Abstract
The purpose of this study was to assess the effects of the cyclooxygenase inhibitor, indomethacin, and some vasoactive agents on the renal functional parameters during the early liver injury induced by four days bile duct ligation (BDL). Wistar rats with four days-BDL and control-sham operated were used. Renal function was measured in anesthetized rat treated with a single dose of indomethacin (control, 0.3, 1.0, 3.0 mg/kg b.w.; i.v.) one hour before clearance studies. Sulindac effects were also evaluated (5 mg/kg b.w., i.p). Isolated rat kidney preparations from control and BDL donor rats were used to study renal vascular response to noradrenaline, carbachol or sodium nitroprusside. The bile duct ligation promoted a diminished renal cortical plasma flow (RCPF) on the fourth day post surgery accompanied with a diminution in the glomerular filtration rate (GFR), increased filtration fraction and increased fractional excretion of water and sodium. Indomethacin 0.3 mg/kg induced an increase in GFR and RCPF, maintaining the high filtration fraction in BDL rats. The other doses did not alter these parameters as compared with bile duct ligated rats without treatment, but indomethacin 3 mg/kg caused a significant increase in filtration fraction. Indomethacin induced dose-dependent diminution in natriuresis in sham and BDL groups. Sulindac did not modify hemodynamic parameters, but induced antinatriuresis and antidiuresis in both experimental groups. Maximal vascular responses to noradrenaline measured in isolated rat kidneys were statistically diminished in BDL-rats as compared with controls (C, n = 7; 35.0 ± 2.3 mmHg ml−1 min; BDL-rats, n = 5; 23.8 ± 0.7 mmHg ml−1 min; p<0.02), without changes in EC15. Maximal relaxation induced by sodium nitroprusside in the phenylephrine (PHE)-pre-constricted renal vasculature in control preparations did not differ from that observed in BDL group (C: n = 6; 49.5 ± 2.3%). Values of EC50 were 1.26 ± 0.07 µM (n = 6) in control preparations and 0.34 ± 0.03 µM (n = 4) in kidneys from BDL-rats (p<0.001). Carbachol induced a biphasic relaxation of PHE-pre-constricted renal vasculature. No differences in maximal responses were found. EC50 value of the second phase in BDL group was significantly decreased compared to control preparations (C: n = 6, 0.47 ± 0.05 µM; BDL: n = 6, 0.22 ± 0.03 µM; p<0.001). The present results show that the altered renal function after a short time post bile duct ligation is determined, at least in part, by increased release of arachidonic derivatives in vascular bed and tubular cells. At this stage of liver injury, the alteration in the renal vascular response to different vasoactive agents is remarkable.
INTRODUCTION
Abnormalities in sodium and water renal excretion are commonly found in cirrhosis, in human as well in animal models. These abnormalities lead eventually to ascites, a common complication of cirrhosis and a major cause of morbidity and mortality Citation[[1]]. Since the peripheral arterial vasodilatation hypothesis has been proposed to explain the mechanism of ascites formation Citation[[2]], the research in this area has progressed substantially Citation[[3]], Citation[[4]]. The hypothesis proposed that primary peripheral arterial vasodilatation, mainly in the splanchnic circulation, leads to the relative underfilling of the arterial circulation Citation[[5]], hyperdynamic circulation Citation[[6]] and the release of numerous humoral and neuronal mediators Citation[[7]], Citation[[8]], Citation[[9]], Citation[[10]].
In animal models, there is a close chronological relationship between the onset of sodium retention and the decrease in blood pressure Citation[[11]], Citation[[12]]. As the endothelium controls the vascular tone, several vasoactive factors (NO, prostaglandins) have been implicated in the pathogenesis of the hemodynamic abnormalities of cirrhosis Citation[[3]], Citation[[13]]. Modification in the responsiveness to endogenous vasoconstrictors including angiotensin II Citation[[14]], norepinephrine Citation[[15]], endothelin Citation[[16]], and vasopressin Citation[[17]] were also described.
Previous work from our laboratory reported abnormalities in rat renal function resembling acute renal failure after a few days of post bile duct ligation Citation[[18]]. The animals did not present ascites, and the systemic blood pressure was similar to control animals in every experimental group. The bile duct ligation promoted a diminished renal cortical plasma flow (RCPF) on the fourth and sixth day post ligation which was accompanied with a diminution in the glomerular filtration rate (GFR) and an increased filtration fraction only on the fourth day. These data were taken as evidence of a greater and more sustained diminution in the RCPF than in the GFR. To validate the early onset of cortical vasoconstriction, the effect of continuous intravenous infusion of dopamine was evaluated in four days bile duct ligated (BDL)-rats. The renal hemodynamic function was normalized with dopamine treatment, although tubular function was not completely recovered Citation[[18]].
Various factors were reported underlying early renal changes promoted by liver disease before any ascites formation. Some authors marked the importance of some bile components leaving access to systemic circulation Citation[[19]], Citation[[20]]. Endogenous vasoconstrictors could be released and so promote the cortical renal vasoconstriction evident on the fourth and sixth days of post bile duct ligation. In this line, thromboxane was increased in urine of BDL-rats Citation[[21]], plasma renin activity was also increased in BDL-rats during the first week following ligation Citation[[22]]. Synthesis of PGI2 and PGE2 is very important to counterbalance the vasoconstrictor effect of angiotensin II, norepinephrine, AVP and increased renal sympathetic tone to maintain renal plasma flow and GFR Citation[[1]].
The present study was undertaken to further investigate the early onset of renal failure observed in rats after four days of bile duct ligation, focusing especially on endogenous prostanoids and the responsiveness of the renal vasculature to noradrenaline, carbachol or sodium nitroprusside.
MATERIAL AND METHODS
Animals
Adult male Wistar rats (250–300 g body weight) were randomly divided in two groups: sham-operated (SO)-controls and BDL-rats during four days as reported Citation[[18]]. In order to be included in the study, BDL-rats had to have hyperbilirubinemia. Serum lactate dehydrogenase (LDH) concentration was used to evaluate the liver injury. All rats were housed in a room with controlled temperature (21–23°C) and light (06–18 h). They were maintained on a standard diet and water ad libitum. All procedures were in accordance with institutional guidelines. Some animals of each group were used to study renal function by clearance techniques, and others were used as kidney donors to study renal vascular responses to different treatments. The animals were anesthetized with sodium pentobarbital (50 mg/kg b.w, i.p.).
Experimental Procedures
Renal Clearance Studies
Rats were prepared for renal clearance studies as previously reported Citation[[18]], Citation[[23]], Citation[[24]]. After surgery, a priming solution (18 mg inulin and 6 mg p-aminohippuric acid (PAH) in saline) was administered i.v., and a sustaining infusion (5% mannitol, 9 mg/ml inulin, 3 mg/ml PAH) was then begun at 5 ml/h and continued through the entire experiment. After equilibration for a 30 min period, urine was collected during two 20-min periods. Blood from the femoral artery was taken at midpoint. GFR was estimated by inulin clearance and RCPF by PAH clearance. The filtration fraction (FF) was calculated from these data. To study the tubular function, fractional excretions of water (FEH2O%), sodium (FENa%) and potassium (FEK%) were estimated.
Experimental Protocols
To study the possible role of arachidonic acid derived products, the inhibitor of cyclooxygenase, indomethacin (INDO) was used. SO and BDL-rats received a single dose of INDO (i.v.) one hour before clearance studies. According to the dose, four different treated groups were studied: basal; 0.3 mg/kg b.w.; 1.0 mg/kg b.w.; and 3.0 mg/kg b.w. These doses were previously used for similar purposes Citation[[25]].
The effects of sulindac (SU) were also evaluated. Clinical studies indicated that SU does not alter the urinary excretion of prostaglandins or alter renal function Citation[[26]]. SO and BDL-rats received a single dose of SU (5 mg/kg b.w., i.p.) one hour before clearance studies, as just reported Citation[[27]].
Renal Vascular Responsiveness to Different Vasoactives
To study renal vascular responsiveness, the isolated perfused kidney model was employed. This model allows studying the renal vascular reactivity in the absence of extra-renal influences Citation[[28]], Citation[[29]].
The right kidney was prepared and perfused as previously described Citation[[28]]. The perfusion medium (pH: 7.4) comprised Krebs Ringer solution containing glucose (10 mM), sodium pyruvate (5 mM) and sodium lactate (5 mM). The medium also contains 0.5 mM cysteine, 0.5 mM glutamic acid, 2.3 mM glycine, and 0.6 mM l-arginine since amino-acid deficiency and glutathione depletion may contribute to disturbances in renal structure and function Citation[[30]]. A close relationship has been demonstrated between renal glutathione levels and renal function Citation[[23]]. The medium was constantly bubbled with 95% O2 and 5% CO2. The whole system operated under thermostatic control at 37°C. Perfusion flow through the isolated kidney was performed with a peristaltic pump (Masterflex, USA) at a constant rate measured with a flowmeter (Gilmont Instruments Inc., USA) inserted in the arterial line. Perfusion pressure was continuously measured at the tip of the arterial cannula by a pressure transducer (Harvard Instruments, USA) and recorded on a multipen recorder (Cole Palmer, USA).
The isolated kidney was perfused for about 30 min until pressure and flow remained constant. After this equilibration period, renal perfusion pressure (RPP) was reduced to 50–60 mmHg, maintaining a renal perfusion flow (RPF) of 10–12 ml min−1. The subsequent experimental time was 60 min, during which RPF was maintained constant and renal vascular response to a vasoactive agent was measured.
Experimental Protocols
Graded doses of noradrenaline (NA) were injected directly in the arterial line, maintaining an open system. NA suffers a considerable uptake and metabolism in renal tissue and thus it seems difficult to quantify cumulative doses. The NA doses range was 0.25–100 nmol. After each dose, the vasculature was allowed to relax, returning RPP to baseline value. Renal vascular resistance (RVR) was calculated as RPP factored by RPF. Changes in RVR from baseline values were considered renal vascular responses to this alpha-adrenergic agonist.
To study vasodilator responses, the tone of preparations was raised by the addition of phenylephrine (PHE) to the perfusate to a final concentration of 2.5 µM, maintaining a close-circuit system. This PHE concentration produces approximately 45% of the maximal contractile response to PHE Citation[[31]]. At the plateau contraction, graded doses of a vasodilator were added to the medium in a cumulative mode. The response was allowed to stabilize before the addition of each incremental dose of the vasodilating agent. Vasodilator responses are reported as a percentage of relaxation from the PHE-induced constriction.
Endothelial-independent relaxation was assessed by the responses to incremental concentrations of sodium nitroprusside (SNP). The perfusate SNP concentration range was 0.001–50 µM.
To investigate endothelial-dependent relaxation, concentration-response curves to carbachol were performed. The perfusate carbachol concentration range was 0.01 nM–50 µM.
Analytical Methods
PAH concentrations in serum and urine were determined as described by Waugh and Beall Citation[[32]]. Inulin concentrations in the same samples were measured by Roe's method Citation[[33]]. Sodium and potassium were measured by flame photometry; and the volume of urine was measured by gravimetry. LDH and bilirubin were determined by standard methods using commercial kits (Wiener Lab., Argentina).
Statistical Analysis
The results are given as mean ± SEM. For studies of renal function, analysis of variance followed by Bonferroni's test were used to assess the significance of differences between groups. A value of p ≤ 0.05 was considered significant.
In renal vascular responsiveness studies, a concentration (or dose) response curve was fitted using a computer program (Grafit, Data Analysis and Graphics Program Version 3.00). For each curve, maximum response and the concentration (or dose) required for the half-maximal effects (EC50) were obtained. Each experimental group presents a mean value ± SEM of the individual parameters of fitted curves, and statistical significance was evaluated using Student's t-test. p ≤ 0.05 was considered statistically significant.
Drugs
Chemicals were of the highest grade available commercially. Indomethacin was obtained as commercially available therapeutic parenteral preparation from Syntial Lab. (Argentina), sulindac was received as kind gifts from Merck Sharp and Dohme (Argentina). Carbachol, phenylephrine chlorohydrate, sodium nitroprusside, and (±) noradrenaline hydrochloride were purchased from Sigma Chemical Company (St. Louis, MO, USA).
RESULTS
Rats tolerated the surgical procedure. Rats’ body weight presented a significant diminution on the fourth day after BDL. Serum bilirubin concentration and LDH significantly increased after bile duct ligation. Data are collected in .
Table 1. Serum Total Bilirubin (TB) and LDH Levels and Renal Functional Parameters in BDL Rats on the Fourth Day After Surgery
Renal Function in BDL-Rats Four Days After Surgery
The mean arterial blood pressure remained unchanged throughout the experiments. Differences were not found among the groups (sham-operated: 111 ± 9 mmHg). Data of renal function in controls and on the fourth day after BDL-rats are presented in .
Effects of Indomethacin and Sulindac on Renal Function of BDL-Rats
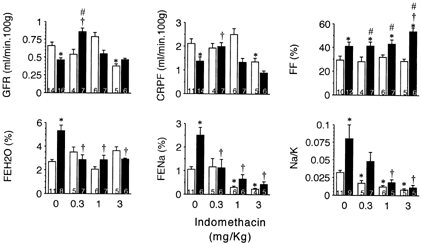
INDO administration improved renal hemodynamic parameters in BDL-rats only at the lower dose employed, 0.3 mg/kg. It is noticeable that INDO 3 mg/kg diminished both GFR and RBF in control rats, but not in BDL animals. FF remained constant in the control group, despite the INDO dose administered. INDO 3 mg/kg caused a significant increase in FF calculated in BDL-rats. INDO induced dose dependent-antinatriuresis in both experimental groups; the drug promoted fractional sodium excretion in ligated-rats similar to those in control rats. This diminution was almost independent of potassium excretion. The relationship Na/K in urine diminished in a dose-related way in both experimental groups. INDO promoted water retention in the BDL group. Data are collected in .
Figure 1.Effects of indomethacin on renal function in sham-operated (□) and bile duct litigated (▄) rats four days after surgery. A single dose of indomethacin was administered i.v. one hour before clearance studies. Data are mean ± SEM. Numbers within the columns indicate the number of animals in the group.*p<0.05 as compared with sham-operated rats without treatment with indomethacin.†p<0.05 as compared with BLD-rats without treatment with indomethacin.#p<0.05 as compared with sham-operated rats treated with equal dose of indomethacin.
SU did not modify hemodynamic parameters in BDL-rats. This drug induced antinatriuresis in both control and BDL-rats (FENa%: Sham, n = 11, 1.05 ± 0.11; BDL, n = 6, 2.50 ± 0.34*; Sham + SU, n = 6, 0.37 ± 0.05*; BDL + SU, n = 8, 0.39 ± 0.07*†; *p<0.05 versus sham; †p<0.05 versus BDL) and is associated with water retention in BDL-rats.
Vascular Responsiveness in the Isolated Perfused Rat Kidney
Perfusion Parameters
After a 30 min equilibration period, no differences in RVR among kidneys obtained from control or BDL-rats were found. Control values (n = 21) for perfusion parameters were RPF = 20.7 ± 1.3 ml/min, RPP = 113 ± 2 mmHg and RVR = 5.6 ± 0.5 mmHg ml−1 min.
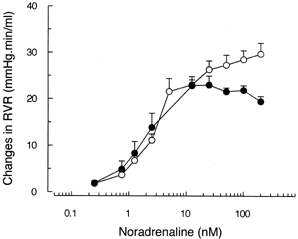
Vasoconstrictor Responses to Noradrenaline
The maximal rise in RVR (mmHg ml−1 min) was statistically diminished in BDL-rats as compared with controls (C: n = 7, 35.0 ± 2.3; BDL-rats: n = 5, 23.8 ± 0.7; p<0.02). Because of this difference in Emax, we calculated EC15 (nmol) instead of EC50. EC15 is the dose necessary to increase RVR in 15 mmHg ml−1 min. No differences were observed among groups (C: n = 7, 4.51 ± 1.00; BDL-rats: n = 5, 4.99 ± 1.64). Curves are shown in .
Figure 2. Changes in renal vascular resistance induced by different doses of noradrenaline (NA) in the isolated perfused rat kidneys of sham-operated (◯, n = 7) and BDL (•, n = 5) rats. Graded doses of NA were injected directly in the arterial line maintaining an open system. Renal vascular resistance (RVR) was calculated as renal perfusion pressure factored by renal perfusion flow. Changes in RVR from baseline values were considered as renal vascular responses to this alpha-adrenergic agonist. Data are mean ± SEM.
Vasodilator Responses
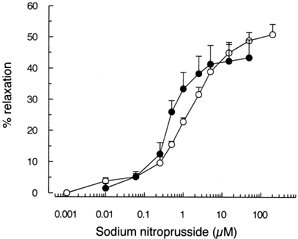
Response to Sodium Nitroprusside in Phenylephrine-Preconstricted Vasculature of the Isolated Rat Kidney
When added in sequentially increasing concentrations, SNP induced a concentration dependent relaxation of the PHE-preconstricted renal vasculature. Maximal relaxation in control preparations did not differ from that observed in BDL group (C: n = 6; 49.5 ± 2.3%; BDL: n = 4, 45.5 ± 5.7%). Values of EC50 were 1.26 ± 0.07 µM (n = 6) in control preparations and 0.34 ± 0.03 µM (n = 4) in kidneys from BDL-rats (p<0.001). Curves are presented in .
Figure 3. Relaxation of phenylephrine-preconstricted renal vasculature induced by sequentially increasing concentrations of sodium nitroprusside in the isolated perfused rat kidneys of sham-operated (◯, n = 6) and BDL (•, n = 4) rats. Vasodilator responses are reported as percentage of relaxation from the phenylephrine-induced constriction. Data are mean ± SEM.
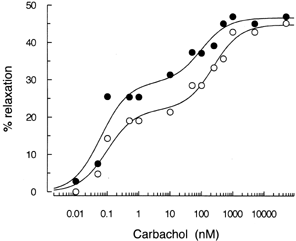
Response to Carbachol in Phenylephrine-Preconstricted Vasculature of the Isolated Rat Kidney
Carbachol caused a biphasic concentration-response curve in both experimental groups. In this sense, it has been reported that carbachol binds to different affinity sites in membranes obtained from guinea pig taenia caecum and in a variety of tissues Citation[[34]]. Typical curves produced by different concentrations of carbachol are shown in . Previous studies from our laboratory have denoted a similar response to carbachol in isolated kidneys from control or diabetic rats Citation[[31]]. No differences in the maximal response were observed in the preparations taken from control (n = 6) (Phase 1: 17.7 ± 3.0%; Phase 2: 26.0 ± 2.7%) or BDL (n = 6) (Phase 1: 22.2 ± 4.0%; Phase 2: 30.2 ± 3.5%)-animals. No statistical differences between groups in EC50 belonging to Phase 1 (C: n = 6, 0.13 ± 0.03 nM; BDL: n = 6, 0.13 ± 0.02 nM) were found, while values of Phase 2 (C: n = 6, 0.47 ± 0.05 µM; BDL: n = 6, 0.22 ± 0.03 µM, p<0.001) were found to be different.
DISCUSSION
In a previous publication from our laboratory, we reported a renal cortical vasoconstriction in jaundiced rats on the fourth and sixth days after BDL, which was reversed by dopamine Citation[[18]]. Other authors have also described reduced renal perfusion, redistribution of renal plasma flow and depression of glomerular filtration rate using the same experimental model as ours Citation[[35]].
Experimental studies on a potential role of catecholamines and prostaglandins as the cause for disturbed renal hemodynamics in BDL-rats have so far led to equivocal results Citation[[36]]. Thromboxane was described to have a role in the early phase of ischemic acute renal failure and also in the renal failure of BDL-rats Citation[[35]]. On the other hand a higher level of PGE2 in plasma and urine of BDL-rats was also described Citation[[21]], Citation[[36]]. Moreover, renal and vascular cytosolic phospholipase A2 activity (the key enzyme in the regulation of prostaglandin synthesis) in rats with cirrhosis and ascites is increased compared with control tissue Citation[[37]].
In the present study, we obtained evidence for the possible role of the products of cyclooxygenases in the development of cortical vasoconstriction at fourth day post BDL by analyzing the effects of different doses of indomethacin and sulindac. The effects observed with INDO 0.3 mg/Kg confirm, at least in part, the participation of a vasoconstrictor product of arachidonic acid during this stage of BDL, indicating a preferential inhibition of the synthesis of a vasoconstrictor agent. The other doses tested did not change hemodynamic parameters as compared with BDL-untreated rats, although the higher dose modified renal hemodynamic in SO-controls. Diuresis and natriuresis developed by BDL were also reversed by INDO in a dose dependent way. A similar effect on natriuresis was promoted in control rats. Wilcox and associates found that pressor responses to infusion of angiotensin II are blunted in the presence of thromboxane receptors antagonists Citation[[38]], Citation[[39]], suggesting that TxA2 may also modulate the hemodynamic effects of other hormonal mediators in the kidney. TxA2 may also regulate epithelial and reabsorptive functions in medullary nephron segments Citation[[40]]. These evidences, together with the present results, suggest that TxA2 might be involved in renal alterations observed at fourth day post bile duct ligation in rats.
On the other hand, an integral role for eicosanoids generated by macula densa-associated COX-2 in mediating renin release was also reported, thus, other prostanoids could be involved in the early cortical vasoconstriction during BDL (i.e., prostacyclin generated by the afferent arteriole mediates renin release). Prostaglandins may also be mediators of macula densa-mediated renin release Citation[[41]]. PGE2 infusion elicits natriuresis and diuresis without significant changes in GFR and RPF, consistent with its inhibition of salt absorption along the nephron Citation[[42]]. This effect may reflect PGE2's well-demonstrated capacity to inhibit salt and water absorption in the thick ascending limb and collecting duct directly Citation[[43]]. In conclusion, the complex effects of indomethacin at higher doses both in controls and BDL-rats appear to be the result of competing hemodynamic and tubular effects of prostanoids, including PGE2, PGI2 and TxA2, similar to that described for the effects on blood pressure Citation[[44]].
The role of eicosanoids derivatives was also studied by use of sulindac. It was described that all NSAID inhibit cyclooxygenase, but the sensitivity of different tissue to each drug may vary. Aspirin, indomethacin, naproxen, and ibuprofen reduced both renal function and urinary prostaglandins excretion. So far, sulindac could be the only NSAID not to inhibit renal cyclo-oxygenase significantly Citation[[45]]. Our results indicate that sulindac does not modify renal hemodynamia in BDL rats. On the other hand, the treatment diminished natriuresis in both experimental groups, giving rise to similar values among them. It has been recognized that medullary tissue synthesized greater amounts of prostaglandins than the cortex. It is generally accepted that the regional heterogeneity of arachidonate metabolism, as well as the lack of vascular communications between the medulla and cortex, dictates that prostaglandins synthesized in the cortex regulate cortical function and prostaglandins synthesized in the medulla regulate medullary function. Selective cyclooxygenase inhibitors (such as sulindac, low-dose of aspirin, sulfinpyrazone) could influence cortical synthesis in a different way than medullary. It has been reported that sulindac sulfide caused a 60–90% reduction in PGE2 excretion rate Citation[[27]]. In the sight of our results, it could be suspected that sulindac impairs PGE2 synthesis in medullary interstitial cells or collecting tubules, while INDO acts on both cortex and medulla avoiding the production of PGI2, PGE2 and TxA2.
It has been reported that angiotensin and vasopressin and other vasoconstrictors stimulate renal prostaglandin synthesis, and renal function becomes “prostaglandin dependent”. Our data reinforce the idea that during the early stage of BDL, constrictor hormones are released which promote a diminished GFR and RCPF and increased FF. As a result arachidonate metabolism could be increased both in cortex (TxA2, PGI2, PGE2) and in medulla (PGE2) Citation[[41]]. The lowest dose of INDO studied could inhibit TxA2 production allowing the expression of vasodilator prostaglandins but maintaining the higher FF as compared with control rats. The other doses could inhibit all the arachidonic derivatives maintaining the higher FF; this pattern could indicate the presence of other vasoconstrictors and/or abnormal vasculature response to them, especially on efferent arteriole.
To analyze this possibility, other experimental groups were studied to assess the response of renal vasculature to noradrenaline or vasodilators. Perfused kidney preparation offers the important advantage of circumventing non-renal factors that influence the “in vivo” model Citation[[29]]. Among them, the isolated system obviates the possible compensatory changes in the cardiocirculatory system.
A role of the sympathetic system in the hemodynamic and renal alterations of liver cirrhosis has been proposed Citation[[46]]. However, little is known about the activation of the sympathetic system in stages previous to altered extracellular fluid volume distribution. It could be assumed an altered action of noradrenaline in BDL-rats. Renal vasculature response to noradrenaline was evaluated “in vitro”. Perfused kidneys from BDL-rats showed similar sensitivity to noradrenaline compared with kidneys taken from control rats, but the maximal response was diminished. Other authors reported that the contractility of blood vessels from cirrhotic rats Citation[[47]] and jaundiced dog Citation[[48]] showed decreased contraction to phenylephrine Citation[[47]] and noradrenaline Citation[[48]]. The decreased reactivity at maximal levels of noradrenaline observed in our BDL-rats could be a consequence of a reduction in receptor sites (down regulation by the agonist) “in vivo”, associated with activation of the sympathetic system from the beginning of bile duct ligation, or a higher activity of endothelium that could counterbalance the noradrenaline contraction Citation[[49]], Citation[[50]]. It has been postulated that induction of iNOS might play a determinant role in the pathogenesis of the arterial vasodilation in cirrhosis Citation[[49]]. It has been shown that eNOS protein is increased in animal models of portal hypertension and that this increase is detectable in cirrhotic rats without ascites Citation[[1]], Citation[[50]]. Our results indicate that BDL significantly influences endothelium dependent vasodilation by carbachol. Sensibility to carbachol is increased in BDL-rats, without changes in the maximal capacity. It was remarkable that our BDL-rats were a model of acute renal failure associated with an early stage of hepatic injury that could be expressing higher levels of NO when stimulated by carbachol. Moreover, in the present study, similar capacity in the dilator response to endothelium-independent donor of vasorelaxing factor (SNP) could be demonstrated in BDL-rats compared with responses in kidneys from controls, but a higher affinity was observed. These data could indicate that NO released by SNP promoted higher response in vascular muscular cells responsiveness to this vasodilating factor in kidneys from BDL-rats.
The present results show that the altered renal function after a short time of bile duct ligation is determined, at least in part, by increased release of arachidonic derivatives in vascular bed and tubular cells. At this stage of liver injury, the alteration in the renal response to different vasoactive agents is remarkable.
ACKNOWLEDGMENT
This work was supported by grants of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP 4845/97) and Agencia Nacional de Promoción de la Ciencia y Tecnología (PICT's 0005-00000-01729). The authors thank Wiener Lab Argentina for analytical kits.
REFERENCES
- Martin P.Y., Schrier R.W. Pathogenesis of Water and Sodium Retention in Cirrhosis. Kidney Int. 1997; 51(suppl 59)S43–S49
- Schrier R.W., Arroyo V., Bernardi M., Epstein M., Enriksen J.H., Rodes J. Peripheral Arterial Vasodilation Hypothesis: A Proposal for the Initiation of Renal Sodium and Water Retention in Cirrhosis. Hepatology 1988; 8: 1151–1157
- Bomzon A., Blendis L.M. The Nitric Oxide Hypothesis and the Hyperdynamic Circulation in Cirrhosis. Hepatology 1994; 20: 1343–1350
- Henriksen J.H. Cirrhosis: Ascites and Hepatorenal Syndrome. Recent Advances in Pathogenesis. J. Hepatology 1995; 23(1)25–30
- Schroeder E.T., Shear L., Sancetta S.M., Gabuzda G.J. Renal Failure in Patients with Cirrhosis of the Liver III. Evaluation of Intrarenal Blood Flow by Para-Aminohippurate Extraction and Response to Angiotensin. Amer. J. Med. 1964; 43: 887–896
- Vorobioff J., Bredfeldt J.E., Grozman R.J. Hyperdynamic Circulation in Portal-Hypertensive Rat Model: A Primary Factor for Maintenance of Chronic Portal Hypertension. Amer. J. Physiol. 1983; 244: G52–G57
- Piszcueta M.P., Piqué J.M., Bosch J., Whittle B.J.R., Moncada S. Effects of Inhibiting Nitric Oxide Biosynthesis on the Systemic and Splanchnic Circulation on Rats with Portal Hypertension. Br. J. Pharmacol. 1992; 105: 184–190
- Schroeder E.T., Anderson G.H., Goldman S.H., Streeten D.H.P. Effect of Blockade of Angiotensin II on Blood Pressure, Renin and Aldosterone in Cirrhosis. Kidney Int. 1976; 9: 511–519
- Ros J., Jimenez W., Lamas S., Clarìa J., Arroyo V., Rivera F., Rodés J. Nitric Oxide Production in Arterial Vessels of Cirrhotic Rats. Hepatology 1995; 21: 554–560
- Bomzon A., Better O.S., Blendis L.M. Renal Alpha-1-Adrenoreceptors in Rats with Obstructive Jaundice. Nephron. 1986; 42: 258–261
- Lopez C., Jimenez W., Arroyo V., Claria J., La Villa G., Asbert M., Gaya J. Temporal Relationship Between the Decrease in Arterial Pressure and Sodium Retention in Conscious Spontaneous Hypertensive Rats with Carbon Tetrachloride-Induced Cirrhosis. Hepatology 1991; 13: 585–589
- Albillos A., Colombato L., Grozmann R. Vasodilation and Sodium Retention in Prehepatic Portal Hypertension. Gastroenterology 1992; 102: 931–935
- Zipser R.D., Lifschitz M.D. Prostaglandin and Related Compounds. The Kidney in Liver Disease, M. Epstein, M.D. Baltimore. Williams and Wilkins. 1988; 393–416
- Castro A., Jimenez W., Claria J., Ros J., Martinez J.M., Bosch M., Arroyo V. Impaired Responsiveness to Angiotensin II in Experimental Cirrhosis: Role of Nitric Oxide. Hepatology 1993; 18: 367–372
- Bichet D.G., Van Putten V.J., Schrier R.W. Potential Role in Increased Sympathetic Activity in Impaired Sodium and Water Excretion in Cirrhosis. New Engl. J. Med. 1982; 307: 1552–1557
- Uchihara M., Izumi N., Sato C., Marumo F. Clinical Significance of Elevated Plasma Endothelin Concentration in Patients with Cirrhosis. Hepatology 1992; 16: 95–99
- Linas S., Anderson R.J., Guggenheim S.J., Robertson G.L., Berl T. Role of AVP in Impaired Water Excretion in Conscious Rats with Experimental Cirrhosis. Kidney Int. 1981; 20: 173–180
- Monasterolo L., Peiretti A., Elías M.M. Rat Renal Functions During the First Days Post-Bile Duct Ligation. Renal Failure 1993; 15: 461–467
- Yarger W.E. Intrarenal Mechanism of Salt Retention after Bile Duct Ligation in Rats. J. Clin. Invest. 1976; 57: 408–418
- Shen J.S., Chen C.F., Liu H.M., Fang H.S. Effects of Rat Bile Infusion on Renal Function in Rat. Renal Physiol. Biochem 1990; 13: 213–222
- Kramer H.J., Schwarting K., Backer A. Impaired Renal Function in Obstructive Jaundice: Enhanced Glomerular Thromboxane Synthesis and Effects on Thromboxane Receptor Blockade in Bile Duct Ligated Rats. Clin. Sci. 1995; 88: 39–45
- Poo J.L., Estanes A., Pedraza-Chaverri J., Cruz C., Uribe M. Effects of Ursodeoxycholic Acid and Hemodynamic and Renal Function Abnormalities Induced by Obstructive Jaundice Rat. Renal Failure 1995; 17: 13–20
- Torres A.M., Rodriguez J.V., Ochoa J.E., Elías M.M. Rat Kidney Function Related to Tissue Glutathione Levels. Biochem. Pharmacol. 1986; 35: 3355–3358
- García V.M.C., Girardi G., Ochoa J.E., Torres A.M., Elías M.M. Early Manifestations of Nephropathy in Alloxan-Treated Rats. Renal Failure 1998; 20: 551–564
- Epstein M. Renal Prostaglandins in the Control of Renal Function in Liver Disease. Amer. J. Med. 1986; 80: 46–55
- Ciabattoni G., Pugliese F., Cinotti G.A., Patrono C. Renal Effects of Anti-Inflammatory Drugs. Eur. J. Reumatol. Inflammation 1980; 2: 210–221
- Zambrasky E.J., Chremos A.N., Dunn J.M. Comparison of the Effects of Sulindac with Other Cyclooxygenase Inhibitors on Prostaglandin Excretion and Renal Function in Normal and Chronic Bile Duct-Ligated Dogs and Swine. J. Pharmacol. Expt. Ther. 1984; 228: 560–566
- Monasterolo L., Ochoa J.E., Trumper L., Elías M.M. Vascular and Tubular Actions of Diazepam in Isolated and Perfused Rat Kidney. Eur. J. Pharmacol 1995; 276: 201–205
- Bekersky I. Use of the Isolated Perfused Kidney as a Tool in Drug Disposition Studies. Drug Metab Rev. 1983; 14: 931–960
- Epstein F., Brosnan J., Tange J., Ross B. Improved Function with Amino Acids in the Isolated Perfused Kidney. Amer. J. Physiol. 1982; 243: F284–F292
- García V., Ochoa E.J., Elías M.M. Effect of Early Stage of Experimental Diabetes on Vascular Functions in Isolated Perfused Kidneys. J. Autonomic Pharmacol. 1999; 18: 97–103
- Waugh W.H., Beall P.T. Simplified Measurements of PAH and Other Arylamines in Plasma and Urine. Kidney Int. 1974; 5: 429–436
- Roe H.H., Epstein J.H., Goldstein N.P. A Photometric Method for the Determination of Inulin in Plasma and Urine. J. Biol. Chem. 1949; 178: 839–843
- Takayanagi I., Koike K., Satoh M., Okayase A. Drug Receptor Mechanisms in Smooth Muscle: β-Chloroethylamine-Sensitive and Resistant Receptor Mechanism. Jpn. J. Pharmacol. 1997; 73: 1–22
- Kramer H.J., Schwarting K., Bäcker A., Meyer-Lehnert H. Renal Endothelin System in Obstructive Jaundice: Its Role in Impaired Renal Function of Bile Duct Ligated Rats. Clin. Sci. 1997; 92: 579–585
- O’Neill P.A., Wait R.B., Kahng K.U. Obstructive Jaundice and Renal Failure in the Rat. The Role of Renal Prostaglandins and the Renin-Angiotensin System. Surgery 1990; 108: 356–362
- Niederberger M., Gines P., Martin P.Y., St. John J., Woytaszek P., Xu L., Tsai P., Nemenoff R.A., Schrier R.W. Increased Renal and Vascular Cytosolic Phospholipase A2 Activity in Rats with Cirrhosis and Ascites. Hepatology 1998; 27: 42–47
- Wilcox C.J., Welch W.J. Angiotensin II and Thromboxane in the Regulation of Blood Pressure and Renal Function. Kidney Int. 1990; 38(Supp 30)S81–S85
- Wilcox C.J., Welch W.J., Snellen H. Thromboxane Mediates Renal Hemodynamics Response to Infused Angiotensin II. Kidney Int. 1991; 40: 1090–1097
- Welch W.J., Wilcox C.J. Potentiation of Tubulo Glomerular Feedback in the Rat by Thromboxane Mimetic: Role of Macula Densa. J. Clin. Invest. 1992; 89: 1857–1865
- Breyer M.D., Zhang Y.H., Guan Y.G., Hao Ch., Hebert R.L., Breyer R.M. Regulation of Renal Function by Prostaglandin E Receptors. Kidney Int. 1998; 54(Suppl 67)S88–S94
- Johnston H.H., Herzog J.P., Lauder D.P. Effect of Prostaglandin E on Renal Hemodynamics, Sodium and Water Excretion. Am. J. Physiol. 1967; 213: 939–946
- Sakairi Y., Jacobson H.R., Noland T.D., Breyer M.D. Luminal Prostaglandin E Receptors Regulated Salt and Water Transport in Rabbit Cortical Collecting Duct. Am. J. Physiol. 1995; 269: F257–F265
- Mistry M., Nasjletti A. Prostanoids as Mediators of Pro-Hypertensive and Antihypertensive Mechanisms. Am. J. Med. Sci. 1988; 295: 263–267
- Mistry C.D., Lote C.J., Gokal R., Currie W.J.C., Vandenburg M., Mallick P. Effects of Sulindac on Renal Function and Prostaglandin Synthesis in Patients with Moderate Chronic Renal Insufficiency. Clin. Sci. 1986; 70: 501–503
- Di Bona G.F. Renal Neural Activity in Hepatorenal Syndrome. Kidney Int. 1984; 25: 841–853
- Weigert A.L., Martin P.Y., Niederberger M., Higa E.M., McMurtry I.F., Gines P., Schrier R.W. Endothelium-Dependent Vascular Hyporesponsiveness Without Detection of Nitric Oxide Synthase Induction in Aortas of Cirrhotic Rats. Hepatology 1995; 22: 1856–1862
- Utkan Z.N., Utkan T., Sarioglu Y., Gonullu N.N. Effects of Experimental Obstructive Jaundice on Contractile Responses of Dog Isolated Blood Vessels: Role of Endothelium and Duration of Bile Duct Ligation. Clin. Exp. Pharmacol. Physiol. 2000; 27: 339–344
- Vallance P., Moncada S. Hyperdynamic Circulation in Cirrhosis: A Role for Nitric Oxide?. Lancet 1991; 337: 776–778
- Martin P.I., Xu D.L., Niederberger M., Weigert A., Tsai P., St. Jone J., Gines P., Schrier R.W. Up-regulation of Endothelial Constitutive NOS: A Major Role in the Increased Production in Cirrhotic Rats. Am. J. Physiol. 1996; 270: F494–F499