Abstract
In vascular smooth muscle, calcium overload is a highly pathogenic event, which increases with advancing age. An increase in the calcium content of arterial wall may be produced in rats by treatment with vitamin D3. The aim of this study was to evaluate the renal clearance of sulfanilamide (a model organic anion, preferentially eliminated by the kidneys) and other parameters of global renal function in rats with arterial calcinosis. Arterial calcinosis was produced in adult rats by means of a single dose of vitamin D3 (300,000 UI/kg bw, i.m.) 5 days before the experiment. Treated rats showed a large increase in calcium content of aortic tissue and an increase in systolic arterial pressure. No modifications were observed in plasma calcium levels and in plasma lipid profiles. Statistically significant decrements were observed in renal clearance of sulfanilamide, in renal blood flow, in fractional excretion of sodium and potassium. A slight decrease, not statistically different, was observed in the glomerular filtration rate. Rats with arterial calcinosis also showed an increment of total calcium levels in renal tissue, in fractional excretion of calcium and in the expression of organic anion transporter 1 (OAT1). Histological studies revealed tubular alterations. In summary, modifications in hemodynamics and tubular parameters are early manifestations of nephropathy in rats with arterial calcinosis, some of which may account for the changes observed in organic anions renal depuration. It is important to mention that the decrease in clearance of organic anions were seen in spite of the increase in expression of OAT1.
Introduction
In vascular smooth muscle, calcium overload is a highly pathogenic event, finally leading to calcification and necrotization of the arterial wall. With advancing age, a 5 to 100 fold accumulation of calcium occurs in human blood vessels higher than those in respective infantile arteries.Citation[[1]], Citation[[2]] Old and/or hypertensive rats have been used to study arterial calcinosis, but these are not ideal models, because in animals vascular calcium overload linked with age or hypertension is less pronounced than in humans.Citation[[3]] An increase in the calcium content of arterial wall may be produced in rats by treatment with vitamin D3.Citation[[4]], Citation[[5]] In this experimental model, calcium overload is not restricted to the arterial wall, and it has been demonstrated in many organs such as heart and kidneys, leading to the impairment of their function.Citation[[6]] Preserved function of kidneys is an important determinant of drug pharmacokinetics.Citation[[7]] Impairment of kidney function produces modifications in the renal elimination of drugs mediated by alterations in blood flow to the kidney, glomerular filtration, active tubular secretion, and passive tubular reabsorption.Citation[[7]] A number of drugs, such as Beta-lactam antibiotics, diuretics, nonsteroidal antiinflammatory drugs and several antiviral drugs exist in vivo as organic anions. Kidneys exhibit an important role in the elimination of organic anions. The organic anion transporter1 (OAT1) is the well-known organic anion/dicarboxylate exchange present in the basolateral membranes from proximal tubule cells.Citation[[8]], Citation[[9]], Citation[[10]]
We have recently evaluated the pharmacokinetics of sulfanilamide (SA, a model of organic anion preferentially eliminated by the kidneys) in rats with arterial calcinosis induced by an overdose of vitamin D3.Citation[[11]] These rats showed modifications in total body clearance of SA. To further clarify these results we propose to evaluate the renal clearance of SA, expression of OAT1 and other parameters of global renal function in rats with calcium overload.
Methods
Animals and Treatment
Adult male Wistar rats (320–380 g body weight, 110–130 days) were randomly divided in two groups: control (C) and treated (T) rats. Treated rats were injected 5 days before the experiment with a single dose of vitamin D3 (300,000 IU/kg b.w., i.m.) in order to induce a vascular calcium overload. Vitamin D3 solutions were prepared in corn oil. Control animals received an identical volume of corn oil by i.m. injection. All animals were housed in rooms with controlled temperature (21–23°C) and regular light/dark cycles (12 h). They were allowed free access to a standard diet and tap water. All procedures were in accordance with institutional guidelines for the care and use of laboratory animals.
Experimental Procedure
Measurement of Arterial Pressure
Systolic arterial pressure was measured employing a Harvard indirect rat tail blood pressure monitor connected to a Harvard student oscillograph in all experimental groups as previously described by us.Citation[[11]], Citation[[12]]
Plasma Composition Study
Blood samples (obtained by cardiac puncture) from animals of all experimental groups were used for the determination of total lipids, triglycerides, cholesterol, and calcium. Total lipids, total cholesterol, triglycerides and calcium were determined spectrophotometrically with commercial reagent kits (Wiener Lab, Rosario, Argentina).
Tissue Calcium Analysis
Samples of the abdominal aorta and kidneys were removed for the analysis of tissue calcium levels. Tissues were weighed and then heated to constant dry weight for 48 h at 120°C. Dry tissue samples were dissolved in nitric acid (14 N) and left for 72 h at room temperature. The samples were then centrifuged (2000 × g, 10 min), and the supernatant removed. Strontium nitrate was added and calcium (µmol/g dry weight) measured by atomic absorption spectrophotometry.
Renal Function Studies. Renal Clearance Studies
These studies were performed as previously described.Citation[[13]], Citation[[14]] Briefly, the animals were anesthetized with sodium thiopental (70 mg/kg b.w.; i.p.). The femoral vein and femoral artery were cannulated (P.E.50. Intramedic, USA) and a bladder catheter (3 mm i.d.) was inserted through a suprapubic incision. Animals were maintained in restraining cages throughout the experiment to facilitate collection of urine. A prime dose of inulin (0.6 mg/kg b.w.) in 1 mL of saline solution was administered through the venous catheter. Then, a solution containing inulin (1.8 g%) and saline solution (0.9 g%) was infused through the venous catheter employing a constant infusion pump (Pump 22; Harvard Apparatus, USA) at a rate of 1 mL/h/100 g b.w. After equilibration for 60 min, urine was collected during two 30 min periods. Blood from the femoral artery was obtained at the midpoint of each clearance period. Arterial blood pressure was estimated throughout the experiments with a manometer inserted in the femoral artery. Inulin concentrations in the urine and blood samples were determined by the procedure of Roe.Citation[[15]] Sodium and potassium were measured by flame photometry and the volume of urine by gravimetry. Osmolality was determined in a freezing point osmometer (Osmomat 030). The glomerular filtration rate (GFR) was calculated from the clearance of inulin. The fractional excretion of water (FE% H2O), sodium (FE% Na+) and potassium (FE% K+) and osmolytes (FE% Osm) were also calculated by conventional formulae for each animal.
Another set of experimental animals (control and treated) were prepared in a similar way in order to study the renal clearance of SA. A prime dose of SA (4 mg/kg b.w.) in 1 mL of saline solution was administered through the venous catheter. The concentration of SA in the infusion solution was 0.6 g%. Determination of plasma and urine SA concentrations was performed by the Bratton Marshall reaction.Citation[[16]] The renal clearance of SA was calculated by conventional formulae for each animal.
Renal Blood Flow Determination
Another set of experimental animals (Control and Treated) were used to evaluate renal blood flow as described by GlennyCitation[[17]] and as employed in our laboratory.Citation[[14]] Oranges microspheres 15 µm in diameter (Molecular Probes) were infused in one shot into the carotid artery (previously catheterized) followed by a washout with 1 mL of saline solution. Arterial reference blood was collected from a catheter inserted in the femoral artery at the rate of 1 mL/min, which was continued for 60 s after injection. Five min after the microspheres injection, the rats were killed and the kidneys removed. Blood samples were digested overnight with 89.2% KOH. Kidney tissue was digested with 22.4% KOH for 24 h. Digested samples were filtered using Poretics polycarbonate filters. The fluorescence dye was extracted with 2-(2-ethoxyethoxy) ethyl acetate. After one hour, fluorescence was measured at 540–560 nm. A known amount yellow-green microspheres (505–515 nm) was added to each sample vial of blood and tissue prior to digestion. These microspheres act as internal standard. Organ blood flow was calculated by the formula: Organ Flow (mL/min) = fl/flref × R (mL/min), where fl is the fluorescence of the tissue, flref is the fluorescence of the reference blood flow sample and R is the withdrawal rate of the reference blood flow sample.
Preparation of Basolateral Membrane Vesicles (BLMV) from Kidney Cortex
The preparations of BLMV from control and treated rats were done by a modification of the method described by Jensen and BerndtCitation[[18]] as previously reported by us.Citation[[14]], Citation[[19]] Approximately 4 g of kidney cortex were placed in a Dounce homogenizer containing 50 mL of 250 mM sucrose, 5 mM Tris-Hepes pH 7.40. After four gentle strokes with the loose fitting pestle, the suspension was homogenized further with a motor-driven Teflon pestle (600 rpm/5 strokes) and spun down for 15 min at 1200 × g. The supernatant was aspirated and spun for 15 min at 22,000 × g. The fluffy beige upper layer of the resulting pellet (crude plasma membranes) was resuspended in about 1 mL of supernatant and added to 19 mL of buffered sucrose. The membrane suspension was homogenized gently through a 16-gauge gavage needle followed by the addition of 3.7 mL of 100% Percoll. The Percoll-membranes mixture was spun for 30 min at 39,250 × g. The top clear layer was discarded and the top-most dark band was removed. This layer was composed primarily of basolateral membranes as established by marker enzyme analysis. BLMV were brought up in KCl buffer (85 mM KCl, 83 mM sucrose, 2 mM Hepes-Tris pH 7.40) at a ratio of 8 mL/g original cortex wet weight. Then, BLMV were pelleted at 30,000 × g for 30 min and washed three times with the KCl buffer indicated. Finally, BLMV were resuspended in 300 uL of 250 mM mannitol, 10 mM Hepes-Tris, pH 7.40. Aliquots of the membranes were stored immediately at −70°C until used (no more than two weeks after membrane preparations). Each preparation represented cortical tissue from six animals.
Immunoblot Technique
Control and treated BLMV were boiled for 3 min in the presence of 1% 2-mercaptoethanol/2% SDS. Samples containing 50 ug were applied to a 8.5% gel, separated by SDS-polyacrylamide gel electrophoresis, and then electroblotted to nitrocellulose membranes. Membranes were stained with Ponceau Red to confirm equal protein loading and transfer between lanes as previously described.Citation[[19]], Citation[[20]], Citation[[21]] The nitrocellulose membranes were incubated overnight with 5% nonfat dry milk in PBST. After being rinsed with PBST, membranes were then incubated with a commercial polyclonal antibody to OAT1 (1.25 ug/mL) for 2 h. Membranes were incubated for 1 h with a peroxidase coupled goat anti-rabbit IgG (Bio-Rad) after being rinsed with PBST. Then, blots were processed for detection using a commercial kit (4Opti-CN, Bio-Rad). To investigate the specificity of the bands, an absorption test was performed. The OAT1 peptide (1.25 ug/mL) was added to the OAT1-antibody solution (1.25 ug/mL) and incubated for 2 h. Using this pre-absorbed antibody, Western blot analysis was performed as described above.
Histopathological Studies
Histopathology of kidneys was performed after fixing in 10% neutral buffered formaldehyde solution for 2 and 4 h and embedding in paraffin, then 4 um thick sections were processed for routine staining with hematoxylin and eosin.
Statistical Analysis
Results are expressed as means ± SEM. Significant differences between groups were determined using Student's t-test. The probability level chosen was p<0.05.
Drugs
All drugs were purchased from Sigma Chemical Company (St. Louis, MO, USA).
Results
shows that treatment with vitamin D3 produced a large increase in calcium content of aortic tissue and an increase in systolic arterial pressure. No modifications were observed in plasma calcium levels and in plasma lipid profile. These data confirm those previously described.Citation[[11]]
Table 1. Systolic arterial pressure, total calcium in aorta and plasma, and plasma lipid profile in control and treated rats (vitamin D3 300,000 IU/kg b.w., i.m., 5 days before the experiment). Results are expressed as means±SD
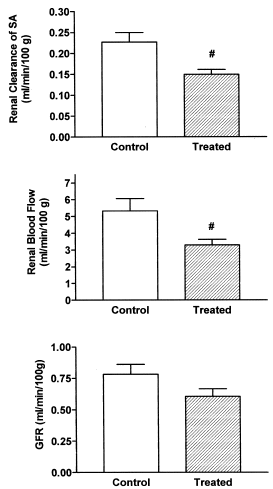
As it is shown in , a statistically significant decrease was observed in renal clearance of SA (30% of control values) and in renal blood flow (40%) in treated rats. A diminished value (not statistically significant) was observed in glomerular filtration rate (20%) in rats with calcinosis.
Figure 1. Renal clearance of SA, renal blood flow and glomerular filtration rate in control and treated rats (vitamin D3 300,000 IU/kg b.w., i.m., 5 days before the experiment). Results are expressed as means ± SEM. (#) p<0.05.
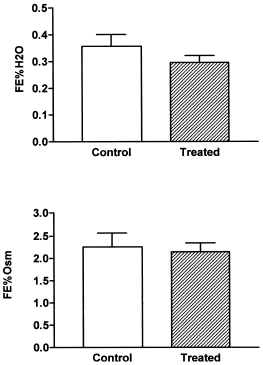
FE% H2O and FE% Osm were not modified after the treatment ().
Figure 2. Fractional excretion of water (FE% H2O) and fractional excretion of osmolytes (FE% Osm) in control and treated rats (vitamin D3 300,000 IU/kg b.w., i.m., 5 days before the experiment). Results are expressed as means ± SEM. (#) p<0.05.
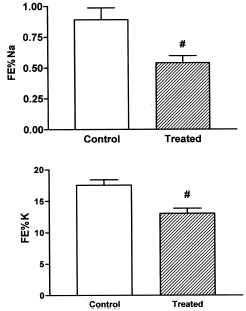
A statistically significant diminution was observed in the FE% Na and FE% K ().
Figure 3. Fractional excretion of sodium (FE% Na) and fractional excretion of potassium (FE% K) in control and treated rats (vitamin D3 300,000 IU/kg b.w., i.m., 5 days before the experiment). Results are expressed as means ± SEM. (#) p<0.05.
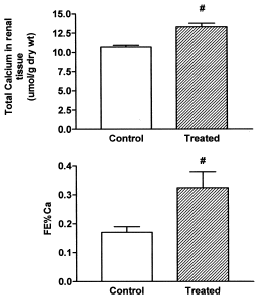
shows a statistically significant increase of total calcium in renal tissue and in the FE% Ca.
Figure 4. Total calcium in renal tissue and fractional excretion of calcium (FE% Ca) in control and treated rats (vitamin D3 300,000 IU/kg b.w., i.m., 5 days before the experiment). Results are expressed as means ± SEM. (#) p<0.05.
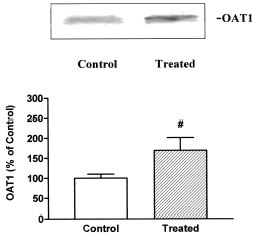
An increment in the expression of OAT1 was observed in renal basolateral plasma membrane vesicles from treated rats (). The OAT1 protein of 57 kDa disappeared when the antibody was pre-absorbed to the synthetic antigen peptide (data not shown).
Figure 5. A: Basolateral membranes (50 ug) from kidneys of control and treated rats were separated on SDS-PAGE. OAT1 was identified using polyclonal antibodies as described in Methods. B: densitometric quantitation of OAT1. Control levels were set at 100%. Each column represents mean ± SEM from experiments carried out in triplicate on 3 different vesicle preparations for each experimental groups. (#) p<0.05.
Histological studies revealed a marked tubular alteration with vacuoles and granules in the cytoplasm, venocapilar congestion in the corticomedullary junction and glomeruli of reduced size. No significant morphologic changes were observed in the interstitium.
Discussion
Previous data from our laboratory had shown an alteration in total body clearance of SA in rats with arterial calcinosis.Citation[[11]] So, it was decided to evaluate the renal clearance of SA and other parameters of global renal function.
Rats with calcinosis presented a large increase in the calcium content in the aorta and an increase in systolic arterial pressure as previously observed. The rats were good in health; in addition, plasma lipid profile appeared to be normal. So it is possible to suggest that the impairment of the arterial wall in this model is arteriosclerotic without an atherosclerotic component.
Treated rats showed an approximately 40% decrease in renal blood flow and a 20% decrease in GFR. The filtration fraction was increased. This indicates a vasoconstrictor response both in afferent and efferent glomerular arterioles. In this connection, it has been described that alterations in calcium metabolism caused by administration of vitamin D3 acutely increases the whole animal pressor response to catecholamines and may selectively constrict regional circulation.Citation[[22]] It has been proposed that this is caused by the inotropic effects on the vitamin D-dependent calcium binding protein.Citation[[22]] As a result of the rise in the filtration fraction, the peritubular protein concentration rises, an peritubular capillary hydrostatic pressure falls.Citation[[23]] These effects lead to an increased reabsorption of sodium chloride, which may explain the decrease in the FE% Na observed in rats with calcinosis. In addition, it is well described that a diminution in sodium load arriving to the distal tubule, decrease potassium secretion, explaining in this way the diminution in the FE% K observed in the treated rats.
We also observed an increase in FE% Ca, which is probably mediated by suppression of parathyroid hormone as has been described by other authors.Citation[[24]] An increment in total renal calcium content was also observed in treated rats. This calcium deposition may also be the cause of a renal vasoconstriction and a decrease in the glomerular ultrafiltration coefficient, as described by Humes et al.Citation[[25]]; explaining, at least in part, the impairment in renal hemodynamic parameters observed in our experimental model.
SA is an organic anion preferentially eliminated by the kidneys, by means of glomerular filtration and proximal secretion.Citation[[26]] Renal clearance of SA was decreased in treated rats, probably due to the diminution observed in renal blood flow, which would determine a lower GFR and a lower proximal secretion. Nevertheless, the diminution in the renal clearance of SA was less pronounced than the diminution in the renal blood flow. At this point, it was of interest to evaluate OAT1 expression in basolateral membrane vesicles from control and treated rats. We observed an increment in the expression of OAT1 in the rats with arterial calcinosis. This suggest a higher tubular secretion of SA in treated rats, which might counter-buff the diminished effect originated by the impairment of renal blood flow in the renal clearance of SA. So the decrease in clearance of SA was seen in spite of the increase in the expression of OAT1. In this connection, it has been described modifications of intracellular calcium levels may regulate the expression of many proteins.Citation[[27]], Citation[[28]]
In summary, both hemodynamic and tubular modifications are early manifestations of nephropathy in rats with arterial calcinosis, some of which may account for the changes observed in the renal elimination of negatively charged compounds.
Acknowledgments
This study was supported by the following grants: FONCYT (PICT 05-06160), Fundación Antorchas, CONICET, Beca Ramón Carrillo-Arturo Oñativia 2001 and Universidad Nacional de Rosario (PID-UNR). The authors also thank Wiener Lab Argentina for analytical kits.
References
- Fleckenstein A., Frey M., Zorn J., Fleckenstein-Grun G. The role of calcium in the pathogenesis of experimental arteriosclerosis. Trends Pharmacol. Sci. 1987; 8: 496–501
- Fleckenstein A., Frey M., Fleckenstein-Grun G. Antihypertensive and arterial anticalcinotic effects of calcium antagonists. Am. J. Cardiol. 1986; 57: 1D–10D
- Henrion D., Chillon J.M., Godeau G., Muller F., Capdeville-Atkinson C., Hoffman M., Atkinson J. The consequences of aortic calcium overload following vitamin D3 plus nicotine treatment in young rats. J. Hypertens. 1991; 9: 919–921
- Fleckestein-Grun G., Thimm F., Frey M., Czirfusz A. Role of calcium in arteriosclerosis-Experimental evaluation of antiarteriosclerotic potencies of Ca antagonists. Basic. Res. Cardiol. 1994; 89: 145–159
- Rucker R.B., Ford D., Riemann W.G., Tom K. Additional evidence for the binding of calcium ions to elastin at neutral sites. Calcif. Tissue Res. 1974; 14: 317–325
- Atkinson J. Vascular calcium overload—physiological and pharmacological consequences. Drugs 1992; 44: 111–118
- Brenner B.B., Rector F.C. The Kidney; 5th Ed. W.B. Saunders Company, PennsylvaniaUSA 1996; 607–625
- Van Aubel R., Masereeuw R., Russel F.G.M. Molecular pharmacology of organic anion transporters. Am. J. Physiol. 2000; 279: F216–F232
- Inui K.-I., Masuda S., Saito H. Cellular and molecular aspects of drug transport in the kidney. Kidney Int. 2000; 58: 944–958
- Berkhin E.B., Humphreys M.H. Regulation of renal tubular secretion of organic compounds. Kidney Int. 2001; 59: 17–30
- Quaglia N.B., Hofer C.G., Torres A.M. Pharmacokinetics of organic anions in rats with arterial calcinosis. Clin. Exp. Pharmacol. Physiol. 2002; 29: 48–52
- Garcia V.M.C., Girardi G., Ochoa J.E., Torres A.M., Elias M.M. Early manifestations of nephropathy in alloxan-treated rats. Renal Fail. 1998; 20: 551–564
- Torres A.M., Rodríguez J.V., Elías M. Vulnerability of the thick ascending limb to glutathione depletion in rat kidney: effects of diuretics and indomethacine. J. Pharmacol. Exp. Ther. 1989; 250: 247–253
- Cerrutti J.A., Quaglia N.B., Torres A.M. Characterization of the mechanisms involved in the gender differences in p-aminohippurate renal elimination in rats. Can. J. Physiol. Pharmacol. 2001; 79: 805–813
- Roe H.H. A photometric method for determination of inulin in plasma and urine. J. Biol. Chem. 1949; 178: 839–844
- Bratton A.C., Marshall E.K., Jr. Determination of p-aminohippurate. J. Biol. Chem. 1939; 128: 537–542
- Glenny R.W. Manual for Using Fluorescent Microspheres to Measure Regional Organ Perfusion. Fluorescent microsphere Resource Center, University of Washington. 1996
- Jensen R.E., Berndt W.O. Epinephrine and norephinephrine enhance p-aminohippurate transport into basolateral membrane vesicles. J. Pharmacol. Exp. Ther. 1988; 244: 543–549
- Cerrutti J.A., Brandoni A., Quaglia N.B., Torres A.M. Sex differences in p-aminohippuric acid transport in rat kidney: role of membrane fluidity and expression of OAT1. Mol. Cell. Biochem. 2002; 233: 175–179
- Shah V., Cao S., Hendrickson H., Yao J., Katusic Z.S. Regulation of hepatic eNOS by caveolin and calmodulin after bile duct ligation in rats. Am. J. Physiol. 2001; 280: G1209–G1216
- Dumont M., Jacquemin E., D'Hont C., Descout C., Cresteil D., Haouzi D., Desrochers M., Stieger B., Hadchouel M., Erlinger S. Expression of the liver Na+-independent organic anion transporting polypeptide (oatp-1) in rats with bile duct ligation. J. Hepatology 1997; 27: 1051–1056
- Bukoski R.D., Wang D., Wagman D.W. Injection of 1,25 (OH)2 vitamin D enhances resistance artery contractile properties. Hypertension 1990; 16: 523–531
- Seldin D.W. Renal handling of calcium. Nephron 1999; 81: 2–7
- Brickman A.S., Coburn J.W., Norman A.W., Massry S.G. Short-term effects of 1,25-dihydroxycholecalciferol on disordered calcium metabolism of renal failure. Am. J. Med. 1974; 57: 28–30
- Humes H.D., Ichikawa I., Troy J.L., Brenner B.M. Evidence for a parathyroid hormone-dependent influence of calcium in the glomerular filtration. J. Clin. Invest. 1978; 61: 32–35
- Fleck Ch., Braunlich H. Interrelationships between excretion of drugs via urine and bile. Progress in Pharmacology and Clinical Pharmacology 1991; 8: 511–529
- Jones G., Strugnell S.A., DeLuca H. Current understanding and the molecular actions of vitamin D. Physiological Reviews 1998; 78: 1193–1222
- Brown A.J., Dusso A., Slatopolsky E. Vitamin D. Am. J. Physiol. 1999; 277: F157–F175