Abstract
The efficiency of human recombinant epoetin in alleviating anemia in hemodialyzed patients has been well documented. However, the effects of rhEPO therapy in correction of antioxidant capacity are not completely explained. In this study we examined both extracellular (plasma) and intracellular (red blood cells) antioxidant potential in hemodialyzed patients before and after three and six months of epoetin treatment by evaluating markers of oxidative stress (malondialdehyde) and antioxidant capacity (thiol groups, superoxide dismutase, and glutathione peroxidase). Six months of treatment with epoetin was followed by significant increases in thiol groups, superoxide dismutase and glutathione peroxidase activities in both plasma and red blood cells of hemodialyzed patients. Hence, during accelerated erythropoiesis, an increase in the number of young hematopoietic cells may replenish erythrocyte superoxide dismutase and glutathione peroxidase activity. However, the consequences of an imbalance between enzymatic antioxidant system (higher superoxide dismutase and lower glutathione peroxidase activity) that exists in these patients are the very high red blood cell and plasma malondialdehyde levels. These results suggest that, in spite of epoetin treatment and improvement in red blood cells and plasma antioxidant capacity, the production of reactive oxygen species overwhelms the intracellular and extracellular antioxidant capacity.
INTRODUCTION
A growing body of evidence indicates that reactive oxygen species (ROS) may be involved in uremic toxicity of patients with end-stage renal disease. In addition, these patients are also chronically exposed to the oxidative stress of ROS resulting from the activation of neutrophils induced by their interaction with the dialysis membranes Citation[[1]], Citation[[2]]. Increased level of malondialdehyde (MDA), one of the end products of lipid peroxidation, has been reported in plasma and red blood cells (RBC) of hemodialysis (HD) patients Citation[[3]], Citation[[4]], Citation[[5]], Citation[[6]]. Proteins are also modified directly by ROS with the formation of oxidized amino acids or indirectly with reactive carbonyl compounds formed by the auto-oxidation of carbohydrates and lipids Citation[[7]]. Precursor carbonyl compounds derived from carbohydrates and lipids are indeed elevated in uremic circulation Citation[[8]], and chronic renal failure patients are in a state of “carbonyl stress” with potentially damaged proteins Citation[[8]]. Besides, hemodialysis is associated with a net reduction of many intracellular and extracellular enzymatic and nonenzymatic antioxidants Citation[[5]], Citation[[6]], Citation[[9]], Citation[[10]].
It has been suggested that some of the complications related to hemodialysis, including cardiovascular complications, atherosclerosis and anemia may be due to ineffective antioxidant systems and/or to increased free oxygen radical production. The use of recombinant human epoetin (rhEPO) to treat anemia in patients on maintenance hemodialysis has been a major advance in the care of these patients. The efficiency of rhEPO in improving anemia in HD patients has been well-documented Citation[[11]]. However, the effects of rhEPO therapy in correction of antioxidant capacity are not completely explained. A few reports with conflicting results of the effect of epoetin treatment on the antioxidant status in HD patients are available. It has been demonstrated that rhEPO enhanced superoxide production in stimulated polymorphonuclear cells in hemodialysis patients, both in vivo and in vitro Citation[[12]], Citation[[13]], while unstimulated whole blood superoxide anion generation did not change significantly following rhEPO therapy Citation[[13]]. Epoetin therapy has been suspected to be responsible for the increased oxidative injury to red blood cells in hemodialysis patients Citation[[3]]. However, it has been also reported that rhEPO could decrease enhanced reactive oxygen metabolites production and suggested that this decrease may protect red blood cells from hemolysis in uremic milieu Citation[[14]]. Moreover, after partial correction of renal anemia using rhEPO, malondialdehyde (MDA) and 4-hydroxynonenal levels fall below values obtained in patients with severe anemia, indicating that anemic state itself makes a secondary contribution to oxidative stress Citation[[15]]. Thus, the role of rhEPO in correction of oxidative stress in HD patients has not been, as yet, established unequivocally.
To get more insight into the possible role of rhEPO therapy in correction of oxidative stress in HD patients, we studied both extracellular and intracellular antioxidant potential in HD patients before and after rhEPO treatment. In the present study we examined the markers of oxidative stress (MDA) and antioxidant capacity (thiol groups, glutathione peroxidase, GSH-Px, and superoxide dismutase, SOD) in plasma and red blood cells of these patients.
PATIENTS AND METHODS
Patients
The average age of 15 hemodialyzed patients (seven females and eight males) included in this study was 43.6 ± 5.8 years, and the patients were on hemodialysis for 6 ± 1.2 years. Hemodialyzed patients suffering from anemia (average initial hemoglobin level 65.7 ± 2.7 g/l) were treated with human recombinant epoetin (rhEPO, 30 U/kg body weight, s.c.) three times a week. Patients were compared before and after three and six months after rhEPO treatment. A control group of 13 healthy volunteers comparable with respect to sex and age of the renal patients was selected. All participants gave their informed consent to enter the study.
Collection and Preparation of Blood Samples
After a 12-h fast, venous blood samples were taken from each patient and control subject. Blood was collected over trace-element-free heparin, and used immediately for the analysis of hematological parameters and activities of antioxidant enzymes. Several aliquots of the same samples were transferred into other tubes to be used for the assays of other plasma or serum parameters, which were performed by routine laboratory techniques. In hemodialyzed patients, blood samples were taken immediately before hemodialysis. Biochemical parameters were checked prior to and three and six months after the beginning of rhEPO treatment.
Isolation of Erythrocytes
Whole blood was immediately centrifuged at 3000 × g at 4°C for 10 min and plasma discharged. The packed red cells were washed in cold saline until supernatant was clear. The cells were lysed with distilled water (1:5 v/v) and three freeze–thaw cycles. The supernatant solution, obtained by centrifugation of cells lysate for 20 min at 10 000 × g, was used for biochemical assays.
Enzyme Assays
Superoxide dismutase (SOD) activity in the plasma and red blood cells was measured by the method of Misra and Fridovich Citation[[16]], based on the ability of SOD to inhibit auto-oxidation of epinephrine at alkaline pH (pH 10.2). One unit of SOD activity was defined as the amount of enzyme, which inhibits the oxidation of epinephrine by 50%.
Glutathione peroxidase (GSH-Px) activity was measured by the coupled assay procedure of Gunzler et al. Citation[[17]], and one unit of enzyme activity is reported as µmol NADPH oxidized/min, assuming 6.22 × 103 to be the molar absorbency of NADPH at 340 nm.
Measurement of Thiol Groups and Malondialdehyde
Plasma thiol (SH) groups concentrations were determined according to the method of Jocelyn et al. Citation[[18]]. Red blood cells non-protein thiol (NP-SH) groups were measured by spectrophotometric assay of Ellman Citation[[19]]. The concentration of thiol groups is expressed in the unit of µmol/ml.
Plasma and red blood cells extent of lipid peroxidation was measured as malondialdehyde (MDA) spectrophotometrically with 2-thiobarbituric acid. Calibration curve was prepared for each run using 1,1,3,3-tetraetoxypropane as a standard. The MDA concentration is expressed as mmol/l.
Laboratory Evaluation
Serum level of creatinine, urea, serum iron, ferritin, and total proteins were determined by routine laboratory techniques. Hematological parameters including hemoglobin, hematocrit and erythrocyte indexes were measured on an automated counter. Reticulocyte counts were measured manually after supravital staining and expressed as a percentage of the red blood cells where at least 1000 cells had been counted.
Statistical Analysis
Data are presented as mean ± S.D. Paired Student's t-test was used in order to determine if the calculated means of the obtained values were significantly changed during the trial. The limit of significance was set up at p< 0.05.
RESULTS
shows the biochemical and hematological data of the patients and healthy controls included in the study. All the patients responded to rhEPO therapy with a progressive increase in their hematocrit, hemoglobin, reticulocytes and red blood cell levels (). The increases in all parameters tested were prompt; the values were highest by the third month. Serum iron and ferritin concentrations, which showed a great dispersion at baseline, showed no significant changes with treatment ().
Table 1. Evaluation of Biochemical and Hematological Parameters in Hemodialyzed Patients During rhEPO Therapy
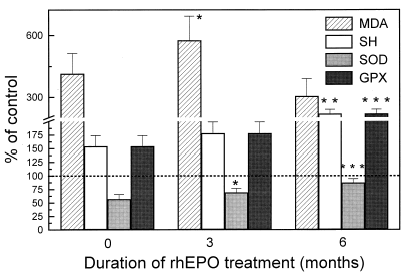
Collectively, the hemodialyzed patients exhibited increased malondialdehyde and non-protein thiol group levels and red blood cells baseline superoxide dismutase hypoactivity, compared to control subjects (, ). In plasma of hemodialyzed patients before rhEPO treatment, MDA level and SOD activity were increased, while low level of thiol groups and diminished GSH-Px activity were observed (, ).
Table 2. Markers of Oxidative Stress and Antioxidant Capacity in Red Blood Cells of Hemodialysis Patients During rhEPO Therapy
Table 3. Markers of Oxidative Stress and Antioxidant Capacity in Plasma of Hemodialysis Patients During rhEPO Therapy
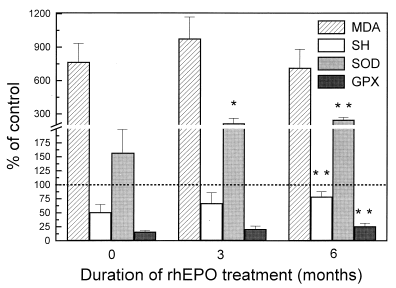
Figure 1. Markers of oxidative stress and antioxidant capacity in red blood cells of hemodialysis patients during rhEPO therapy, expressed as % of control. *p<0.05, *p<0.01, ***p<0.001 versus pretreatment values.
By the third month of rhEPO therapy, a significant increase in the red blood cell superoxide dismutase activity was already evident (, ), coinciding with increase in reticulocyte count (). The activity of this enzyme reached its maximum level by the sixth month, together with significant increase in reticulocyte counts () and red blood cells GSH-Px activity (, ). After an initial increase, which reached its maximum by the third month, the red blood cells MDA level decreased slightly during the following months, but still remained three times higher than control values (, ). During rhEPO therapy, red blood cell non-protein thiol groups progressively increased (, ).
Three months after starting with rhEPO therapy, plasma MDA levels and SOD activity are still significantly higher in hemodialyzed patients than in healthy subjects (, ). At the same time, thiol groups content as well as GSH-Px activity in treated patients were very low compared to healthy subjects (, ). A 6-month treatment with rhEPO was followed by significant increase in plasma thiol groups, SOD and GSH-Px activities (, ) compared to pretreatment values.
DISCUSSION
The evolution of hematological parameters in our patients is similar to that described in the extensive literature about the correction of nephrogenic anemia with rhEPO Citation[[11]]. Besides, a 6-month treatment with rhEPO was also followed by significant increases in antioxidant capacity in red blood cells and plasma of HD patients.
During epoetin therapy, a significant increase in the RBC SOD activity was evident, followed by similar changes in red blood cells and plasma GSH-Px activities, although the values obtained for red blood cells SOD as well as for plasma GSH-Px activities after rhEPO therapy are still lower than in healthy controls. Increased availability of antioxidants, reflected in increasing RBC SOD and GSH-Px activities, previously has been reported in hemodialyzed patients Citation[[20]] and in chronic ambulatory peritoneal dialysis patients Citation[[21]] during epoetin treatment. In theory, no induction of antioxidant enzymes occurs in RBC, which lack the ability of de novo protein synthesis. This argument has been used by some authors to explain the particular susceptibility of RBC to peroxidative membrane damage induced by uremia Citation[[22]], Citation[[23]], Citation[[24]]. However, it is known that stimulation of erythropoiesis during epoetin therapy increases RBC precursors where de novo synthesis can occur. Thus, in our patients, the elevated SOD and GSH-Px activities may be due to an increased number of young cells, such as reticulocytes, which are known to contain elevated levels of the above-mentioned enzymes Citation[[25]].
It is generally believed that blood plasma is exposed to more severe oxidative stress than intracellular fluids Citation[[26]]. Thiol groups of serum proteins, including serum albumin, have been suggested to be a “sacrificial” antioxidant in plasma and extravascular spaces Citation[[27]]. The results presented in this study demonstrated that the concentration of protein thiol groups (P-SH) in plasma was markedly reduced in HD patients before rhEPO therapy. A 6-month treatment with rhEPO was followed by significant increase in plasma thiol groups. At the same time rise in RBC non-protein SH groups was also observed. Our data together with those of Ludat et al. Citation[[28]] who found a significant increase in whole-blood glutathione level after rhEPO treatment, show diminished secondary free radical damage to thiol groups of peptides and proteins during the correction of nephrogenic anemia.
Very low plasma GSH-Px activity, found in hemodyalized patients as consequence of its impaired synthesis in the damaged renal cells Citation[[29]], significantly increased during rhEPO treatment, but its level still remained several times lower than in healthy subjects. Therefore, an imbalance between superoxide dismutase and glutathione peroxidase activity in HD patients treated with rhEPO still exists. Lack of coordinated action of plasma antioxidant enzymes, superoxide dismutase and glutathione peroxidase, may result in accumulation of free radical species and in unscheduled oxidation of susceptible molecules. Our data on increased plasma and red blood cell MDA levels after three months of epoetin treatment suggest that, despite improvement in RBC and plasma antioxidant capacity, susceptibility of intracellular and extracellular lipids to oxidation remains significant. An increased MDA level during the initial phase of rhEPO therapy had been also reported in children Citation[[30]] while Cavdar et al. Citation[[31]] showed unchanged MDA plasma concentrations. By the sixth month of epoetin therapy malondialdehyde levels slightly decreased in our patients, but remained above the normal range.
In summary, the use of epoetin to correct anemia and rejuvenate the red blood cell population induces a significant increase in the endogenous RBC and plasma antioxidants in hemodialysis patients. However, after correction of anemia lipid peroxidation products are still higher than those in healthy subjects, indicating that the free radical production in these patients overwhelms the capacity of intracellular and extracellular antioxidant systems.
REFERENCES
- Klahr S. Oxygen Radicals and Renal Diseases. Miner. Electrolyte Metab. 1997; 23: 140–143
- Luciak M., Trznadel K. Free Oxygen Species Metabolism During Hemodialysis with Different Membranes. Neprol. Dial. Transplant. 1991; 6: 66–70
- Zachee P., Ferrant A., Daelemans R., Coolen L., Goosens W., Lius R.L., Couttenye M., De Broe M.E., Boogertes M.A. Oxidative Injury to Erythrocytes, Cell Rigidity and Splenic Hemolysis in Hemodialyzed Patients. Nephron. 1993; 65: 288–293
- Dasgupta A., Hussain S., Ahmad S. Increased Lipid Peroxidation in Patients on Maintenance Hemodialysis. Nephron. 1992; 60: 56–59
- Peuchant E., Carbonneau M.A., Dubourg I. Lipoperoxidation in Plasma and Red Blood Cells of Patients Undergoing Hemodialysis. Vitamins, A, E and Iron Status. Free Radic. Biol. Med. 1994; 16: 339–346
- Mimić-Oka J., Simić T., Djukanović L.J., Reljić Z., Davičević Ž. Alteration in Plasma Antioxidant Capacity in Various Degrees of Chronic Renal Failure. Clin. Nephrol. 1999; 51: 233–241
- Rice-Evans C.A. Formation of Free Radicals and Mechanisms of Action in Normal Biochemical Processes and Pathological States. Free Radical Damage and Its Control, C.A. Rice-Evans, R.H. Burdon. Elsevier, New York 1994; 131–151
- Inagi R., Miyata F. Oxidative Protein Damage with Carbohydrates and Lipids in Uraemia. Blood Purif. 1999; 17: 95–98
- Mimić-Oka J., Simić T., Ekmeščić V., Dragičević P. Erythrocyte Glutathione Peroxidase and Superoxide Dismutase Activities in Different Stages of Chronic Renal Failure. Clin. Nephrol. 1995; 44: 44–48
- Fiorillo C., Oliviero C., Rizzuti G., Nediani C., Pacini A., Nassi P. Oxidative Stress and Antioxidative Defenses in Renal Patients Receiving Regular Hemodialysis. Clin. Chem. Lab. Med. 1998; 36: 149–153
- Nimer S.D., Wolcott D.L. Recombinant Human Epoetin and Renal Anemia: Molecular Biology, Clinical Efficacy and Nervous System Effects. Ann. Intern. Med. 1991; 114: 402–416
- Hung-Chun Chen, Jer-Chia Tsai, Juei-Hsiung Tsai, Yung-Hsiung Lai. Recombinant Human Epoetin Enhances Superoxide Production by FMLP-Stimulated Polymorphonuclear Leukocytes in Hemodialysis Patients. Kidney Intern 1997; 52: 1390–1394
- Luciak M., Pawlicki L., Kedziora J., Trznadel K., Blaszcyk J., Buczynski A. Whole Blood Superoxide Anion Generation and Efficiency of Some Erythrocyte Antioxidant Systems During Recombinant Human Epoetin Therapy in Uremic Anemia. Free Radic. Biol. Med. 1991; 10: 397–401
- Bozfakioglu S., Alptekin N., Seckin S., Ark E., Kocak-Toker N. Red Cell Lipid Peroxidation and Antioxidant System in Chronic Renal Failure Patients Treated with Recombinant Human Erythropoietin. Nephron. 1992; 61: 228–229
- Sommeburg O., Grune T., Hampl H., Reidel E., Ehrich J.H.H., Siems W.G. Does Treatment of Renal Anemia with Recombinant Human Erythropoietin Influence Oxidative Stress in Hemodialysis Patients?. Clin. Nephrol. 2000; 53: S23–S29
- Misra H.P., Fridovich I. The Role of Superoxide Anion in the Auto-Oxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972; 247: 3170–3175
- Gunzler W.A., Kremers H., Flohe L. An Improved Coupled Test Procedure for Glutathione Peroxidase in Blood. Z. Klin. Chem. Klin. Biochem. 1974; 12: 444–448
- Jocelyn P.C. Spectrophotometric Assay of Thiols. Methods in Enzymol. 1987; 143: 44–67
- Ellman G.L. Tissue Sulfhydryl Groups. Arch. Biochem.Biophys. 1959; 74: 443–450
- Delmas-Beauvieux M.C., Combe C., Peuchant E., Carbonneau M.A., Duborg L., Precigout V., Aparicio M., Clerc M. Evaluation of Red Blood Cell Lipoperoxidation in Hemodialysed Patients During Epoetin Therapy Supplemented or Not with Iron. Nephron. 1995; 69: 404–410
- Canestrani F., Buoncristiani U., Galli F. Redox State, Antioxidative Activity and Lipid Peroxidation in Erythrocytes and Plasma of Chronic Ambulatory Peritoneal Dialysis Patients. Clin. Chim. Acta 1995; 234: 127–136
- Asamaya K., Shiki Y., Ito H., Hasegana O., Miyao A., Hayashiba H. Antioxidant Enzymes and Lipoperoxidation in Blood in Uremic Children and Adolescents. Free Radic. Biol. Med. 1990; 9: 105–109
- Giardini O., Taccone-Galucci M., Lubrano R., Ricciardi-Tenore G., Bandino D., Silvi I., Ruberto U., Casciani C.U. Evidence of Red Blood Cell Membrane Lipid Peroxidation in Hemodialysis Patients. Nephron. 1984; 36: 235–237
- Taccone-Galucci M., Giardini O., Lubrano R. Red Blood Cell Lipid Peroxidation in Pre Dialysis Chronic Renal Failure. Clin. Nephrol. 1984; 27: 238–241, 1987
- Glass G.A., Gershon D. Decreased Enzymatic Protection and Increased Sensitivity to Oxidative Damage in Erythrocytes as a Function of Cell and Donor Aging. Biochem. J. 1984; 218: 531–537
- Halliwell B., Gutteridge M.C. Antioxidants of Human Extracellular Fluids. Arch. Biochem. Biophys. 1990; 280: 1–8
- Halliwell B. Reactive Oxygen Species in Living Systems. Source, Biochemistry and Role. Am. J. Med. 1991; 9: 114–122
- Ludat K., Sommeburg O., Grune T., Siems W.G., Riedel E., Hampl H. Oxidation Parameters in Complete Correction of Renal Anemia. Clin. Nephrol. 2000; 53: S30–S35
- Yoshimura S., Suemizu H., Nomoto Y. Plasma Glutathione Peroxidase Deficiency Caused by Renal Dysfunction. Nephron. 1996; 73: 207–211
- Turi S., Nemeth I., Varga I., Bodrogi T., Matkovics B. The Effect of Erythropoietin on the Cellular Defence Mechanism of Red Blood Cells in Children with Chronic Renal Failure. Pediatr. Nephrol. 1992; 6: 536–541
- Cavdar C., Camsari T., Semin I., Gonenc S., Acikgoz O. Lipid Peroxidation and Antioxidant Acitivty in Chronic Hemodialysis Patients Treated with Recombinant Human Erythropoietin. Scand. J. Urol. Nephrol. 1997; 31: 371–375