Abstract
We examined the effect of aqueous murine kidney extract (MKE) on the growth of mast cells prepared from the liquid culture of human umbilical cord blood CD34+ cells in the presence of a combination of recombinant human stem cell factor (SCF) and interleukin-6 (IL-6). Cultured cells were mature mast cells that expressed CD117 antigen on their surface, a specific marker for human mast cell, and they contained 6.53 pg histamine per cell. Adding MKE resulted in a 53% inhibition of mast cell growth and a 40% decrease in histamine content in mast cells in a serum-free liquid culture stimulated by SCF and IL-6. The inhibitory molecule for the growth of human mature mast cells was estimated at about 30 kDa of protein from gel-filtration HPLC. This fraction also inhibited the growth of murine peritoneal cells-derived mast cells. These results suggest that MKE contains regulator(s) that suppress the growth of mast cells and histamine synthesis, and that act beyond species specificity.
INTRODUCTION
Mast cells are produced by the proliferation and differentiation of hematopoietic stem cell in the bone marrow Citation[[1]], Citation[[2]]. In humans, because circulating mast cell progenitors express hematopoietic surface markers such as the CD34 antigen, cord blood (CB) as well as fetal liver mononuclear cells are also a source of mast cell progenitors Citation[[3]], Citation[[4]]. However, mast cells do not circulate in the blood as recognizable mature cells Citation[[5]]. In the differentiation process, immature mast cells leave the hematopoietic tissue and grow to various types of mature mast cells in various tissues Citation[[2]], Citation[[6]]. Mast cells can be generated in vitro into an almost pure population in the presence of a combination of stem cell factor (SCF) and interleukin-6 (IL-6) Citation[[3]], Citation[[7]]. SCF has been cloned as a ligand for c-kit that is expressed on the surface of hematopoietic stem or progenitor cells, or both, and mast cells Citation[[8]], and has been identified as a critical factor of human mast cell proliferation, differentiation and maturation. Mature mast cells are multi-functional cells containing various physiologically active substances, such as cytokines, proteases, and histamine, and they have an important role in allergic reaction and immunity reaction Citation[[9]], Citation[[10]]. However, the identity of the circulating progenitors has not been clarified, and little is known of the differentiation pathways that lead to mature, tissue mast cells and cytokines that promote survival or suppress the regulatory activity of human mast cells.
In a previous study, we reported that aqueous murine kidney extract (MKE) inhibits proliferation of murine peritoneal mast cells in a serum-free culture with SCF and IL-3 and suppresses intracellular histamine contents Citation[[11]]. Also we reported that MKE shows colony-promoting activity (CPA) of granulocyte-macrophage colony-forming units (CFU-GM) from murine bone marrow cells in serum-free cultures stimulated by IL-3 and erythropoietin (Epo), and acts synergistically with granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), IL-1, IL-3 and IL-11 Citation[[12]]. We found also that MKE promotes colony formation of human megakaryocyte colony-forming units (CFU-Meg) in plasma clot culture with recombinant human thrombopoietin alone Citation[[13]]. These studies suggested that the CPA detected in MKE for murine CFU-GM and for human CFU-Meg are derived from the same molecule(s), indicating that factor(s) detected in MKE affect both murine and human hematopoiesis in vitro. In this study, we investigated the effect of MKE on the proliferation of mature mast cells prepared from CD34+ cells of human CB.
METHODS
Preparation of MKE
MKE was prepared as described by Murakami et al. Citation[[11]]. Briefly, kidneys were removed from male ddY strain mice (8–14 weeks of age, Japan SLC Inc., Shizuoka, Japan) and were homogenized with distilled water at a concentration of 12% (w/v) using a Polytron (Kinematica, Switzerland) at 6 × 103 rpm for 30 s at three 30 s intervals at 0°C. The homogenates were stirred for one night at 4°C and were centrifuged at 9 × 103 g at 4°C for 30 min. The supernatants were adjusted to pH 4.0 with acetic acid and the insoluble material was removed by centrifugation. The clear supernatant was dialyzed against Dulbecco's phosphate buffered saline (PBS, without Ca2+ and Mg2+), was concentrated to 1/5 volume using an ultrafiltration membrane (molecular weight cut off: 10 000, Amicon) and was stored at 120°C until used.
Cytokines and Antibodies
Recombinant human SCF and human IL-6 were kindly provided by Kirin Brewery Co. Ltd. (Tokyo). Doses of these factors were SCF, 100 ng/ml; and IL-6, 50 ng/ml. Fluorescence-labeled monoclonal antibody (MoAb), fluorescein isothiocyanate conjugated antihuman CD34, phycoerythrin-cyanin 5 fluorochrome tandem conjugated antihuman CD117 (CD117-PC5) were purchased from Beckman Coulter Immunotech (Marseille, France).
Collection of CB and Isolation of CD34+ Cells
CB was collected at the end of full-term deliveries, after obtaining informed consent from the mothers, using a sterile collection bag containing anticoagulant citrate-phosphate dextrose according to the guidelines of the Tokyo Cord Blood Bank. Low-density mononuclear CB cells were separated by centrifuging on Ficoll-Paque (1.077 g/ml, Amersham Pharmacia Biotech AB, Uppsala, Sweden) for 30 min at 300 g at room temperature and were washed twice with PBS containing 5 mM EDTA. Low-density mononuclear cells were processed for CD34+ cell enrichment using a magnetic cell sorting MACS CD34+ progenitor cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). At the end of the procedure, CD34+ cell recovery was 0.13–66%, and the purity was in the range of 88–93%, as measured using a flow cytometer (EPICS XL, Beckman Coulter, Fullerton, CA, USA).
Pre-culture for Human Mast Cells
Mature mast cells derived from human CD34+ cells were obtained from a pre-culture as described by Saito et al. Citation[[14]] with minor modifications. CD34+ cells (2.4 × 105 cells) were suspended in 8 ml X-VIVO 10 (Bio Whittaker, Walkersville, Maryland) supplemented with 5% fetal calf serum (FCS, Intergen Co., Purchase, NY), SCF and IL-6 in a 25-cm2 plastic flask (Falcon, Becton Dickinson Labware, Lincoln Park, NJ). Each flask was incubated at 37°C in a humidified atmosphere of 5% CO2 for more than 10 weeks. The purity of CD117+ cells was analyzed by a flow cytometer, and showed a range of 90.7–93.4%. Cultured cells were resuspended with Iscove's modified Dulbecco's medium (IMDM, GIBCO BRL, Life Technologies, Inc., Rockville, MD) containing 30% FCS. The cells were made specimen using cytospined on slide glass at 650 rpm for 5 min and were stained with May-Grünwald-Giemsa or Wright-Giemsa-acid toluidine blue for morphological examination. Cultured cells generated in SCF and IL-6 showed metachromasia using acid toluidine blue.
Mature Mast Cell Culture
Serum-free liquid cultures of human mature mast cells was made using the method of Murakami et al. Citation[[11]] with minor modifications. The cultures contained a 1 × 105/ml concentration of mature mast cells, SCF and IL-6 in 0.5 ml IMDM with additives of 100 U/ml penicillin, 100 µg/ml streptomycin, (all from GIBCO BRL), 2.0% deionized bovine serum albumin (BSA, Sigma, St. Louis, MO), 600 µg/ml human holo-transferrin (Sigma), 100 µg/ml bovine serum lipoprotein (Sigma), 10 µg/ml human insulin (Sigma), 100 mM β-mercaptoethanol (Wako), and 304–608 µg/ml of MKE or PBS in wells of 24-well plates (Falcon). Each plate was incubated at 37°C in a humidified atmosphere of 5% CO2 for 14 days. The viable cells were counted using a hemacytometer with trypan-blue solution. The mast cell inhibitory activity (MIA) was calculated as the ratio of the number of cells cultured with MKE to the number of cells cultured without MKE.
Flow Cytometric Analysis
The expression of cell surface antigens was analyzed using a flow cytometer with MoAbs of CD117-PC5. Briefly, the cells were incubated with saturated concentrations of the relevant MoAbs for 20 min at room temperature, were washed, and were analyzed using a flow cytometer. For each experiment, negative controls were performed using isotype-matched irrelevant control MoAbs.
Determination of Histamine
The histamine content in each sample was measured using a Histamine Enzyme Immunoassay Kit (EIA kit, Immunotech, France) according to the manufacturer's instructions.
Gel-Filtration HPLC (GF-HPLC)
MKE was applied to a TSK gel G3000SWXL column (7.5 mm ID × 30 cm, TOSOH) previously equilibrated with PBS at a flow rate of 30 ml/h. Fractions of 0.5 ml were collected, and the absorbance at 280 nm and the activity of each fraction were measured. Thyroglobulin (650 kDa), ferritin (450 kDa), egg albumin (45 kDa) and cytochrome C (12.5 kDa) were each eluted as standard proteins.
Protein Assay
The protein contents of the samples were measured using Lowry's method Citation[[15]] with BSA as the standard protein. MKE contained 15.2 mg protein/ml. Doses of MKE were represented by the concentration of protein as BSA.
Statistical Analysis
The results were expressed as the mean for data from two or more separate experiments. The significance of the difference between groups was determined using the Student's t test.
RESULTS
Effect of MKE on the Growth of Human Mature Mast Cells in Liquid Culture
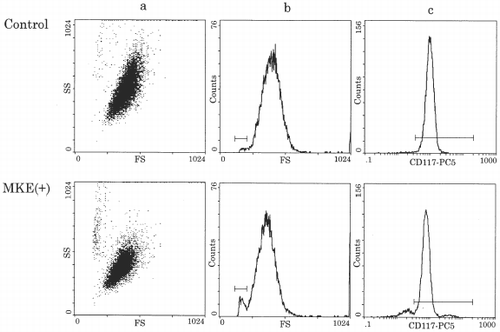
In the control culture, mature mast cells proliferated to approximately 1.3 × 105 cells after 14 days culture in a serum-free condition with SCF and IL-6, indicating a 1.3-fold increase in the control (). Adding MKE (304–608 µg) suppressed the total number of cells from 86 to 45% of the control dose dependently. When 456 µg MKE or more were added, the total number of cells was less than the number of cells at the start. To see the cell population and the expression of CD117 in harvested cells, flow cytometric analysis was performed (). The cells of the control culture showed almost one population by plotting cell size (forward scatter) against granularity (side scatter). MKE-treated cells showed two populations with an increase in small cells or debris, and lower granularity in the main population (a,b). The expression of CD117 antigen was detected as 98.4% positive in the control and 91.4% positive in the culture with MKE (c). The small cells or debris did not express the CD117 antigen. At this time, a significantly high number of cells without nuclei (20–35%) were in the MKE-treated cells from the results of the morphological study using May-Grünwald-Giemsa staining (data not shown).
Figure 1. Relationship between MKE concentration and the number of mast cells in serum-free liquid cultures. Human mature mast cells (1 × 105/ml) were suspended in serum-free condition in the presence of 100 ng/ml SCF and 50 ng/ml IL-6 with the indicated concentrations of MKE, and were incubated for 14 days. Numbers represent the mean ± S.E. of 6 wells from two separate experiments. *p<0.05, **p<0.01.
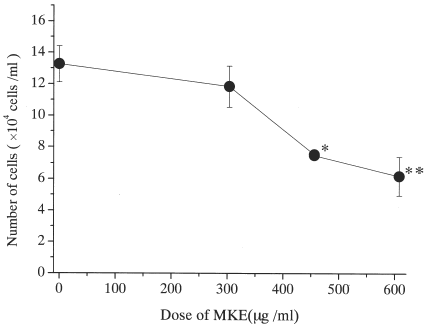
Figure 2. Representative flow cytometric analysis of cultured mast cells. Human mature mast cells (1 × 105/ml) were cultured in serum-free liquid culture condition for 14 days, then harvested cells were treated with anti-human CD117-PC5 MoAb and were analyzed by a flow cytometer.
The histamine content in harvested cells and medium were measured using an EIA kit (). The intracellular histamine content of the control increased from 6.53 to 8.11 pg/cell and histamine in the conditioned medium also increased from 3.3 to 154.6 ng/well. The histamine content in MKE-treated cells decreased in 54–59% of the control, but no difference was observed in the dose of MKE. However, the concentration of histamine detected in the conditioned medium increased 1.6–1.8 times higher than the control. The total histamine decreased to 74–59% of the control with MKE dose dependently.
Table 1. Histamine Concentration in Cultured Cells and Conditioned Medium Harvested from Serum-Free Liquid Culture
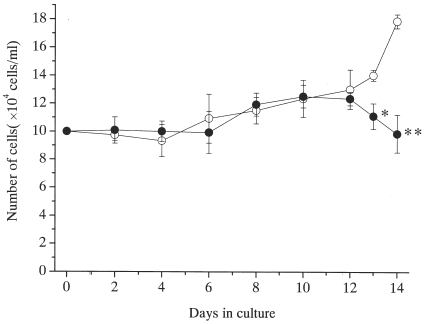
Cultured cells were harvested, and the total number of cells was evaluated at 2, 4, 6, 8, 10, 12, 13 and 14 day (). The number of cells slightly increased until day 12 in spite of the presence or the absence of MKE. After day 13, the mast cell number decreased in the culture with MKE.
Figure 3. Relationship between culture period and number of mast cells with or without of MKE. Human mature mast cells (1 × 105/ml) were cultured with 100 ng/ml SCF and 50 ng/ml IL-6 in the presence (•) or the absence of (◯) MKE (608 µg/ml). Numbers represent the mean ±S.E. of 6 wells from two separate experiments. *p<0.05, **p<0.0005.
Properties of the Active Molecule(s) in MKE
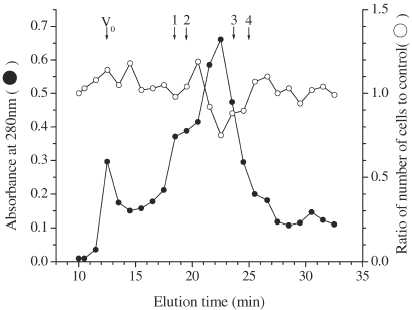
To see the properties of mast cell inhibitory factor(s) (MIF) in MKE for human mast cells, MKE was heated and trypsinized. The active molecule(s) in MKE was sensitive to trypsin and heat at 56°C for 30 min (), indicating a heat-unstable protein. The molecular weight of MIF in MKE was estimated using GF-HPLC with a G3000SWXL column (). The MIA was detected in a fraction that was eluted at 22.5 min, indicating about 30 kDa from the results of calibrating using a standard protein (data not shown). Also, the MIA on the growth of mast cells derived from murine peritoneal cells was also detected in the same fraction (data not shown).
Table 2. Effect of Trypsinized and Heated MKE on the Proliferation of Mast Cells
Figure 4. Elution profile of MIA in MKE on TSKgel-G3000SWXL. Sample: MKE 100 µl (1.52 mg), Flow rate: 30 ml/h, Fractionation: 0.5 ml/fraction. Each number indicates the elution position of the standard protein for calibration. 1: BSA (68 kDa), 2: albumin from egg (45 kDa), 3: chymotrypsinogen (25 kDa) and 4: cytochrome C (12.5 kDa).
DISCUSSION
Human mature mast cells produced in this study were prepared from CB CD34+ cells according to the method of Saito et al. Citation[[16]], Citation[[17]] who showed that mature human mast cells contain 3.5–9 pg histamine in each cell. In our study, mast cells from the culture of CB CD34+ cells supported by SCF and IL-6 contained 6.53 pg histamine in each cell, which was consistent with the reports of Saito et al. Citation[[16]], Citation[[17]]. From the results of the morphological study, flow cytometric analysis and intracellular histamine analysis, the cells used in this study were probably mature mast cells.
Our previous report showed that MKE contains mast cell inhibitory factor(s) that suppress the growth of mast cells prepared from murine peritoneal cells and eliminate the histamine content in mast cells Citation[[11]]. In this study, we found that MKE acts on the growth of human mature mast cells as in the previous report, but the inhibitory level of MKE for human mast cells was lower than that detected in murine culture. Mature mast cells have a weak potential to grow in vitro Citation[[18]]. In this study, the increase in total cell number after 14 days of culture showed only a 1.3-fold increase in the control culture. No differences were observed in total cell number until day 12 in spite of the presence or the absence of MKE (). After day 13, MKE had decreased significantly the number of mast cells. Although murine mast cells derived from peritoneal cells resulted in a 33-fold increase over the initial input Citation[[11]], a similar decrease in total cell number was observed in the later period of the culture, indicating that the MIF in MKE may act on both human and murine mast cells through the same mechanism. Although MKE showed an MIA not indicating species specificity, these results suggest that the lower suppressive effect of MKE to human mature mast cells is due to the differences in proliferative potential in both cells, or species specificity.
Histamine synthesis in mast cells continued to the later period of the culture. Because the conditioned medium from the culture with MKE contained a higher concentration of histamine than that from the control, histamine synthesis may also continue until late in the culture. These results suggest that the MIF of MKE is not a toxic molecule(s), and acts as a hematopoietic regulator in the differentiation of mast cell. Besides these results, we observed many cells without nuclei from their morphology and a decrease in granularity in cells culture with MKE. Apoptosis starts from reduction of cell size accompanied by a structural change in nuclei, such as nuclear concentration, unlike necrosis Citation[[19]]. We observed the nucleus disappearance and the outline of a cell collapse, which is part of apoptosis. However, DNA fragmentation in general apoptosis depends on the kind of cell, and no homogenate has been reported. Little is known of the death of mast cells, and almost all details are not well understood. Therefore, although concluding that the inhibitory effects of MKE on mast cells were due to apoptosis in this study is difficult, a possibility exists that the MKE action induces mast cell apoptosis rather than including necrosis from our histochemical, morphological, and biochemical studies. As obtaining large numbers of human mast cells for various analyses of cell death was difficult, we made no estimation about them. However, to know if MKE-induced mast cell death is apoptosis, we examined DNA fragmentation and cell-cycle analysis using murine mast cells. No apoptosis event was observed (data not shown). Detailed studies of the mechanisms of mast cell death by MIF in MKE are required to understand mast cell physiology.
MIF in MKE was approximately 30 kDa protein from the results of GF-HPLC (), suggesting that MIF in MKE has a potential to suppress mast cell growth from mice and humans. Our previous studies showed that MKE contains colony-promoting factor for CFU-GM from murine bone marrow cells, and that MKE promotes the clonal growth of human mature CFU-Meg and the maturation of megakaryocytes. Furthermore, our previous reports indicated that MKE does not contain G-CSF, granulocyte-macrophage-CSF, M-CSF, IL-1, IL-3, IL-4, IL-6, IL-11, SCF and Epo judged from the biological and chemical properties, the target cells, and the mechanism of action of factors in MKE Citation[[12]]. IFN-γ inhibits the proliferation of human mast cells derived from CB CD34+ cells Citation[[20]], Citation[[21]]. We identified also that IFN-γ is not included in MKE by a neutralization experiment using a murine anti-IFN-γ antibody Citation[[11]]. Therefore, we suggest that MKE contains at least more than two hematopoietic regulatory factors. Possibilities exist that multiple factors detected in MKE are known proteins not identified as hematopoietic regulators or that unknown proteins are hematopoietic regulators, but the details of the MIF in MKE is not clear. A more detailed study is necessary to investigate the active molecules in MKE together with the role of the kidney in hematopoiesis.
ACKNOWLEDGMENT
We thank Maternity Nurse Reiko Fukushi and Nurse Chieko Fukushi of Fukushi Maternity Center in Goshogawara-shi, Aomori-ken, Japan, for collecting and providing the CB. We also thank all mothers and babies who gave their umbilical cord blood for this study.
REFERENCES
- Kitamura Y., Hatanaka K., Murakami M., Shibata H. Presence of Mast Cell Precursor in Peripheral Blood of Mice Demonstrated by Parabiosis. Blood 1979; 53: 1085–1088
- Kitamura Y., Yokoyama M., Matsuda H., Ohno T., Mori K.J. Spleen Colony-Forming Cell as Common Precursor for Tissue Mast Cells and Granulocytes. Nature (London) 1982; 291: 159–160
- Mitsui H., Furitsu T., Dvorak A., Irani A.A., Schwartz L.B., Inagaki N., Takei M., Ishizaka K., Zsebo K.M., Gillis S., Ishizaka T. Development of Human Mast Cells from Umbilical Cord Blood Cells by Recombinant Human and Murine c-Kit Ligand. Proc. Natl. Acad. Sci. USA 1993; 90: 735–739
- Irani A.A., Craig S., Nilsson G., Ishizaka T., Schwartz L.B. Characterization of Human Mast Cells Developed In Vitro from Fetal Liver Cells Co-cultured with Murine 3T3 Fibroblasts. Immunology 1992; 77: 136–143
- Valent P., Spanblochl E., Sperr W.R., Sillaber C., Zsebo K.M., Agis H., Strobl H., Geissler K., Bettelheim P., Lechner K. Induction of Differentiation of Human Mast Cells from Bone Marrow and Peripheral Blood Mononuclear Cells by Recombinant Human Stem Cell Factor/Kit-Ligand in Long-Term Culture. Blood 1992; 80: 2237–2245
- Kitamura Y. Heterogeneity of Mast Cells and Phenotypic Change Between Subpopulations. Annu. Rev. Immunol. 1989; 7: 59–76
- Yanagida M., Fukamachi H., Ohgami K., Kuwaki T., Ishii H., Uzumaki H., Amano K., Tokiwa T., Mitsui H., Saito H. Effects of T-Helper 2-Type Cytokines, Interleukin-3 (IL-3), IL-4, IL-5, and IL-6 on the Survival of Cultured Human Mast Cells. Blood 1995; 86: 3705–3714
- Neu S., Geiselhart A., Kugi S., Baur F., Niethammer D., Handgretinger R. Isolation and Phenotypic Characterization of CD117-Positive Cells. Leukemia Research 1996; 20: 963–971
- Mekori Y.A., Metcalfe D.D. Mast Cells in Innate Immunity. Immunol. Rev. 2000; 173: 131–140
- Schmidt-Choudhury A., Meissner J., Seebeck J., Goetzl E.J., Xia M., Galli S.J., Schmidt W.E., Schaub J., Wershil B.K. Stem Cell Factor Influences Neuro-Immune Interactions: The Response of Mast Cells to Pituitary Adenylate Cyclase Activating Polypeptide Is Altered by Stem Cell Factor. Regul. Pept. 1999; 15: 73–80
- Murakami M., Kashiwakura I., Hayase Y., Takagi Y. Inhibitory Effect of Murine Kidney Extracts on the Proliferation of Murine Mast Cells. Biol. Pharm. Bull. 1999; 22: 1153–1157
- Bamba T., Kashiwakura I., Hayase Y., Takagi Y. Biological Properties of the Colony-Promoting Activity in Extracts Prepared from Murine Kidney. Yakugaku Zasshi 1996; 116: 719–727
- Murakami M., Kashiwakura I., Hayase Y., Takahashi T.A., Takagi Y. Effect of Murine Kidney Extracts on the Proliferation of Hematopoietic Progenitor Cells in Human Umbilical Cord Blood. Biol. Pharm. Bull. 2000; 23: 1136–1142
- Saito H., Ebisawa M., Tachimoto H., Shichijo M., Fukagawa K., Matsumoto K., Iikura Y., Awaji T., Tsujimoto G., Yanagida M., Uzumaki H., Takahashi G., Tsuji K., Nakahata T. Selective Growth of Human Mast Cells Induced by Steel Factor, IL-6, and Prostaglandin E2 from Cord Blood Mononuclear Cells. J. Immunol. 1996; 157: 343–350
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951; 193: 265–275
- Saito H., Ebisawa M., Sakaguchi N., Onda T., Iikura Y., Yanagida M., Uzumaki H., Nakahata T. Characterization of Cord-Blood-Derived Human Mast Cells Cultured in the Presence of Steel Factor and Interleukin-6. Int. Arch. Allergy Immunol. 1995; 107: 63–65
- Shichijo M., Inagaki N., Nakai N., Kimata M., Nakahata T., Serizawa I., Iikura Y., Saito H., Nagai H. The Effects of Anti-asthma Drugs on Mediator Release from Cultured Human Mast Cells. Clin. Exp. Allergy 1998; 10: 1228–1236
- Galli S.J., Dvorak A.M., Marcum J.A., Ishizaka T., Nabel G., Der Simonian H., Pyne K., Goldin J.M., Rosenberg R.D., Cantor H., Dvorak H.F. Mast Cell Clones: A Model for the Analysis of Cellular Maturation. J. Cell. Biol. 1982; 95: 435–444
- Kitanaka C., Kuchino Y. Caspase-Independent Programmed Cell Death with Necrotic Morphology. Cell Death and Differentiation 1999; 6: 508–515
- Kirshenbaum A.S., Worobec A.S., Davis T.A., Goff J.P., Semere T., Metcalfe D.D. Inhibition of Human Mast Cell Growth and Differentiation by Interferon Gamma-1b. Exp. Hematol. 1998; 26: 245–251
- Yanagida M., Fukamachi H., Takei M., Hagiwara T., Uzumaki H., Tokiwa T., Saito H., Iikura Y., Nakahata T. Interferon Promotes the Survival and FcRI-Mediated Histamine Release in Cultured Human Mast Cells. Immunology 1996; 89: 547–552