Abstract
Hemodialysis patients have low 5-year survival rates of approximately 60%, and the most common cause of death is cardiovascular diseases. Their population may be considered, therefore, as an accelerated model in analyzing the risk factors for cardiovascular diseases. We previously reported the role of blood pressure, one of the most important risk factors for cardiovascular diseases, in determining the prognosis of hemodialysis patients. In this study, we examined the effect of cardiomegaly detected on chest roentgenogram or electrocardiogram before initiating hemodialysis therapy on survival after introduction to maintenance hemodialysis. One hundred and sixty hemodialysis patients who had no history of ischemic heart disease or arrhythmia were followed up for 88.9 ± 4.0 months, among whom 69 died. Heart enlargement, defined on chest roentgenogram, was detected in 104 patients, and left ventricular hypertrophy, defined on electrocardiogram, was detected in 105 patients. The presence of either finding shortened their survival. However, Cox's proportional hazards model and logistic multiple regression analysis identified only left ventricular hypertrophy as one of the significant determinants for survival, but heart enlargement was not independent. Correction of systolic hypertension on the maintenance phase had no significant favorable effect on survival in patients with left ventricular hypertrophy, while it improved in those with heart enlargement. This finding, together with those above from Cox's model and logistic analysis strongly suggests that risk from left ventricular hypertrophy is independent of, but one from heart enlargement is dependent on hypertension.
INTRODUCTION
In patients with end-stage renal disease, cardiovascular events remain the major cause of mortality and morbidity Citation[[1]], Citation[[2]]. The mortality rate of patients on hemodialysis was 3.5 times higher than that of the general population Citation[[3]] and has begun to increase recently, probably due to increased cardiovascular complications Citation[[1]], Citation[[2]]. Their population may be considered, therefore, as an accelerated model in analyzing the risk factors for cardiovascular diseases, making it possible to identify them even in a small number of patients and in a short observation period. Left ventricular hypertrophy, LVH, is a relatively common finding in end-stage renal disease Citation[[4]]. The Framingham study demonstrated that LVH by electrocardiogram conferred an increased mortality independent of other cardiovascular risk factors Citation[[5]]. LVH has been repeatedly shown as an important marker of risk for death and other adverse events in patients with hypertension Citation[[6]] as well as in normal subjects Citation[[7]].
We previously reported the role of blood pressure, as one of the most important risk factors for cardiovascular diseases, in determining the prognosis of hemodialysis patients Citation[[8]], Citation[[9]]. In this study, we report the incidence of heart enlargement on chest roentgenogram and LVH on electrocardiogram in patients introduced to dialysis therapy, and the effect of heart enlargement and LVH on their survival.
METHODS
Patients
One hundred and ninety-five patients with chronic renal failure, who had been introduced to hemodialysis therapy at the National Cardiovascular Center Hospital between 1977 and 1987 and discharged from there to branch hospitals for maintenance hemodialysis, were studied retrospectively. Twenty-five patients who had history of angina pectoris or myocardial infarction, nine patients with arrhythmia and one patient with transplanted kidney were excluded from this study. Myocardial infarction was diagnosed by either electrocardiogram or echocardiogram, angina pectoris by both symptom and electrocardiogram. Thus, a total of 160 patients (94 males and 66 females) were studied. When a questionnaire on survival as of January 1994 was mailed, answers from all 160 respondents could be recollected. Thus, recovery rate was 100%. The clinical characteristics of the patient cohort, at the time of the initiation of hemodialysis, are shown in . Their age and the distribution of the primary renal disease were similar to the overall Japanese hemodialysis population Citation[[1]]. The primary renal diseases that caused chronic renal failure included 90 cases of chronic glomerulonephritis, 29 of diabetic nephropathy, 13 of nephrosclerosis, 7 of polycystic kidney disease, 3 of pyelonephritis, 3 of lupus nephritis, and 15 of unknown origin. Of the 160 patients, 69 had died during the observation period (88.9 ± 4.0 months). The cause of death was determined by the death certificate, resulting in 31 classified as uremia, 20 as cardiovascular diseases, 2 as infection, 2 as malignancy, 8 as others, and 6 as unknown. We analyzed the data with respect to survival or death without further analyzing the primary causes of death. The primary renal diseases of 69 dead cases included 23 cases of chronic glomerulonephritis, 27 of diabetic nephropathy, 9 of nephrosclerosis, 1 of polycystic kidney disease, 1 of pyelone-phritis, 1 of lupus nephritis, and 7 of unknown origin.
Table 1. Baseline Characteristics of 160 Patients
Risk Factors Examined
Several parameters on both introduction and maintenance phases of hemodialysis were examined as risk factors. The introduction phase was defined as within two months before introduction to dialysis therapy, and the maintenance phase as from two weeks after the introduction to dialysis until discharge from the National Cardiovascular Center. The risk factors examined included age, primary renal disease (diabetic nephropathy or not), habit of smoking more than 10 cigarettes per day, and cerebrovascular accident and atherosclerotic obliteration of peripheral arteries to lower extremities. Cerebrovascular accident was diagnosed by both neurological findings and computerized tomography, and atherosclerotic obliteration by both symptomatic claudication and low ankle/brachial pressure index. Serum total cholesterol and albumin concentrations were measured on the introduction phase. Chest X-ray was recorded close to inception of dialysis therapy and heart enlargement was defined as the cardiothoracic ratio, CTR, larger than 50%. Electrocardiogram, ECG, recorded on the introduction phase was classified into 4 degrees of change (ECG score); 0: normal, 1: high voltage on left ventricular leads (SV1 + RV5 ≥ 3.5 mV), 2: slightly ST depression (0.05 ˜ 0.1 mV), and 3: severe ST depression (≥0.1 mV). Left ventricular hypertrophy (LVH) on ECG was defined as ECG score more than 1. M-mode echocardiograms were performed close to inception of dialysis therapy in 74 patients. Blood pressure for the introduction phase was defined as the average value measured by doctors, with the patient in the sitting position after resting more than 5 min, at the last three visits to the hospital as outpatients before hospitalization for introduction to dialysis therapy. Blood pressure for the maintenance phase was defined as the average value measured by nurses, with the patient in the supine position after resting more than 5 min, before starting the last 3 dialysis sessions at the National Cardiovascular Center. Antihypertensive medications were used in 72 patients on maintenance phase.
Statistical Analysis
Kaplan-Meier Analysis and Cox's Proportional Hazards Regression Model
Cumulative survival curves were computed based on Kaplan-Meier Analysis, and pairwise multiple comparisons of the estimated survival distribution were made based on Cox-Montel Method between the presence and absence of heart enlargement or LVH. Cox's proportional hazards regression model identified the relative risk of each risk factor for cumulative survival. Age, gender and other risk factors such as primary renal disease, habit of smoking, cerebrovascular accident, atherosclerotic obliteration, serum total cholesterol concentrations, serum albumin concentrations, cardiothoracic ratio, left ventricular hypertrophy and blood pressure were included as the variables of the multivariate models.
Logistic Multiple Regression Analysis
Since the annual mortality rate within three years after the introduction to hemodialysis therapy was higher than that after four years, a total of 160 hemodialysis patients were divided into two groups: the survivors (n = 123) who survived more than three years after the introduction to hemodialysis and the non-survivors (n = 37) who died within three years. Based on the logistic multiple regression analysis, the contribution of each risk factor in determining patient death within three years after introduction to hemodialysis therapy was estimated. In this analysis, 32 dead cases who survived more than three years after the introduction and died thereafter were considered survivors. The follow-up period of all survivors exceeded three years.
Data are represented as mean ± SEM. One way analysis of variance followed by Fisher's PLSD and Student's t test for unpaired data were utilized for statistical analysis. A p value of less than 0.05 was considered to indicate statistical significance.
All statistical analyses were performed with StatView version 5.0 (SAS Institute Inc).
RESULTS
Characteristics and Mortality
showed the baseline characteristics of all patients, patients with diabetes mellitus (DM) and patients without diabetes mellitus (NDM). Age (p<0.01), blood pressure (p<0.01), creatinine (p<0.01), albumin (p<0.01), CTR (p<0.01) and ECG score (p<0.05) were different between DM and NDM.
Of the 160 consecutive hemodialysis patients, 69 died during the follow-up period of 88.9 ± 4.0 months. A cumulative survival curve was computed, showing that the 3-year survival rate was 77 ± 3%, the 5-year survival rate was 71 ± 4%, and the 10-year survival rate was 57 ± 4%.
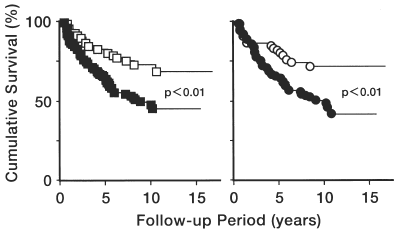
Effect of Heart Enlargement and LVH on Survival Curve
The survival rate of patients with heart enlargement (n = 104) was significantly worse than that of patients (n = 56) without heart enlargement (p<0.01) (, left panel). The 5-year survival rates in the respective groups were 64 ± 5% and 80 ± 5%. The worst survival rate in patients with heart enlargement was seen only in NDM (p<0.02) but not in DM (data were not shown). Survival of patients with LVH (n = 105) was significantly worse than that of patients without LVH (n = 55) (p<0.01) (, right). The 5-year survival rates in the respective groups were 64 ± 5% and 80 ± 6%. The worst survival in patients with LVH was seen only in NDM (p<0.02) but not in DM (data were not shown). Left ventricular mass index (LVMI) was estimated in 74 patients, 189.1 ± 8.4 g/m2 in DM (n = 19) and 163.9 ± 7.0 g/m2 in NDM (n = 55). Survival of patients with LVMI>120 g/m2 (n = 67) was worse than that of patients with LVMI<120 (n = 7) (p<0.05). The worst survival in patients with LVMI>120 was seen only in NDM (p<0.05) but not in DM.
Effect of Blood Pressure on Survival Curve
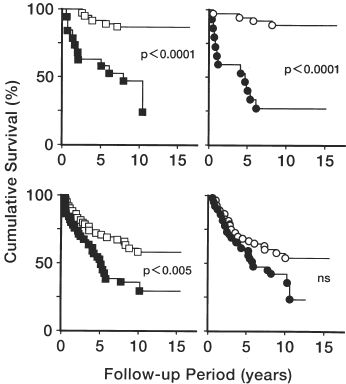
In both patients with and without heart enlargement, high systolic blood pressure (≥160 mmHg) on the maintenance phase shortened their survival (, left panels). On the other hand, in patients with LVH, high systolic blood pressure on the maintenance phase had no effect on the already poor survival (, right on lower panel), though it did shorten in those without LVH (, right on upper panel). Similarly, in patients with high systolic blood pressure (≥160 mmHg) on the maintenance phase, neither heart enlargement nor LVH had significant effect on their already poor survival, while in those without high blood pressure, both heart enlargement (p<0.01) and LVH (p<0.01) significantly shortened their survival.
Figure 2. Effect of systolic blood pressure on the maintenance phase on cumulative survival curve of hemodialysis patients with and without cardiomegaly detected on chest X-ray or ECG. Comparison between the absence and presence of heart enlargement on chest X-ray was shown in the left panels, while comparison between the absence and presence of LVH on ECG was shown in the right panels. The absent cases were shown in the upper panels, while present cases were shown in the lower panels. Open symbols indicate the patients with mSBP below 160 mmHg, and closed symbols indicate those with mSBP more than 160 mmHg.
Relative Risk by Cox's Proportional Hazards Model
Relative risks of mortality were calculated based on Cox's proportional hazards models (). It was highest at 3.3 (p<0.0001) for diabetic nephropathy, followed by 2.5 (p<0.001) for age (older than 60 years), and 2.0 (p<0.005) for systolic blood pressure on the maintenance phase (higher than 160 mmHg), 1.3 (p<0.05) for LVH defined by ECG in all patients. It was 3.3 (p<0.001) for age, 2.1 (p<0.05) for systolic blood pressure on the maintenance phase and 1.4 (p<0.05) for LVH in NDM. In DM, these were not revealed as relative risks. It is important to note that there was no significant risk estimated for heart enlargement (p = 0.16). We did not find any associations between mortality risk and the other conditions, including cerebrovascular accident, atherosclerotic obliteration, smoking habits and hypercholesterolemia.
Table 2. Relative Risk of Clinical Variables on Survival Based on Cox's Proportional Hazards Regression Model
Attributable Rate by Logistic Multiple Regression Analysis
The contribution of each risk factor on the death within three years after the introduction to hemodialysis therapy was determined by logistic multiple regression analysis. The contribution from diabetic nephropathy as the cause of renal failure was 35% (p<0.001), followed by 16% (p<0.01) of age at introduction of hemodialysis (older than 60 years), 9% (p<0.001) of systolic blood pressure on the maintenance phase (higher than 160 mmHg), and 8% (p<0.05) of LVH defined by ECG. There were no significant contributions estimated from heart enlargement, serum cholesterol, smoking habits, cerebrovascular accident or atherosclerotic obliteration.
DISCUSSION
We investigated the effect of roentgenographic heart enlargement and electrocardiographic left ventricular hypertrophy on survival of hemodialysis patients, and showed that LVH was a more important determinant of outcome than heart enlargement in such patients without past history of ischemic heart disease or arrhythmia. This finding is consistent with a previous report that heart enlargement on chest X-ray was not as ominous as LVH on ECG Citation[[10]]. The Framingham study identified LVH as a risk factor independent of others such as systolic high blood pressure Citation[[5]], being also compatible with present findings. shows that the correction of systolic hypertension on the maintenance phase had no significant favorable effect on survival in patients with LVH, while it improved in those with heart enlargement on chest X-ray. This finding, together with those above from Cox's proportional hazards model and logistic multiple regression analysis, strongly suggests that a risk from LVH is independent, but one from heart enlargement is dependent on hypertension and possibly also on LVH.
In our study, chest X-ray and ECG were performed in all patients, but echocardiogram was performed in half of them. However, Chicos et al. demonstrated that CTR and heart size both quantitated from plain chest film were correlated with the left ventricular end-diastolic volume Citation[[13]]. Therefore, we used CTR to determine heart enlargement. Although ECG was less sensitive than echocardiograms as a means of detecting anatomical hypertrophy Citation[[11]], LVH detected by ECG has identified patients at high risk for cardiovascular morbid events and has been considered a significant risk factor for future morbid events independent of age, blood pressure or resting ventricular function in men with mild hypertension Citation[[5]], Citation[[6]]. Therefore, we used both chest X-ray and ECG to detect heart enlargement and LVH, respectively, and to identify the risk factors for survival after introduction to maintenance hemodialysis therapy. LVH on echocardiogram was also revealed as risk factor like LVH on ECG.
LVH is reported as one of the major risk factors for cardiovascular morbidity and mortality Citation[[4]], Citation[[14]], Citation[[15]], Citation[[16]], Citation[[17]], Citation[[18]], Citation[[19]] in patients with end stage renal disease, compatible with present data. Echocardiographic observation showed left ventricular mass hypertrophy in 74% and left ventricular dilation in 36% of patients, and these findings were the strongest predictors of late mortality Citation[[20]]. Approximately one-third of patients with LVH had congestive heart failure at the time of initiation of dialysis therapy Citation[[15]], Citation[[21]], and 20% had ischemic heart disease. The pathogenesis of LVH in hemodialysis patients is very complex, involving many etiopathogenetic factors Citation[[4]]. Presence of arteriovenous fistulae, salt and water retention and anemia increased preload Citation[[18]], Citation[[22]], Citation[[23]], while the changes in blood pressure and physical properties of large arteries increased afterload Citation[[22]]. Other putative risk factors for LVH may include age, aluminum accumulation, hyper-parathyroidism and some unknown uremic toxins Citation[[22]], Citation[[24]], Citation[[25]], Citation[[26]], Citation[[27]], Citation[[28]]. In addition to LVH, we also demonstrated that diabetic nephropathy, age and systolic blood pressure on the maintenance phase were the independent predictors for survival of dialysis patients. Diabetic nephropathy and age were non-correctable factors, but systolic blood pressure and LVH were the factors correctable by dialysis therapy. Prolonged antihypertensive therapy with strict body fluid volume control is reported to considerably reduce LVH in chronically hemodialyzed patients Citation[[29]], Citation[[30]], Citation[[31]], Citation[[32]]. Partial regression of LVH can also be achieved by correction of anemia with erythropoietin Citation[[23]], Citation[[32]], Citation[[33]]. In order to improve the survival of dialysis patients, based on the regression of LVH, therefore, treatment of both hypertension and anemia seems at least essential. The hemodialysis population seems to provide us with a useful model, suitable for identifying the risk factors for cardiovascular diseases in a relatively small number of patients and a short observation period.
ACKNOWLEDGMENT
This study was supported by Research Grants for Cardiovascular Diseases (C-1994-6 & C-1995-3) and for “Progressive Renal Disease” from “Specially Selected Diseases by the Ministry of Health and Welfare Research Project”, as well as for Scientific Research Expenses for Health and Welfare Programs and Funds for Comprehensive Research on Long Term Chronic Disease (Renal Failure), all from the Ministry of Health and Welfare of Japan.
REFERENCES
- Maeda K. For Statistical Survey Committee: An Overview of Dialysis Treatment in Japan (as of Dec. 31, 1996). J. Jpn. Soc. Dial. Ther. 1998; 31: 1–24
- United States Renal Data System. USRDS 1997 Annual Data Report U.S. Department of Health and Human Service. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD 1997, August
- Harnett J.D., Kent G.M., Foley R.N., Parfrey P.S. Cardiac Function and Hematocrit Level. Am J. Kidney Dis. 1995; 25: S3–S7
- Harnett J.D., Parfrey P.S. Left Ventricular Dysfunction in Dialysis Subjects. Principles and Practice of Dialysis, W.L. Henrich. Williams & Wilkins, Baltimore 1994; 170–180
- Kannel W.B., Gordon T., Offutt D. Left Ventricular Hypertrophy by Electrocardiogram. Prevalence, Incidence, and Mortality in the Framingham Study. Ann. Int. Med. 1969; 71: 89–105
- Casale P.N., Devereux R.B., Milner M., Zullo G., Harshfield A., Pickering T.G., Laragh J.H. Value of Echocardiographic Measurement of Left Ventricular Mass in Predicting Cardiovascular Morbid Events in Hypertensive Men. Ann. Int. Med. 1986; 105: 173–178
- Levy D., Savage D.D., Garrison R.J., Anderson K.M., Kannel W.B., Castelli W.P. Echocardiographic Criteria for Left Ventricular Hypertrophy: The Framingham Heart Study. Am. J. Cardiol. 1987; 59: 956–960
- Tomita J., Kimura G., Inoue T., Inenaga T., Sanai T., Kawano Y., Nakamura S., Baba S., Matsuoka H., Omae T. Role of Systolic Blood Pressure in Determining Prognosis of Hemodialyzed Patients. Am. J. Kidney Dis. 1995; 25: 405–412
- Kimura G., Tomita J., Nakamura S., Uzu T., Inenaga T. Interaction Between Hypertension and Other Cardiovascular Risk Factors in Survival of Hemodialyzed Patients. Am. J. Hypertens. 1996; 9: 1006–1012
- Kannel W.B. Framingham Study Insights into Hypertensive Risk of Cardiovascular Disease. Hypertens. Res. 1996; 18: 181–196
- Reichek N., Devereux R.B. Left Ventricular Hypertrophy: Relationship of Anatomic, Echocardiographic and Electrocardiographic Findings. Circulation 1981; 63: 1391–1398
- Devereux R.B., Alonso D.R., Lutas E.M., Gottlieb G.J., Campo E., Sachs I., Reichek N. Echocardiographic Assessment of Left Ventricular Hypertrophy: Comparison to Necropsy Findings. Am. J. Cardiol. 1986; 57: 450–458
- Chikos P.M., Figrey M.M., Fisher A.L. Correlation Between Chest Film and Angiographic Assessment of Left Ventricular Size. Am. J. Roentgenol. 1977; 128: 367–373
- Foley R.N., Parfrey P.S. Cardiac Disease in Chronic Uremia: Clinical Outcome and Risk Factors. Adv. Ren. Replace Ther. 1997; 4: 234–248
- Foley R.N., Parfrey P.S., Harnett J.D., Kent G.M., Martin C.J., Murray D.C., Barre P.E. Clinical and Echocardiographic Cardiovascular Disease in End Stage Renal Disease: Prevalence, Associations and Prognosis. Kidney Int. 1995; 47: 186–192
- Harnett J.D., Murphy B., Collingwood P., Purchase L., Kent G.M., Parfrey P.S. The Reliability and Validity of Echocardiographic Measurement of Left Ventricular Mass Index in Hemodialysis Patients. Nephron. 1993; 71: 212–214
- Silberberg J.S., Barre P.E., Prichard S.S., Sniderman A.D. Impact of Left Ventricular Hypertrophy on Survival in End-Stage Renal Disease. Kidney Int. 1989; 36: 286–290
- Silberberg J.S., Rahal D.P., Patton R., Sniderman A.D. Role of Anemia in the Pathogenesis of Left Ventricular Hypertrophy in End-Stage Renal Disease. Am. J. Cardiol. 1989; 64: 222–224
- Parfrey P.S., Harnett J.D., Griffiths S.M., Taylor R., Hand J., King A., Barre P.E. The Clinical Course of Left Ventricular Hypertrophy in Dialysis Patients. Nephron. 1990; 55: 114–120
- Foley R.N., Parfrey P.S., Harnett J.D., Kent G.M., Murray D.C., Barre P.E. The Prognostic Importance of Left Ventricular Geometry in Uremic Cardiomyopathy. J. Am. Soc. Nephrol. 1995; 5: 2024–2031
- Harnett J.D., Foley R.N., Kent G.M., Murray D.C., Barre P.E., Parfrey P.S. Congestive Heart Failure in Dialysis Patients: Prevalence, Incidence, Prognosis and Risk Factors. Kidney Int. 1995; 47: 884–890
- Harnett J.D., Kent G.M., Barre P.E., Taylor R., Parfrey P.S. Risk Factors for the Development of Left Ventricular Hypertrophy in a Prospectively Followed Cohort of Dialysis Patients. J. Am. Soc. Nephrol. 1994; 4: 1486–1490
- Silberberg J.S., Racine N., Barre P., Snidermann A. Regression of Left Ventricular Hypertrophy in Dialysis Patients Following Correction of Anemia with Recombinant Human Erythropoietin. Can. J. Cardiol. 1990; 6: 1–4
- Stefenelli T., Mayr H., Bergler-Klein J., Globits S., Woloszczuk W., Niederle B. Primary Hyperparathyroidism; Incidence of Cardiac Abnormalities and Partial Reversibility After Successful Parathyroidectomy. Am. J. Med. 1993; 95: 197–202
- London G.M., Fabiani F., Marchais S.J., De Vernejoul M.-C., Guerin A.P., Safer M.E., Metivier F. Uremic Cardiomyopathy: An Inadequate Left Ventricular Hypertrophy. Kidney Int. 1987; 31: 973–980
- London G.M., De Vernejoul M.-C., Fabiani F., Marchais S.J., Guerin A.P., Metivier F., Chappuis P., Llach F. Association Between Aluminum Accumulation and Cardiac Hypertrophy in Hemodialyzed Patients. Am. J. Kidney Dis. 1989; 13: 75–83
- Rambusek M., Amann K., Mall G., Ritz E. Structural Causes of Cardiac Dysfunction in Uremia. Renal Failure 1993; 15: 421–428
- Weisenee D., Low-Friedrich I., Richle M., Bereiter-Hahn J., Schoeppe W. In Vitro Approach to Uremic Cardiomyopathy. Nephron. 1993; 65: 392–400
- Weidmann P., Maxwell M.H. Hypertension. Clinical Aspects of Uremia and Dialysis, S.G. Massry, A.L. Sellers. Charles C. Thomas, Illinois 1976; 100–139
- Campese V.M., Chervu I. Hypertension in Dialysis Subjects. Principles and Practice of Dialysis, W.L. Henrich. Williams & Wilkins, Bartimore 1994; 148–169
- Cannella G., Paoletti E., Delfino R., Peloso G., Malinari S., Taverso G.B. Regression of Left Ventricular Hypertrophy in Hypertensive Dialyzed Patients on Long-Term Antihypertensive Therapy. Kidney Int. 1993; 44: 881–886
- Zehnder C., Zuber M., Sulzer M., Meyer B., Straumann F., Jenzer H.R., Blumberg A. Influence of Long-Term Amelioration of Anemia and Blood Pressure on Left Ventricular Hypertrophy in Hemodialyzed Patients. Nephron. 1992; 64: 202–206
- Wizemann V., Schaffer R., Kramer W. Follow-Up of Changes Induced by Anemia Compensation in Normotensive Hemodialysis Patients with Left Ventricular Hypertrophy. Nephron. 1993; 64: 202–206