Abstract
N-acetylcysteine (NAC) repletes intracellular stores of reduced glutathione and may be a scavenger of oxygen free radicals. We report a 52-year-old female who developed acute renal insufficiency after administration of one dose of 150 mg of cisplatin for treatment of squamous cell cancer of the esophagus. Her blood urea nitrogen and creatinine rose from 12 and 0.7 mg/dL, respectively, to 24 and 1.8 mg/dL on day 5 after cisplatin. On that day the patient was begun on NAC, starting with a loading dose of 140-mg/kg-body weight followed by 70 mg/kg every 4 h for 4 days. Two days after starting NAC her renal function began to improve, and although she failed to complete a full course of the drug, by day 10 her serum creatinine had fallen to 0.8 mg/dL. A previous report showed that N-acetylcysteine might reverse cisplatin-induced renal toxicity. Our case supports this hypothesis.
Cisplatin (cis-diaminedichloroplatinum) is an inorganic metal complex, which is used clinically for the treatment of solid tumors especially of the head and neck, certain lymphomas, testicular and ovarian tumors. Nephrotoxicity compromises its usefulness as a therapeutic agent. Measures to prevent cisplatin-induced nephrotoxicity include early administration of intravenous normal saline, sometimes with mannitol.Citation[[1]], Citation[[2]] N-acetylcysteine (NAC), a thiol donor, has been shown to inhibit cisplatin-induced glutathione depletion, hydrogen peroxide accumulation, and free radical-induced lipid peroxidation.Citation[[3]] NAC was recently reported to reverse cisplatin-induced acute renal failure.Citation[[4]] We report a second case where NAC appears to have hastened recovery from acute renal failure due to cisplatin.
CASE REPORT
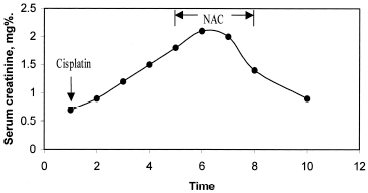
A 52-year-old woman with squamous-cell carcinoma of the esophagus was admitted for elective chemotherapy to the hospital. Laboratory tests performed at the time of her admission showed: blood urea nitrogen (BUN) 12 mg%; creatinine (Cr) 0.7 mg%; sodium 143 mmol/L; potassium 4.4 mmol/L; chloride 108 mmol/L; bicarbonate 22 mmol/L. She received a total of 100 mg/m2 (150 mg) of cisplatin. Hydration with 150 mL/h of normal saline and 12.5 g of mannitol was given. However, as seen in , on the fourth day after completion of the chemotherapy her BUN and Cr rose to 18 mg/dL and 1.5 mg/dL. On the fifth day, when Cr was 1.8 mg/dL, a trial of NAC, at the doses used to treat acetaminophen toxicity, was begun. The initial dose of NAC, 140-mg/kg body weight, was followed by 70 mg/kg every 4 h. BUN and Cr levels peaked at 27 and 2.1 mg/dL, respectively, on the following day and then started to decline (). On the third day of NAC therapy BUN and Cr had declined to 18 and 1.4 mg/dL (). The patient left the hospital against medical advice on day 4 of NAC. Blood chemistries from the oncology clinic on day 10 showed that Cr had fallen to 0.8 mg/dL.
DISCUSSION
The kidney is not only responsible for the majority of cisplatin excretion but is also the primary site of cisplatin accumulation.Citation[[5]] The effects of cisplatin on the kidney include acute and chronic renal failure, renal magnesium wasting, and polyuria. The mechanism by which cisplatin produces cellular injury is not known but may involve metabolites of cisplatin. The primary target associated in the rat is the proximal tubule S3 segment,Citation[[6]] but in humans the S1 and S2 segments, distal tubule, and collecting ducts, mainly in the corticomedullary region of the kidney where the concentration of platinum is highest, may also be affected. Appearance of tubular cell proteins in the urine may precede the fall in renal function.Citation[[7]]
In vitro studies using primary cultures of rabbit renal proximal tubule cells showed that while DNA synthesis, protein synthesis, glucose transport, Na+−K+-ATPase activity, and cell viability were all inhibited by cisplatin, DNA synthesis was the most sensitive.Citation[[8]] This suggests that cisplatin nephrotoxicity may involve inhibition of DNA and protein synthesis as well as transport functions. Furthermore, the lack of complete return of renal function following cisplatin treatment in vivo may result from interference of cisplatin with the normal proliferative response that occurs after injury.
Like other heavy metals, platinum binds to sulfhydryl groups of proteins such as renal ATPase and GGT, inhibiting their activity and resulting in toxicity. Mitochondrial injury in proximal tubules occurs early following in vitro or in vivo exposure to cisplatin and may result in adenine nucleotide depletion and cell death.Citation[[9]], Citation[[10]] The mitochondrial injury is associated with the loss of mitochondrial glutathione concentration.Citation[[11]] In the intracellular milieu, cisplatin's chloride ions may exchange for cellular SH moieties, resulting in glutathione depletion, hydrogen peroxide accumulation, and lipid peroxidation.Citation[[3]], Citation[[11]], Citation[[17]], Citation[[18]] Cisplatin-induced lipid peroxidation, in addition to causing direct cellular injury, may further contribute to renal dysfunction by generating vasoconstrictive E2- and F2- isoprostanes, which can be inhibited by NAC.Citation[[3]] Hence, sulfhydryl amino acids such as NAC have been explored as possible means of reversing cisplatin-induced nephrotoxicity.Citation[[12]]
NAC increases hepatic reduced glutathione; hence it is used to treat acetaminophen overdose.Citation[[13]] Glutathione forms a complex with cisplatin, and exogenous glutathione lowers cellular uptake of cisplatin.Citation[[12]] In addition, NAC directly reduces reactive oxygen species, which have been proposed to be involved in the genotoxic and cytotoxic actions of cisplatin.Citation[[14]] NAC was shown to abolish the nephrotoxicity of cisplatinCitation[[15]] and ameliorate ischemic acute renal failure in the rat.Citation[[19]]
Cisplatin nephrotoxicity is dose-dependent, and the recovery of renal function after administration of modest cisplatin dosages normally occurs after a 2-week delay.Citation[[16]] The immediate improvement in our patient's renal function during NAC therapy suggests that the NAC may have ameliorated the renal injury. NAC appears to have potential usefulness as salvage therapy in the management of cisplatin induced acute renal failure.
REFERENCES
- Ostrow S., Egorin M.J., Hahn D., Markus S., Aisner J., Chang P., LeRoy A., Bachur N.R., Wiernik O.H. High-Dose Cisplatin therapy Using Mannitol Versus Furosemide Diuresis: Comparative Pharmacokinetics and Toxicity. Cancer Treat. Rep. 1981; 65: 73–78
- Hayes D.M., Cvitkovic E., Golbey R.B., Scheiner E., Helson L., Krakof I.H. High Dose Cisplatin Diamine Dichloride: Amelioration of Renal Toxicity by Mannitol Diuresis. Cancer 1977; 39: 1372–1381
- Salahudeen A., Poovala V., Parry W., Pande R., Kanji V., Ansari N., Morrow J., Roberts J. Cisplatin Induces N-Acetyl Cysteine Suppressible F2-Isoprostane Production and Injury in Renal Tubular Epithelial Cells. J. Am. Soc. Nephrol. 1998; 9(8)1448–1455
- Sheikh-Hamad D., Timmins K., Jalali Z. Cisplatin-Induced Renal Toxicity: Possible Reversal by N-Acetylcysteine Treatment. J. Am. Soc. Nephrol. 1997; 8: 1640–1645
- Borch R.F. The Nephrotoxicity of Antineoplastic Drugs. Toxicology of the Kidney; 2nd Ed. 1993; pp 283–301
- Safirstein R., Miller P., Dikman S., Lyman N., Shapiro C. Cisplatin Nephrotoxicity in Rats: Defect in Papillary Hypertonicity. Am. J. Physiol. 1982; 241: F175–F185
- Feinfeld D.A., Fuh V.L., Safirstein R. Urinary Glutathione-S-Transferase in Cisplatin Nephrotoxicity in the Rat. J. Clin. Chem. Clin. Biochem. 1986; 24: 529–532
- Courjault F., Leroy D., Coquery I., Toutain H. Platinum Complex-Induced Dysfunction of Cultured Renal Proximal Tubule Cells. Arch. Toxicol. 1993; 67: 338–346
- Gordon J.A., Gattone V.H. Mitochondrial Alterations in Cisplatin Induced Renal Failure. Am. J. Physiol. 1986; 264: F991–F998
- Brady H.R., Zeidel M.I., Kone B.C. Differential Actions of Cisplatin on Renal Proximal Tubule and Inner Medullary Collecting Duct Cells. J. Pharmacol. Exp. Ther. 1993; 219: 1421–1428
- Zhang R.K. Organic Anion Transport and Action of Glutamyl-Transpeptidase in Kidney Linked Mechanistically to Renal Tubule Uptake of Inorganic Mercury. Toxicol. Appl. Pharmacol. 1995; 132: 289–289
- Kronig R., Lichtenstein A.K., Nagami G.T. Sulphur-containing Amino Acids Decrease Cisplatin Cytotoxicity and Uptake in Renal Tubule Epithelial Cell Lines. Can. Chemo. and Pharmacol. 2000; 45(1)43–49
- Flanagan R.J., Meredith T.J. Use of N-Acetylcysteine in Clinical Toxicology. Am. J. Med. 1991; 91: 131s–139s
- Aruoma O.I., Halliwell B., Hoey B.M., Butler J. The Antioxidant Action of N-Acetylcysteine: Its Reaction with Hydrogen Peroxide, Hydroxyl Radical, Superoxide, and Hypochlorous Acid. Free Radic. Biol. Med. 1989; 6: 593–597
- Appenroth D., Winnefeld K., Schroter H., Rost M. Beneficial Effect of Acetylcysteine on Cisplatin Nephrotoxicity in Rats. J. Appl. Toxicol. 1993; 13: 189–192
- Nanji A.A., Stewary D.J., Mikhael N.Z. Hyperuricemia and Hypoalbuminemia Predispose to Cisplatin-induced Nephrotoxicity, Cancer Chemother. Pharmacol. 1986; 17: 274–276
- Sugihara K., Nakano S., Gemba M. Stimulatory Effect of Cisplatin on Production of Lipid Peroxidation in Renal Tissues. Jpn. J. Pharmacol. 1987; 43: 247–252
- Hanneman J., Baumann K. Cisplatin Induced Lipid Peroxidation and Decrease of Gluconeogenesis in Rat Kidney Cortex: Different Effects of Antioxidants and Radical Scavengers. Toxicology 1988; 51: 119–132
- DiMari J., Megyesi J., Udvarhelyi N., Price P., Davis R., Safirstein R. N-Acetyl Cystine Ameliorates Ischemic Renal Failure. Am. J. Physiol. 1997; 272: F292–F298