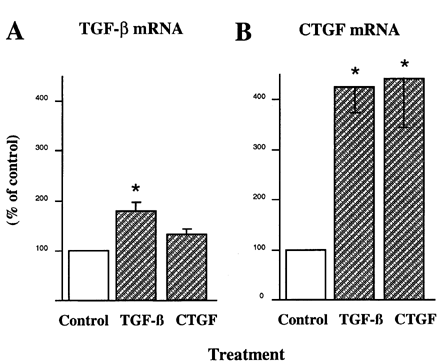
We next examined TGF-β as a possible regulatory factor in MC expression of CTGF message. Exogenous TGF-β exposure increased the expression of CTGF greater than 4-fold, whereas TGF-β1 mRNA was increased 80% (A). Exposure to CTGF failed to alter the level of TGF-β mRNA, but surprisingly auto-induced CTGF message (B). In our First experiments designed to examine the regulation of CTGF by high glucose levels, we found that MC continuously propagated in 5 mM glucose, when switched for 14 d to growth media containing 35 mM glucose, increased their levels of CTGF message nearly sevenfold Citation[[24]].
Figure 1. Regulation of CTGF and TGF-β mRNA by exogenous TGF-β and CTGF. Serum-deprived mesangial cells were incubated for 24 hr in the presence of 2 ng/mL TGF-β or 20 ng/mL rhCTGF. RNA was extracted for Northern analyses and probed for, TGF-β1 or CTGF. The mRNA bands from replicate experiments were quantified by densitometric analysis, and the results normalized to the values of the ribosomal RNA. n = 4, *P < 0.05 vs. control.
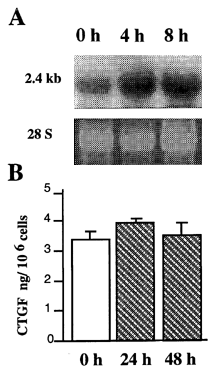
To ascertain whether mRNA expression was associated with production of CTGF protein, similar experiments were conducted and the conditioned media studied. In unstimulated MC cultures, two faint CTGF bands were detected at the level of the recombinant standard, approximately 36 and 39 kD (). The intensities of these full-length CTGF bands increased markedly following treatment with either TGF-β or high glucose, and a smaller CTGF molecule(s) (18–20 kDa) was strongly induced by TGF-β. This smaller moiety corresponds in size to half of the full-length CTGF molecule. When an ELISA was also used to quantify CTGF in supernatants, unstimulated MC secreted 2.3 ng/106 cells in a 24 hr period. This baseline level increased 2.5- and 2-fold following exposure to TGF-β or high glucose, respectively (). The effect of high glucose levels was not due to an osmolar effect since exposure to 5 mM glucose plus 15 mM mannitol had no effect on the amount of CTGF () or the distribution of secreted CTGF forms, as determined by immunoblotting (data not shown).
Figure 2. Qualitative analysis of CTGF protein and its induction in MC. Cells grown in a medium containing 5 mM glucose were serum-depleted and then cultured for an additional 48 hr in the presence of 2 ng/mL TGF-β or 20 mM glucose. Control cultures received fresh media containing 5 mM glucose, without added TGF-β. Conditioned media were pooled and heparin-sepharose purified, then immunoblotted using a polyclonal antibody raised against full length rhCTGF Citation[[24]]. Duplicate lanes represent media from different pools of media.
![Figure 2. Qualitative analysis of CTGF protein and its induction in MC. Cells grown in a medium containing 5 mM glucose were serum-depleted and then cultured for an additional 48 hr in the presence of 2 ng/mL TGF-β or 20 mM glucose. Control cultures received fresh media containing 5 mM glucose, without added TGF-β. Conditioned media were pooled and heparin-sepharose purified, then immunoblotted using a polyclonal antibody raised against full length rhCTGF Citation[[24]]. Duplicate lanes represent media from different pools of media.](/cms/asset/58ff9a2e-8cce-4c89-b543-63f9e2ef49cd/irnf_a_11378947_uf0002_b.gif)
Table 2. Quantification of CTGF Protein and Its Induction in MC
Because the stimulation of ECM production in MC by high glucose has been previously shown by our laboratory and others to be mediated in largely by TGF-β Citation[[11]], Citation[[13]], we examined the possibility that this cytokine was responsible for the observed effect on CTGF production. Again high glucose had a stimulatory effect on CTGF secretion (). However, neutralization of TGF-β activity in these cultures blocked the induction of CTGF. Constitutive secretion of CTGF, i.e., in the presence of normal concentrations of glucose, appeared somewhat reduced by the presence of TGF-β antibody, however this change was not statistically significant. Also nonsignificant was the difference in CTGF levels in normal glucose- and high glucose-treated cells when TGF-β was neutralized ().
Table 3. Role of TGF-β in High Glucose-Induced CTGF
To determine if cyclic strain is capable of altering MC expression of CTGF, cells were grown on collagen-coated flexible-bottom plates then subjected to either stretch or maintained under static conditions, as we have previously described Citation[[11]]. In an attempt to mimic conditions of MC stretch during possible low frequency oscillations in intraglomerular pressure, all experiments were carried out using alternating cycles of 10 sec stretch and 10 sec relaxation (50 mHz) and an average elongation of approximately 8% over the entire culture surface. Our previous work had shown that 24–48 hr of cyclic stretch were required for elevation in TGF-β activity Citation[[17]]. Therefore, to ascertain whether stretch could stimulate CTGF expression independent of TGF-β, mRNA levels were determined after 4 and 8 hr. Cyclic strain induced a rapid and marked induction of the CTGF message, with levels increasing more than 2-fold at 4 hr and 8 hr of stretch (A), and remaining elevated after 48 hr of stretch (not shown). When in similar experiments the secretion of CTGF protein was determined, unlike mRNA, there was no detectable influence by stretch (B).
Figure 3. Effect of cyclic stretching on the CTGF mRNA and protein levels in MC. Cells cultured overnight on collagen coated flexible-bottomed dishes were subjected to cyclic stretching or control, static conditions. At the indicated periods, (A) RNA was extracted and probed for CTGF message, or (B) media was assayed for CTGF protein by ELISA.
CTGF Expression in Experimental Diabetic Nephropathy
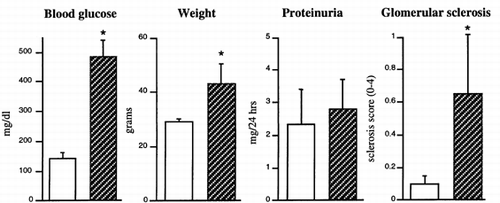
To determine if CTGF is upregulated in early diabetic nephropathy, we studied diabetic db/db mice and their nondiabetic db/m littermates. The db/db mouse carries a defective receptor gene for leptin, and becomes obese at 3 to 4 weeks of age developing hyperglycemia Citation[[25]]. Associated nephropathy includes proteinuria and mesangial expansion with increased mesangial matrix that develops by 5–7 mo Citation[[26]]. At 5 mo of age, or approximately 3.5mo after the onset of diabetes, animals were evaluated for blood glucose levels, total weight, proteinuria and mesangial expansion. Mean blood glucose levels, body weights, but not the level of proteinuria were significantly greater in the db/db animals (). Inspection of the renal tissue by light microscopy demonstrated that the diabetic animals exhibited noticeable, but minimal, glomerular changes consistent with early diabetic glomerulosclerosis, i.e., mild mesangial matrix expansion without apparent tubulointerstitial disease. Semiquantitative analysis demonstrated that the observed mesangial expansion in the diabetic animals, while statistically significant, was of mild intensity ().
Figure 4. Measurement of changes in db/db mice at 5 mos. Protein excretion was the mean of 2 consecutive 24-hr urine collections. Mesangial sclerosis was scored on a scale of 0–4. (0, no lesion; 1 minimal mesangial expansion; 2, mesangial expansion and/or basement membrane thickening; 3, marked mesangial thickening, some collapsed lumina, occasional lobule with full sclerosis; 4, diffuse collapse of capillary lumina, and sclerosis involving 75% or more of the tuft) from PAS stained kidney sections (a total of 100–150 glomeruli per kidney). *Values are significantly different from control, p < 0.05. Open bars are control db/m and stripped bars are diabetic db/db mice.
Northern analysis of whole kidney RNA indicated that the CTGF message levels were increased 2-fold in diabetic mice with similar changes in fibronectin mRNA levels (). In comparison, analysis of microdissected glomeruli by quantitative RT-PCR identified a low transcript level of CTGF in the glomeruli of control animals that was dramatically increased (27-fold) in the mice with diabetes. The upregulation of glomerular CTGF mRNA was accompanied by a nearly 5-fold increase in the amount of fibronectin mRNA. Measurement of the “housekeeping gene” GAPDH demonstrated that these changes were not due to dissimilar sizes of glomeruli in the two animal groups.
Table 4. Effect of Diabetes on CTGF and Fibronectin Transcript Levels in db/db Mice; Comparison of Whole Kidney and Glomeruli
DISCUSSION
Our demonstration that CTGF mRNA is expressed in the whole kidney of normal animals, and that its level is high in comparison to the heart and brain, suggests that endogenously produced cytokine may be involved in normal turnover of renal ECM. However, the low levels of constitutive CTGF mRNA expression demonstrated in cultured MC suggest that this cell type may have a controlling mechanism for CTGF formation different from that of other cells in the kidney. The low levels of CTGF message observed in MC under unstimulated conditions is associated with an apparent release of small quantities of CTGF protein into the culture medium, present as 36 and 38kD molecular species. This is similar in size to that secreted by vascular endothelial and fibroblast cells Citation[[18]], Citation[[27]]. The larger protein is equivalent to the full-length CTGF molecule predicted from gene analysis, whereas the smaller peptide may represent a differential N-glycosylation (unpublished observation).
A casual role for CTGF was demonstrated with the finding that exogenous CTGF stimulates MC to produce the ECM components, fibronectin and collagen type I. The finding that high extracellular glucose concentrations, TGF-β, and cyclic stretching all increase CTGF mRNA, and all but the latter simultaneously induce the production and/or secretion of CTGF protein indicate that all may be regulatory factors. This may be particularly important in diabetic glomerulosclerosis where all three of these elements are present. Based on these findings, CTGF would also expected to be important in non-diabetic renal sclerosis, since both TGF-β, and increased pressure are commonly present.
TGF-β and CTGF were able to autoinduce their own expression in MC. This is the first time such an action for CTGF has been noted. These findings suggest that once stimulated by TGF-β, CTGF mRNA levels in MC may remain elevated even in the absence of additional TGF-β activity producing a continued enhancement of ECM synthesis and deposition. This could help explain, in other studies, the prevalent inability to totally block ECM production in MC and in the mesangium by TGF-β neutralization Citation[5-6], Citation[[10]]. With a strong upregulation of CTGF protein, as occurs in response to TGF-β, there is a marked induction of a small molecular weight CTGF species, (∼ 18 kD), approximately half in size, of the full-length CTGF molecule. The structure and biological role of this CTGF fragment is unknown. However, its size and properties indicate that it contains both the thrombospondin 1 and the C-terminal modules of CTGF Citation[[19]].
The observed induction of CTGF by high glucose could have resulted from a direct effect, or have been mediated through the action of TGF-β. However, our neutralization studies demonstrated a role for the cytokine in the process, since incubation with TGF-β antibodies resulted in a complete blockade of CTGF stimulation. Our other studies showing that cyclic stretch induced within 4 hr a marked increase in CTGF transcript levels suggests that this form of induction is likely independent of TGF-β, since synthesis and activation of the latter require 48–72 hr of mechanical stimulation Citation[[17]]. The inability to measure changes in CTGF protein following stretch was unexplained. However, it is possible that in this case translational control of CTGF may be independent of transcription. Alternatively, increased CTGF protein may remain cell or matrix bound.
The studies here reported on the quantitative glomerular expression of CTGF mRNA in db/db mice, strongly suggest that CTGF action is a factor in the initiation of glomerular ECM deposition in non-insulin-deficient diabetes. While CTGF mRNA is expressed in normal glomeruli, the levels are dramatically upregulated (28-fold) after a short period of diabetes and before the onset of overt glomerular disease. In this study, CTGF mRNA upregulation occurred at a time when glomerular fibronectin mRNA levels were increased but glomerular mesangial expansion was minimal and proteinuria insignificant. As compared to glomeruli, the much lower upregulation of CTGF observed in the whole kidney suggests that the CTGF is, at least in the early phases of nephropathy, primarily involved in the induction of the glomerular alterations. However, in the more advanced stages of diabetic nephropathy, CTGF may be also an important inducer of tubulointerstitial disease. In an in vitro model of calcium oxalate nephrolithiasis, that renal tubular epithelial cells respond to calcium oxalate by upregulating the CTGF gene along with other genes involved in matrix turnover Citation[[28]]. A similar response also occurs in the same cells following mechanical wounding Citation[[29]]. Also, a positive relationship has been reported between the number of positive CTGF mRNA expressing interstitial cells and the extent of tubulointerstitial lesions in human biopsies Citation[[30]].
In summary, these studies suggest that, in addition to enhanced glomerular TGF-β expression, CTGF upregulation is an important factor in exaggerated deposition of mesangial matrix. This CTGF upregulation is likely to be maximal under conditions that include both high glucose concentrations and cellular mechanical stain, and may occur by pathways that are both dependent and independent of a preceding TGF-β stimulation. This is likely to have the greatest effect on the progression of glomerulosclerosis ultimately leading to kidney failure in diabetes where hyperglycemia occurs in combination with impaired autoregulation of intraglomerular pressure.
ACKNOWLEDGMENT
This work was supported by a Juvenile Diabetes Foundation International Grant #1-1998-243 (to Dr. Riser).
REFERENCES
- Fine L G, Hammerman M R, Abboud H E. Evolving role of growth factors in the renal response to acute and chronic disease. J Am Soc Nephrol 1992; 2: 1163–1170
- Yamamto T, Nakamura T, Noble N, Ruoslahti E, Border W A. Expression of transforming growth factor is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci 1993; 90: 1814–1818
- Sharma K, Ziyadeh F N. Renal hypertrophy is associated with upregulation of TGF-β1 gene expression in diabetic BB rat and NOD mouse. Am J Physiol 1994; 267: F1094–F1101
- Isak Y, Fujiwara Y, Ueda N, Kaneda Y, Kamada T, Imai E. Glomerulosclerosis induced by in vivo transfection of transforming growith factor-β or platelet-derived growth factor gene into the rat kidney. J Clin Invest 1993; 92: 2597–2601
- Border W A, Okuda S, Languino L R, Sporn M B, Ruoslahti E. Suppression of experimental glomerulonephritis by transforming growth factor-β. Nature 1990; 346: 371–374
- Sharma K, Jin Y, Guo J, Ziyadeh F N. Neutralization by TGF-β antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 1996; 45: 522–530
- MacKay K, Striker L J, Stauffer J W, Doi T, Agodoa L Y, Striker G E. Transforming growth factor-β: Murine glomerular receptors and responses of isolated glomerular cells. J Clin Invest 1989; 83: 1160–1167
- Bollineni J A, Reddi A S. Transforming growth factor-β1 enhances glomerular collagen synthesis in diabetic rats. Diabetes 1993; 42: 1673–1677
- Mauer S M, Steffes M W, Ellis E N, Sutherland D ER, Brown D M, Goetz F C. Structural-functional relationships in diabetic nephropathy. J Clin Invest 1984; 74: 1143–1155
- Ziyadeh F N, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-β. J Clin Invest 1994; 93: 536–542
- Riser B L, Cortes P, Yee J, Sharba A K, Asano K, Barbero A, Narins R G. Mechanical strain- and high glucose-induced alterations in mesangial cell collagen metabolism: role of TGF-β. J Am Soc Nephrol 1998; 9: 827–836
- Riser B L, Ladson-Wofford S, Sharba A, Drake K, Guerin C J, Cortes P, Yee J, Choi M, Segarini P R, Narins R G. TGF-β receptor expression and binding in rat mesangial cells: modulation by glucose and cyclic mechanical strain. Kidney Int 1999; 54: 428–439
- Ayo S H, Radnik R A, Garoni J A, Glass W F, II, Kreisberg J I. High glucose causes an increase in extracellular matrix proteins in cultured mesangial cells. Am J Pathol 1990; 136: 1339–1348
- Hayashi K, Epstein M, Loutzenhiser R, Forster H. Impaired myogenic responsiveness of the afferent arterole in streptozotocin-induced diabetic rats: Role of eicosanoid derangements. J Am Soc Nephrol 1992; 2: 1578–1586
- Bidani A K, Griffin K A, Picken M, Lansky D M. Continuous telemetric blood pressure monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol 1993; 265: F391–F398
- Riser B L, Cortes P, Zhao X, Berstein J, Dumler F, Narins R G. Intraglomerular pressure and mesangial stretching stimulate extracellular matrix formation in the rat. J Clin Invest 1992; 90: 1932–1943
- Riser B, Cortes P, Heilig C, Grondin J, Ladson-Wofford S, Patterson D, Narins R G. Cyclic stretching force selectively up-regulates transforming growth factor-β isoforms in cultured rat mesangial cells. Am J Path 1996; 148: 1915–1923
- Bradham D M, Igarashi A, Potter R L, Grotendorst G R. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biology 1991; 114: 1285–1294
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS 1993; 327: 125–130
- Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst G R. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Derm 1996; 107: 404–411
- Igarashi A, Nashiro K, Kikuchi K, Sato S, Ihn H, Fujimoto M, Grotendorst G R, Takehara K. Connective tissue growth factor gene expression in tissue sections from localized scleroderma, keloid, and other fibrotic skin disorders. J Invest Derm 1996; 106: 729–733
- Oemar B S, Werner A, Garnier J M, Do D D, Godoy N, Nauck M, Marz W, Ruppv J, Pech M, Luscher T. Human connective tissue growth factor is expressed in advanced atheroschlerotic lesions. Circulation 1997; 4: 831–839
- Grotendorst G R, Okochi H, Hayashi N. A novel transforming growth factor response element controls the expression of the connective tissue growth factor gene. Cell Growth & differentiation 1996; 7: 469–480
- Riser B L, DeNichilo M, Cortes P, Baker C, Yee J, Narins R G. Regulation of connective tissue growth factor (CTGF) activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Amer Soc Nephrol 2000; 11: 25–38
- Hummel K P, Dickie M M, Coleman D L. Diabetes, a new mutation in the mouse. Science 1966; 153: 1127–1128
- Cohen M P, Sharma K, Jin Y, Hud E, Wu V Y, Tomaszewski J, Ziyadeh F. Prevention of diabetic nephrology in db/db mice with glycated albumin antagonists. J Clin Invest 1995; 95: 2338–2345
- Steffen C, Ball-Mirth D K, Harding P A, Bhattacharyya N, Pillai S, Brigstock D R. Characterization of cell-associated and soluble forms of connective tissue growth factor (CTGF) produced by broblasts cells in vitro. Growth Factors 1998; 15: 199–213
- Hammes M S, Lieske J C, Pawar S, Spargo B H, Toback F G. Calcium oxalate monohydrate crystals stimulate gene expression in renal epithelial cells. Kidney Int 1995; 48: 501–509
- Pawar S, Kartha sec Toback F G. Differential gene expression in migrating renal epithelial cells after wounding. J Cellular Physiol 1995; 165: 556–565
- Ito Y, Aten J, Bende R J, Oemar B S, Rabelink T J, Weening J J, Goldschmeding R. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int 1998; 53: 853–861