Abstract
Objective: Treatment of anemia with recombinant human erythropoietin (rHuEpo) in hemodialysis patients has been associated with improvement of several abnormalities in hypothalamic–pituitary function. The aim of the present study is to investigate the effects of long term erythropoietin therapy on the hypothalamic–pituitary–thyroid hormone axis in patients undergoing continuous ambulatory peritoneal dialysis (CAPD). Design: Single center, prospective study. Patients and methods: Ten patients who were clinically stable and had been on CAPD were evaluated. Eleven age and sex matched healthy volunteers were chosen as controls. All of the patients were clinically euthyroid. All patients were on CAPD therapy and none of them had received rHuEpo treatment previously. In all patients after basal estimations of free T3, free T4, TSH, GH and prolactin levels, a bolus of 400 µg TRH was administered intravenously. Levels of TSH, GH and prolactin were measured in blood samples collected every 30 min of the 3 h test period. After the treatment with rHuEpo, TRH test with the same protocol was repeated. Results: Before the improvement in serum hemoglobin levels with rHuEpo treatment, the patients on CAPD showed abnormal hypothalamic–pituitary–thyroidal functions, including delayed and prolonged TSH (NS), paradoxically elevated GH (p < 0.001) and increased and prolonged prolactin (p = 0.001) responses to TRH. After improvement of anemia with rHuEpo no significant difference was found between the patients and control groups for baseline TSH levels. In the patients peak TSH level and AUC of TSH secretion were significantly reduced after the treatment (p < 0.05 for both). Furthermore the improvement in anemia did not eliminate the paradoxic GH and prolonged prolactin responses to TRH administration. Conclusion: Some hypothalamic–pituitary–thyroid function abnormalities including delayed and blunted TSH, increased and prolonged prolactin and paradoxical GH responses to TRH administration were observed in uremic patients treated with CAPD and the improvement in anemia with rHuEpo seems to cause slight changes on the hypothalamic–pituitary–thyroid axis and peripheral thyroid hormones.
INTRODUCTION
A variety of pituitary thyroid function test abnormalities, including depressed serum triiodothyronine (T3) concentrations,Citation[[1]], Citation[[2]] low normal serum thyroxine (T4) levelsCitation[[1]], Citation[[3]] and blunted serum thyroid stimulating hormone (TSH) responses to exogenous thyrotropin-releasing hormone (TRH)Citation[[4]] have been reported in uremic patients. Despite the low or low–normal levels of T3 and T4, TSH levels have frequently been found in normal range.Citation[[5]] On the other hand, patients with end stage renal disease (ESRD) frequently have anemia, primarily due to erythropoietin deficiency, and correction of anemia with recombinant human erythropoietin (rHuEpo) reverses some of the endocrine alterations.Citation[[4]] It has been reported that blunted serum TSH responses to exogenous TRH normalized after correction of anemia with rHuEpo in hemodialysis patients.Citation[[4]] In spite of some studies investigating hypothalamic–pituitary–thyroid axis,Citation[[6]], Citation[[7]], Citation[[8]], Citation[[9]], Citation[[10]], Citation[[11]], Citation[[12]] as far as we know, effects of long-term rHuEpo on the hormonal axis has not been evaluated in CAPD patients. CAPD has been demonstrated to achieve improved metabolic control in ESRD compared to hemodialysis.Citation[[13]] Therefore, CAPD might be expected to improve the disturbance in hypothalamic–pituitary–thyroid axis found in ESRD. Based on this background we decided to investigate the effects of long-term rHuEpo therapy on hypothalamic–pituitary–thyroidal functions in CAPD patients.
MATERIALS AND METHODS
Ten clinically euthyroid male patients on CAPD were included in the study. Eleven healthy volunteers, age and sex matched, were studied as controls. Patients with goiter, known thyroid disease, diabetes mellitus or with any other severe systemic illness were excluded. The underlying diseases were chronic glomerulonephritis (n = 3), hypertension (n = 2) and unknown (n = 5). All patients were on CAPD therapy for a mean duration of 11 months, and none of them had received rHuEpo treatment before. All patients received calcium carbonate and oral iron supplementation. Eight of the patients were hypertensive and being treated with calcium channel blockers and/or alpha-receptor blockers. In all patients after basal estimations of free T3, free T4, TSH, GH and prolactin levels, a bolus of 400 µg TRH (Ferring GmbH D-24109 Kiel, Germany) was administered intravenously. Levels of TSH, GH and prolactin were measured in blood samples collected every 30 min of the 3 h test period. Pre-study hematocrits were below 23 vol %. All patients received a total dose of 150 U/kg BW rHuEpo (EPREX, CILAG AG. International 6300 Zug/Switzerland) s.c., per week on three different days. The rHuEpo dose was readjusted every 2–4 weeks depending on the response. The aim of rHuEpo therapy was to achieve a hemoglobin value of at least 11 g/dL. After achieving the target hemoglobin level with exogenous rHuEpo, TRH test with the protocol as described above was repeated. Iron, total iron binding capacity, ferritin and blood chemistries were measured in each patient monthly. Blood samples were centrifuged immediately, and the serum was stored at −20°C until assayed. Complete blood count was measured in the Cell-Dyn/1700 (Abbott Diagnostic Division, IL60064 USA), and serum chemistry determinations were made using a Konelab 60i analyzer (Kone Instruments, Ruukintie 18 FIN-02320 ESPOO). Iron and iron binding capacity were measured in the Perkin Elmer (Oak Brook Instruments, Illinois, USA) with spectrophotometric technique. All specimens for individual hormones were run in one assay. Commercially available kits were used to measure free T3, free T4 and TSH (Amersham, UK), GH (Immunotech, Marseille, France), and prolactin (Active Prolactin IRMA DSL-4500, Diagnostic Systems Laboratories, Inc. Texas, USA). The intra-assay and inter-assay coefficients of variations were: 5.8 and 9.8% for free T3; 6.5 and 7.5% for free T4; 5.3 and 8.6% for TSH; 0.66 and 13.43% for GH and 5.2 and 7.3% for prolactin.
The results are reported as means ± SEM. The TSH, GH and prolactin responses to TRH between 0 and 180 min were also expressed as area under the curve (AUC) estimated by trapezoidal rule. For statistical evaluation of the TSH, GH and prolactin response after TRH administration, the obtained values were analyzed by using the Wilcoxon's signed rank test. For comparisons between patients and control subjects the Mann-Whitney U test was employed. A p value of < 0.05 was regarded as statistically significant.
RESULTS
Hematological Changes
The average levels of hemoglobin and hematocrit before the rHuEpo treatment were 7.65 ± 0.29 g/dL and 22 vol% in patients, respectively. The mean duration of rHuEpo treatment was 15.2 ± 1.16 weeks (ranging from 8 to 20 weeks). After rHuEpo was used, the average levels of hemoglobin and hematocrit increased to 11.42 ± 0.07 g/dL and 34 vol%, respectively. Post rHuEpo tests were performed 8 weeks after the hematocrit reached a value of 33 vol%. As a result, the time interval between the pre and post rHuEpo studies was 23.2 ± 1.16 weeks.
Residual Renal Functions and Adequacy of Peritoneal Dialysis
The mean values for residual renal creatinine clearances before and after the treatment were 1.05 ± 0.71 mL/min and 0.95 ± 0.63 mL/min (ranges 0–6.00 mL/min), respectively. The Kt/V urea indices decreased from 2.22 ± 0.11 to 2.15 ± 0.09 (p < 0.05) between the two evaluation time points. Nevertheless, there was no need to change the standard CAPD prescription throughout the study.
Baseline Hormone Levels
Comparisons of mean thyroid function tests, basal levels of GH and prolactin for CAPD patients before and after rHuEpo treatment and healthy controls are summarized in . Both free T3 and free T4 levels of CAPD patients were lower before and after rHuEpo treatment than the controls; but the reduction was statistically significant for only free T4 levels (p < 0.05). For free T3, the values in the patients were 2.62 ± 0.13 before the treatment and 2.74 ± 0.26 pg/mL after the treatment. For free T3, the control value was 3.09 ± 0.21 pg/mL. For free T4, the values in the patients were 1.07 ± 0.06 before the treatment and 1.12 ± 0.06 ng/mL after the treatment. For free T4, the control value was 1.41 ± 0.10 ng/mL.
Table 1. Baseline and Stimulated Hormonal Concentrations in Uremic Patients on CAPD Therapy and Healthy Controls
Before the rHuEpo treatment, basal levels of TSH, GH and prolactin were significantly higher (2.47 ± 0.44 uIU/L, 18.18 ± 11.34 mIU/L and 27.66 ± 4.11 ng/mL) than the controls (1.30 ± 0.17 uIU/L, 1.34 ± 1.26 mIU/L and 12.40 ± 3.23 ng/mL) (p < 0.05, p = 0.001 and p < 0.05, respectively). When compared with the pretreatment values, after the improvement in anemia with rHuEpo, no differences were found between basal TSH (2.11 ± 0.43 uIU/L) levels, however basal GH (10.88 ± 6.47 mIU/L) and prolactin (23.70 ± 3.85 ng/mL) levels were still higher than controls (p < 0.001 and p < 0.05, respectively).
TSH Responses to TRH
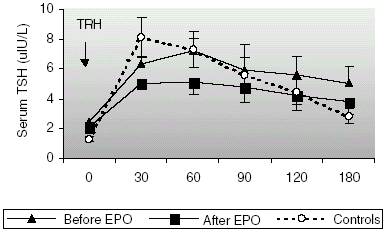
Baseline TSH concentrations were significantly higher in CAPD patients (2.47 ± 0.44 uIU/L) than in the controls (1.30 ± 0.17 uIU/L, p < 0.05), however they were still in normal ranges. The changes in TSH concentration after TRH administration are shown in . An expected rise in TSH concentration, with a peak level at 30 min was observed in all of the control subjects. Before rHuEpo treatment, a delayed TSH response to TRH was observed in majority of the patients (peak TSH level was reached at 30 min in 3, at 60 min in 6 patients and at 90 min in 1 patient). AUC of TSH secretion before rHuEpo treatment was slightly higher in patients (1027.98 ± 204.35 and 938.31 ± 150.09 uIU h/L (NS), respectively) than in healthy volunteers (). AUC of TSH secretion reduced to a level of 785.80 ± 148.45 uIU h/L (NS) after the treatment. After the treatment, peak TSH level and AUC of TSH secretion reduced significantly (p < 0.05 and p < 0.05, respectively) and the delayed TSH response was again observed in half of the patients (peak TSH level was reached at 30 min in 5 patients and at 60 min in the other 5 patients). TSH values reached a maximum of 7.53 ± 1.65 uIU/L before rHuEpo, 5.27 ± 1.03 uIU/L after rHuEpo and of 8.56 ± 1.38 uIU/L in controls (NS) after TRH administration.
Prolactin Responses to TRH
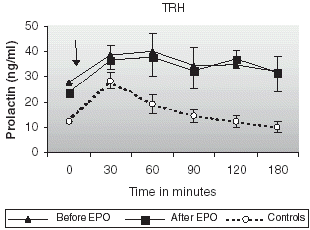
The changes in prolactin secretion after TRH administration are shown in . Baseline prolactin levels in patients were significantly higher before and after the treatment than those found in controls (27.66 ± 4.11 and 23.70 ± 3.85 vs. 12.40 ± 3.23 ng/mL, p = 0.001 for both; ). Prolactin concentrations rose with a peak level at 30 min in healthy controls. An increased and prolonged rise in prolactin levels was observed in CAPD patients before and after improving anemia with rHuEpo. AUC of prolactin secretion was significantly higher in patients (6316.14 ± 1133.85 and 6185.51 ±1094.59 ng/mL, respectively) than in healthy controls (2885.65 ± 388.65 ng/mL, p = 0.001).
GH Responses to TRH
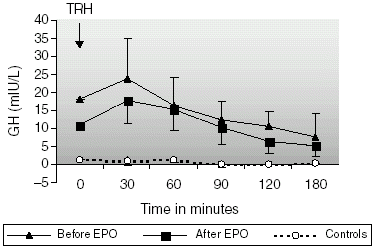
All patients had elevated basal levels of GH with paradoxical response to TRH. Baseline GH levels were significantly higher (18.18 ± 11.34 mIU/L) than in healthy subjects (1.34 ± 1.26 mIU/L, p < 0.001; ) before rHuEpo treatment. After the treatment with rHuEpo, basal GH levels reduced from 18.18 ± 11.34 to 10.88 ± 6.47 mIU/L (NS), but not normalized (p < 0.05 vs. normals). The changes in GH concentration after the administration of TRH are shown in . AUC of GH secretion before rHuEpo treatment was significantly higher in patients (2547.66 ± 912.35 mIU h/L) than in controls (115.45 ± 48.99 mIU h/L, p < 0.001). The improvement in anemia with rHuEpo did not eliminate the paradoxical response but reduced its magnitude. AUC of GH secretion reduced from 2547.66 ± 912.35 to 1907.41 ± 643.79 mIU h/L (NS) after the treatment, but it was still significantly greater than the control values (p<0.001).
DISCUSSION
In uremic state, the hypothalamic–pituitary–thyroid hormone axis as well as peripheral thyroid hormone metabolism is altered without coexisting thyroid disease.Citation[[1]], Citation[[3]], Citation[[5]] Dialysis therapy does not have a remarkable normalizing effect on thyroid hormone metabolism.Citation[[5]] Despite some differences between the two dialytic modalities, serum total T4, free T4 index, total T3, TBG and serum TSH levels and TSH responses to TRH were found to be similar in patients undergoing CAPD and hemodialysis therapy,Citation[[1]], Citation[[6]], Citation[[7]] while serum TBG concentrations were found to be lower in CAPD patients due to the peritoneal lossesCitation[[6]] in the previous reports.
Studies in uremic patients undergoing maintenance hemodialysis have demonstrated that long-term therapy with rHuEpo had been associated with improvement in several abnormalities in the hypothalamic-pituitary functions, including normalization of the response of thyrotropin (TSH) to thyrotropin-releasing hormone (TRH).Citation[[2]] On the other hand, it has been shown that patients on regular hemodialysis frequently had asymptomatic derangements in serum thyroid hormone levelsCitation[[14]], Citation[[15]] but rHuEpo did not seem to improve them.Citation[[15]]
We have investigated the effects of long-term rHuEpo therapy on the hypothalamic–pituitary–thyroid axis in patients on CAPD. Similar to most other reports we found that our patients on CAPD had low levels of serum free T4 (p = 0.001) and low-normal levels of free T3 (NS) before and after correction of anemia with rHuEpo when compared with healthy controls. Our patients showed significantly higher levels of TSH before the treatment (p = 0.001). After rHuEpo treatment, baseline TSH levels were again higher than controls but the difference was not statistically significant. We observed a delayed and blunted TSH response to TRH administration in our group of patients on CAPD. Correction of anemia with rHuEpo did not seem to have a positive effect on these abnormalities, even TSH responsiveness to TRH reduced significantly after the treatment. There was a significant decrease in peak TSH level (p < 0.05) and AUC of TSH release reduced significantly (p < 0.05).
As observed in the present study, the paradoxical increase in GH levels after TRH administration has been described in various reports. It has been suggested by Ramirez et al. that correction of anemia with rHuEpo normalizes the baseline GH levels and eliminates the paradoxical GH response to TRH. In the present study, rHuEpo therapy did not eliminate the paradoxical GH response to TRH administration but reduced its magnitude (statistically not significant). Furthermore the increased and prolonged prolactin response to TRH that observed in our patients on CAPD was not normalized after the treatment.
In conclusion data reported here demonstrate that patients on CAPD have high-normal free T3, lowered free T4 and elevated TSH levels. Moreover they have some hypothalamic–pituitary–thyroid function abnormalities including delayed and blunted TSH, increased and prolonged prolactin and paradoxical GH responses to TRH administration. Our results indicate that correction of anemia with rHuEpo seems to cause slight changes on the hypothalamic–pituitary–thyroid axis and peripheral thyroid hormones.
REFERENCES
- Lim V.S., Fang V.S., Katz A.I., Refetoff S. Thyroid Dysfunction in Chronic Renal Failure. A Study of the Pituitary Thyroid Axis and Peripheral Turnover Kinetics of Thyroxine and Triiodothyronine. J. Clin. Invest. 1977; 60: 522–534
- Diez J.J., Iglesias P., Selgas R. Pituitary Dysfunction in Uremic Patients Undergoing Peritoneal Dialysis: A Cross Sectional Descriptive Study. Adv. Perit. Dial. 1995; 11: 218–224
- Ramirez G., Jubiz W., Gutch C.F., Bloomer H.A., Siegler R., Kolff W.J. Thyroid Abnormalities in Renal Failure. Ann. Int. Med. 1973; 79(4)500–504
- Ramirez G., Bittle P.A., Sanders H., Bercu B.B. Hypothalamo-Hypophyseal Thyroid and Gonadal Function Before and After Erythropoietin Therapy in Dialysis Patients. J. Clin. Endocrinol. Metab. 1992; 74: 517–524
- Kaptein E.M. Thyroid Hormone Metabolism and Thyroid Diseases in Chronic Renal Failure. Endocrine Reviews 1996; 17: 145–163
- Pagliacci M.C., Pelicci G., Grignani F., Giammartino C., Fedeli L., Carobi C., Buoncristiani U., Nicoletti I. Thyroid Function Tests in Patients Undergoing Maintenance Dialysis: Characterization of the ‘Low-T4 Syndrome’ in Subjects on Regular Hemodialysis and Continuous Ambulatory Peritoneal Dialysis. Nephron 1987; 46: 225–230
- Robey C., Shreedhar K., Batuman V. Effects of Chronic Peritoneal Dialysis on Thyroid Function Tests. Am. J. Kidney Dis. 1989; 12: 9–103
- Lin C.C., Chen T.W., Ng Y.Y., Chou Y.H., Yang W.C. Thyroid Dysfunction and Nodular Goiter in Hemodialysis and Peritoneal Dialysis Patients. Perit. Dial. Int. 1998; 18(5)516–521
- Dusunsel R., Poyrazoglu H.M., Gunduz Z., Kurtoglu S., Kiris A., Gunes T. Evidence of Central Hypothyroidism in Children on Continuous Ambulatory Peritoneal Dialysis. Adv. Perit. Dial. 1999; 15: 262–268
- Thysen B., Gatz M., Freeman R., Alpert B.E., Charytan C. Serum Thyroid Hormone Levels in Patients on Continuous Ambulatory Peritoneal Dialysis and Regular Hemodialysis. Nephron 1983; 33: 49–52
- Semple C.G., Beastall G.H., Henderson I.S., Thomson J.A., Kennedy A.C. Thyroid Function and Continuous Ambulatory Peritoneal Dialysis. Nephron 1982; 32: 249–252
- Kerr D.J., Singh V.K., Tsakiris D., McConnell K.N., Junor B.J.R., Alexander W.D. Serum and Peritoneal Dialysate Thyroid Hormone Levels in Patients on Continuous Ambulatory Peritoneal Dialysis. Nephron 1986; 43: 163–168
- Gokal R. History of Peritoneal Dialysis. Textbook of Peritoneal Dialysis, 2nd Ed., R. Gokal, R. Khanna, R. Krediet, K. Nolph. Kluwer Academic Publishers, DordrechtThe Netherlands 2000; 1–19
- Davis F.B., Spector D.A., Davis P.J., Hirsch B.R., Walshe J.J., Yoshida K. Comparison of Pituitary-Thyroid Function in Patients With End-Stage Renal Disease and in Age- and Sex-Matched Controls. Kidney Int. 1982; 21: 371–378
- Goffin E., Oliveira D.B.G., Raggatt P., Evans D.B. Assessment of the Thyroid Function of Patients Undergoing Regular Hemodialysis. Nephron 1993; 65: 568–572