Abstract
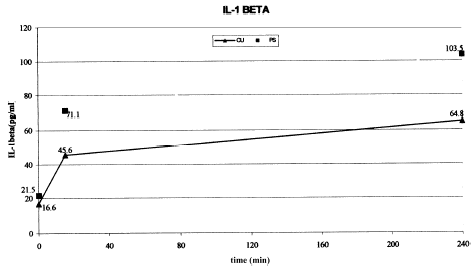
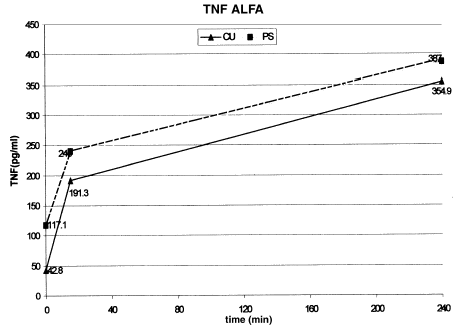
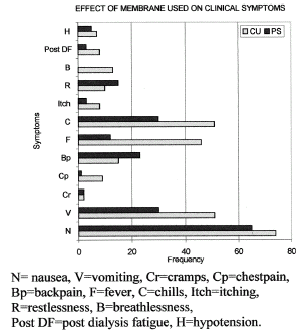
Background. Intradialytic symptoms including hypotension have been reported during dialysis and it has been suggested that these are related to the release of cytokines like IL-1β and TNFα by blood mononuclear cells when they get activated either due to contact with the dialyzer membrane or by compliment activation. Objective. To study the relationship between hemodialysis symptoms, cytokine production, and dialyzer membrane composition. Method. In a randomized prospective crossover study, 20 ESRD patients on maintenance hemodialysis were studied over cuprophan (CU) and polysulfone (PS) low flux dialyzer membranes for three weeks each undergoing a biweekly dialysis schedule of 4 h sessions. Serial IL-1β and TNFα levels were measured over 0, 15, 240 min during the first use of the dialyzer for all patients on both membranes. Intradialytic symptoms were monitored in a total of 240 dialysis sessions. Results. IL-1β levels increased from 16.6 ± 2.2 to 64.8 ± 25.1 pg/mL on CU and 21.5 ± 3.7 to 103.5 ± 30.7 pg/mL on PS membrane over the 4-h dialysis session. Similarly TNFα increased from 42.8 ± 4.5 to 354.9 ± 80.4 pg/mL on CU and 117.1 ± 53.7 to 387.0 ± 78.0 pg/mL on PS membrane. IL-1β levels increased significantly with PS membrane while TNFα rise was significant with both the membranes. Nausea was the most common symptom occurring in 138 dialysis sessions (57.5%). Vomiting, chest pain, fever, chills, and breathlessness occurred significantly more during dialysis with CU membrane as compared with PS membrane (P<0.01). Nausea, cramps, back pain, itching, restlessness, post dialysis fatigue, and hypotension did not differ between the two membranes. The mean rise in the cytokine levels during the first 15 min of sessions where the symptoms occurred, when compared with the mean rise in sessions where the symptoms did not occur, did not reveal any significant difference. Cytokine release did not correlate with the occurrence of intradialytic symptoms. Conclusion. Both CU and PS membranes increase circulating cytokine levels. More intradialytic clinical symptoms are seen in dialysis with CU as compared with PS membrane but the rise in cytokines IL-1β and TNFα does not appear to be responsible for them.
Introduction
Hemodialysis is an important treatment modality in chronic renal failure patients. It has continuously evolved starting from regenerated cellulose membrane with a small pore size and large thickness to modified plate like dialyzers, followed by cellulosic hollow fiber membranes in 1970s.Citation[[1]] Cellulosic membranes have hydroxyl groups, which interact with compliment leading to its activation.Citation[[2]] Synthetic membranes like polyamide and polysulfone differed in their polymeric composition and hydrophobicity. Dialysis is associated with intradialytic and long-term complications. Inflammatory mediators from mononuclear cells are considered responsible. While membrane induced compliment activation may prime monocytes by inducing cytokine mRNA, transport properties of membrane, and microbiological quality of dialysate determine cytokine release.Citation[[3]] Direct contact of the dialysis membrane with peripheral blood mononuclear cells may also stimulate them depending on the nature of the dialysis membrane. IL-1β and TNFα are the common cytokines involved in the inflammatory response. Circulating cytokines are the active form and just increased transcription does not contribute to clinical complications. According to the “Interleukin hypothesis” IL-1β produced is a cause of acute responses observed in patients during hemodialysis.Citation[[4]] IL-1β can produce fever and hypotension and increase release of acute phase proteins.Citation[[5]] TNFα can cause hypotension, fever, leukopenia, and metabolic dysfunction that may produce shock.
If dialysis results in increased cytokine production what role do they play in the hemodialysis related effects like hypotension and fever? Can the use of so-called “Biocompatible” membranes decrease the release of cytokines and the incidence of dialysis related symptoms? With the advent of dialyzers made from various polymers and synthetic materials there is much to choose from while prescribing dialysis. This study was therefore undertaken to compare the two commonly used dialyzer membranes; low flux polysulfone (PS) and cuprophan (CU) on cytokine release and intradialytic symptoms.
Subjects and Methods
In this randomized crossover prospective study 20 patients of end stage renal disease (ESRD) stabilized on maintenance hemodialysis for more than one month duration were selected after obtaining informed written consent. Patients suffering from significant pulmonary or cardiac disorders, acute or chronic infective disorders or on immunosuppressant drugs were excluded from the study.
Patients were divided in two groups, A and B of 10 patients each and dialyzed biweekly over one membrane for 3 weeks before crossover to the other membrane for another 3 weeks. Both membranes were of comparable surface area and hydraulic permeability (). Acetate dialysate at a flow rate of 500 mL/mt was used and blood flow was kept at 250 mL/mt. Samples for IL-1β and TNFα were drawn during the use of a fresh dialyzer in all patients at 0, 15, 240 mt of the dialysis session from the arterial side of the dialysis circuit, plasma separated and stored at −80°C immediately.Citation[[6]] During each dialysis session (total 240 dialysis sessions) intradialytic clinical symptoms were recorded in the study performa. Cytokine assays were done using ELISA kits (BioSource Int., Inc., USA). Correlation of symptoms with the first 15 min rise in IL-1β and TNFα was done by calculating the average rise in the respective cytokine levels during the first 15 min in those sessions where the symptoms occurred and then comparing it with the average rise in those sessions where the symptoms did not occur, to see for any significant difference.
Table 1. Dialyzer properties
Calculating mean and standard deviation in different dialysis groups did statistical analysis. T-test for paired values and Mann-Whitney U test for % data were used. Changes were considered significant when P<0.05.
Results
Twenty-four patients of ESRD undergoing regular hemodialysis were enrolled out of which 20 completed the study and the results for the same are presented. The mean age was 41 ± 13 yrs. with mean weight of 54.1 ± 13.9 Kg. Patient had a mean duration of 3.4 ± 1.9 months of maintenance dialysis before enrollment in the study. Out of the 20 patients 7 were of chronic glomerulonephritis, 4 of diabetic nephropathy, 2 each of hypertensive nephropathy and obstructive uropathy, one case of polycystic kidney disease, and 4 of unknown etiology. There were 18 males and 2 females.
Among the cytokine levels measured, IL-1β levels increased progressively over the 240 min session on both type of membranes. As compared with the baseline value the rise reached significance with in 15 min of start of the dialysis session on PS membrane (P = 0.024) while on CU it reached borderline significance only later at 240 min (P = 0.057). The absolute increase in the levels of IL-1β in the first 15 min and over 240 mt was more with PS membrane. Thus the two membranes differed in their potential to increase circulating IL-1β levels with PS causing more increase (). Plasma free TNFα levels increase was more rapid in CU achieving significance at 15 min (P = 0.014) while at 240 min both membranes have shown a significant rise ().
Table 2. IL-β and TNFα levels during hemodialysis (pg/mL)
Among clinical symptoms nausea was the most common symptom, which occurred in 62% sessions on CU, and 54% on PS membrane, the difference was not significant. Vomiting, chest pain, fever, chills, and breathlessness occurred significantly more during dialysis with CU membrane as compared with PS (). Cramps, back pain, itching, restlessness, post dialysis fatigue, and hypotension did not significantly differ. There was no correlation between cytokine rise and dialytic symptoms. Paradoxically the levels were significantly less among patients who suffered from the symptoms of fever, chills, itching, breathlessness, post dialysis fatigue, and hypotension ().
Table 3. Frequency of clinical symptoms on the two membranes
Table 4. Comparison of cytokine rise in symptomatic sessions with rise in asymptomatic sessions (pg/mL)
Discussion
Despite technical advancements in dialysis equipment patients undergoing maintenance hemodialysis are still severely affected by intradialytic and long-term complications.Citation[[7]] According to US Renal Data System (USRDS) there has been a substantial reduction in the use of cellulose membranes from approximately 70% in 1990 to less than 20% in 1996.Citation[[8]] The newer synthetic dialyzers are considered better compared with the cellulose-based dialyzers, being more biocompatible—not causing any clinically significant host response.Citation[[9]] This might not be true. Factors other than the dialyzer membrane can influence the biocompatibility of dialysis like dialyzer reuse, sterilants, and materials used in reprocessing. Only few prospective randomized studies are available to address the clinical effects of dialysis membranes. Many technical aspects have to be taken into account in such studies and type and quality of dialysate, blood flow, dialysate flow, dialysis duration, and patient characteristics have to be taken into account. Thus the dialysis conditions and dialysate composition were kept constant to study the cytokine releasing properties of the membranes.
In our study there was a progressive increase in IL-1β levels on both membranes () as shown by others,Citation[[3]] but significant rise was found only with PS. Reports have shown that if a sterile bicarbonate dialysate solution is used then there in no significant rise in cytokine levels on either CU or PS membranes.Citation[[10]] We used acetate dialysate in the crossover study because the availability of bicarbonate dialysis is not universal, especially at secondary health care centers. Moreover, studies have shown that concentrations of IL-1β was not influenced by either the membrane or the dialysate composition and fell to 21 and 22% of the predialysis levels with CU and PS membranes respectively.Citation[[11]] Circulating levels of IL-1β during in vitro incubation has been reported to be increased,Citation[[12]], Citation[[13]] decreased,Citation[[14]] or normal levels.Citation[[15]], Citation[[16]], Citation[[17]] These mixed reports make the picture unclear. Various reasons for this could have been the presence of circulating IL-1Ra, transient rise in blood levels or inadequate assay procedures. Anemia and malnutrition can also contribute to decrease cytokine production.
TNFα was found to be significantly elevated on both membranes (). There was no significant difference between the two membranes. Jorres et al.Citation[[6]] observed no significant rise in TNFα levels on either PS or CU membranes or the high inter and intra-individual variability was assigned to the nonstandardized sampling procedures. Rise in circulating TNFα during hemodialysis has been shown in various studiesCitation[[12]], Citation[[18]], Citation[[19]] while others have demonstrated the contrary.Citation[[20]], Citation[[21]] According to them contaminated dialysate, inter-assay variation, sample handling, and heparin use might be responsible for increased TNFα levels, which do not reflect circulating TNFα levels. Blood samples taken in EDTA, by chelating calcium, have been reported not to show in vitro production of TNF.Citation[[22]] All these technical aspects were taken into account during this study. Base line levels of TNFα were high in both the groups. This might reflect an inadequate clearance rather than increased production.
IL-1β and TNFα are potent mediators of acute phase inflammatory response. Activated circulating monocytes are the main source. It has been shown that both cell associated and spontaneous release of IL-1β are greater in patients treated with cellulosic membranes.Citation[[23]] Increased transcription of mRNA for IL-1β and TNFα have been noted with cellulosic membranes. Although measuring production of IL-1β and TNFα in cells removed from patients is a worthwhile approach, such determinations may be misleading in terms of the implications for the patients since the circulating cytokines are the active form, which can produce immediate complications. Thus circulating cytokines were evaluated in this study. Several naturally occurring specific and nonspecific inhibitors of cytokines have been found. These could alter the biological relevance of a given cytokine present in plasma.Citation[[24]] Lipoproteins, lipids, α2 macroglobulin are some of the naturally occurring non-specific inhibitors. Same cells that synthesize IL-1 and TNF also produce molecules that block their action e.g., IL-1 receptor antagonist (IL-1Ra) and soluble TNF receptors (TNF-SR). In patients on dialysis, monocytes are said to be in a state of chronic activation this might influence their potential to release cytokines on fresh contact with a dialyzer or any other foreign agent.Citation[[25]] Also the released cytokines might be absorbed on the dialyzer surface itself thus showing very less circulating levels.Citation[[26]] All these confounding factors make the interpretation of the results difficult.
Among intradialytic symptoms nausea was the commonest (). Vomiting, chest pain, fever, chills, and breathlessness occurred significantly more during dialyses with CU membrane as compared with PS membrane. JorstadCitation[[27]] found polysulfone to be more biocompatible to cuprophan on clinical parameters, apart from the biochemical parameters. This was probably due to more compliments activating property of cuprophan membranes. Chanard et al.Citation[[28]] reported decrease in hypotension, vomiting, cramps, and headache on AN69 membrane as compared with cuprophan. In contrast, othersCitation[[29]], Citation[[30]], Citation[[31]] found no significant difference in intradialytic patient well being and pattern of complications on CU and PS membranes. Collins et al.Citation[[32]] also suggest that hypotension, headache, nausea vomiting, and muscle cramps do not depend on the type of dialyzer used. Pruritus does not appear to be due to the dialysis membraneCitation[[33]] as was the case in our study. In all the above-described studies major stress was on the incidence of hypotension. It was reported in 15 to 50% of the dialysis sessions.Citation[[34]] Hypotension and cardiovascular instability have been reported as most frequent side effects and are being related to induction of IL-1β and TNFα.Citation[[4]] Cytokine induced nitric oxide release as a vasodilator was blamed. Larger dialyzer surface area, ultrafiltration rates, and blood flow rates lead to increased incidence of hypotension. These factors had been considered while planning our study. Hypotension was seen in 7 sessions on CU and 5 sessions on PS out of 120 sessions on each membrane in the present study.
Monocyte activation with cytokine production is a well-known event in the course of dialysis treatment but its relationship to symptoms of dialysis or long-term pathologic changes in chronic hemodialysis patients is still under evaluation.Citation[[35]] Cytokine production depends on the balance between inducers and inhibitors while the clinical effects would depend on the interaction with the uremic environment and cellular metabolism. The production of cytokines during dialysis has been reported to be responsible for acute complications like fever, headache, hypotension as well as for long term complications such as dialysis related amyloidosis, increased susceptibility to infections and increased muscle protein catabolism, altered sleep pattern and decreased responsiveness to erythropoietin.Citation[[4]]
In our study, rise in cytokines in first 15 min was compared with dialytic symptoms. Rise in levels of IL-1β was paradoxically low in the patients who suffered from symptoms like fever, chills, itching, breathlessness, and post dialysis fatigue as compared with those where these symptoms occurred. This shows a poor correlation between symptom occurrence and IL-1β levels rise. Similarly TNFα levels increase did not show any specific correlation with the monitored intradialytic symptoms. These results put a doubt to the cause effect relationship of cytokines IL-1β and TNFα to various symptoms. Others also have not found any correlation between cytokine rise and occurrence of clinical symptoms.Citation[[11]] According to Cappelli et al.Citation[[35]] it is not possible to link a specific symptom to a definite stimulus like dialysate contamination or compliment activation and cytokine release. Our study had a follow up of only 3 weeks on each membrane thus it was difficult for us to comment on the membrane effect on long term complications like amyloidosis, bone disease, malnutrition, infection rate, reduction in residual renal function, and mortality.
In conclusion, evidence gathered from present study shows a significant IL-1β rise with PS membrane while TNFα rise was significant with both the membranes reflecting a mixed response to cytokine production of both the membranes. Nausea is a common intradialytic symptom. Vomiting, chest pain, fever, chills, and breathlessness occurred significantly more with CU compared to PS membrane. IL-1β and TNFα levels correlate poorly and may not be responsible for intradialytic symptoms. But it should be kept in mind that these results cannot be generalized to all types of cellulosic membranes.
The use of cellulosic membrane dialyzers should not be discontinued in the belief that they are bioincompatible, though it does not mean that they are superior to more biocompatible membranes. Biocompatibility is the quality of being mutually tolerant with life and is not absolute. Thus it is the different degree of bioincompatibility of the membranes, which is important. The regenerated cellulose membrane hemodialyzers provide a reasonable, cost effective alternative for the life sustaining procedure of dialysis and more so in developing countries where cost factor may be decisive in the decision to take the patient on a maintenance dialysis program. A role of regenerated cellulose membrane undoubtedly exists they should not be considered as a second-class treatment. Perhaps the future lies in developing better membranes, which are both more cost effective, and biocompatible.
References
- Lipps B., Stewart R., Perkins H. The hollow fiber artificial kidney. Trans. Am. Soc. Artif. Intern. Organs 1967; 13: 200–207
- Walton D., Cheung A. Membrane biocompatibility. Clinical Dialysis 3, A. Nissenson, R. Fine, D. Gentile, C.T. Norwalk. Appleton and Lange. 1995; 93–120
- Colton CK. Analysis of membrane processes for blood purification. Blood Purif. 1987; 5(4)202–251
- Henderson L.W., Koch K.M., Dinarello C.A., Shaldon S. Hemodialysis hypotension the interleukin hypothesis. Blood Purif. 1983; 1: 3–8
- Gesualdo L., Pertosa G., Grandaliano G., Schena F.P. Cytokines and bioincompatibility. Nephrol. Dial. Transplant. 1998; 13(7)1622–1626
- Jorres A., Froese P., Fischer C., Safak H., Gahl G.M., Muller C., Vienken J. Variables associated with the assessment of systemic tumor necrosis factor alpha levels during hemodialysis. Int. J. Artif. Organs 1992; 15(11)653–657
- Pollak V.E., Charoenpanich R., Robson M., Kant K.S. Dialyzer membranes: syndromes associated with first use and effects of multiple use. Kidney Int. suppl. 1988; 24: S49–S52
- Hakim R.M. Influence of the dialysis membrane on outcome of ESRD patients. Am. J. Kidney Dis. 1998; 32(6 suppl 4)S71–75
- Klinkmann H., Vienken J. Membranes for dialysis. Nephrol. Dial. Transplant. 1995; 10(suppl 3)39–45
- Gardinali M., Calcagno A., Conciato L., Agostoni A., Rosti A., Cori P., Vozzo N., Moroni A., Anelli A., Zoni U. Complement activation in dialysis: effects on cytokines, lymphocyte activation and beta 2 microglobulin. Int. J. Artif. Organs 1994; 17(6)337–344
- Dunati D., Degiannis D., Homer L., Raska K., Jr., Roskova J. Production and kinetics of interleukin-1 in hemodialysis. In vivo and in vitro study. Am. J. Nephrol. 1991; 11(6)451–458
- Herbelin A., Urena P., Nguyen A.T., Zingraff J., Descamps-Latscha B. Influence of first and long term dialysis on uremia associated increased basal production of interleukin-1 and tumour necrosis factor alpha by circulating monocytes. Nephrol. Dial. Transplant 1991; 6(5)349–357
- Herbelin A., Nguyen A.T., Zingraff J., Urena P., Deschamps-Latsha B. Influence of uremia and hemodialysis on circulating interleukin-1 and tumour necrosis factor alpha. Kidney Int. 1990; 37: 116–125
- Pereira B.J., Poutsiaka D.D., King A.J., Strom J.A., Narayan G., Levey A.S., Dinarello C.A. In vitro production of interleukin-1 receptor antagonists in chronic renal failure: CAPD and HD. Kidney Int. 1992; 42(6)1419–1424
- Powell A.C., Bland L.A., Oettinger C.W., McAllister S.K., Oliver J.C., Arduino M.J., Favero M.S. Enhanced release of TNF alpha but not IL-1beta from uremic blood after endotoxin stimulation. Lymphokine Cytokine Res. 1991; 10(5)343–346
- Homes C., Evans R., Ross D., Frankamp P. Plasma interleukin-1 and tumour necrosis factor levels during high flux dialysis with cellulose triacetate membranes (abstract). Kidney Int. 1990; 37: 301
- Powell A.C., Bland L., Oettinger C.W. Plasma interleukin-1 beta and tumour necrosis factor alpha do not increase during unfavorable dialysis conditions (abstract). J. Am. Sol. Nephrol. 1990; 1: 373
- Mege J.L., Olmer M., Purgus R., Bertocchio P., Farnarier C., Kaplanski S., Capo C., Bongrand P. Hemodialysis membranes modulate chronically the production of TNF alpha, IL-1beta, and IL6. Nephrol. Dial. Transplant. 1991; 6(11)868–875
- Chollet-Martin S., Stamatakis G., Bailly S., Mery J.P., Gougerot-Pocidalo M.A. Induction of tumor necrosis factor alpha during hemodialysis. Influence of membrane type. Clin. Exp. Immunol. 1991; 83(2)329–332
- Davenport A., Crabtree J., Androjna C., Will E.J., Davison A.M. Tumour necosis factor does not increase during routine cuprophane haemodialysis in healthy well nourished patients. Nephrol. Dial. Transplant. 1991; 6(6)435–439
- Mandolfo S., Tetta C., David S., Gervasio R., Ognibene D., Wratten M.L., Tessore E., Imbasciati E. In vitro and in vivo biocompatibility of substituted cellulose and synthetic membranes. Int. J. Artif. Organs 1997; 20(11)603–609
- McLaughlin P.J., Davies H.M., Aikawa A. Tumour necrosis factor in normal plasma. Lancet 1990; 336(8721)1014–1015
- Haeffner-Cavaillon N., Cavaillon J.M., Ciancioni C., Bacle F., Delons S., Kazatchkine M.D. In vivo induction of interleukin-1 during hemodialysis. Kidney Int. 1989; 35(5)1212–1218
- Dinarello C.A. Cytokines: agents provocateurs in hemodialysis?. Kidney Int. 1992; 41(3)683–694
- Zaoui P., Hakim R.M. The effects of the dialysis membrane on cytokine release. J. Am. Soc. Nephrol. 1994; 4(9)1711–1718
- Goldfarb S., Golper T. Proinflammatory cytokines and hemofiltration membranes. J. Am. Soc. Nephrol. 1994; 5(2)228–232
- Jorstad S. Biocompatibility of different hemodialysis and plasmapheresis membranes. Blood Purif. 1987; 5(2–3)123–137
- Chanard J., Brunois J.P., Melin J.P., Lavaud S., Toupance O. Long term results of dialysis therapy with a highly permeable membrane. Artif. Organs 1982; 6: 261–266
- Locatelli F., Mastrangelo F., Redaelli B., Ronco C., Marcelli D., LaGreca G., Orlandini G. Effects of different membranes and dialysis technologies on patient treatment tolerance and nutritional parameters. The Italian Cooperative Dialysis Study Group. Kidney Int. 1996; 50(4)1293–1302
- Bergamo Collaborative Dialysis Study Group. Acute Intradialytic well-being: results of a clinical trial comparing polysulfone with cuprophan. Kidney Int. 1991; 40(4)714–719
- Skroeder N.R., Jacobson S.H., Lins L.E., Kjellstrand C.M. Biocompatibility of dialysis membranes is of no importance for objective or subjective symptoms during or after hemodialysis. ASAIO Trans. 1990; 36: M637–M639
- Collins D.M., Lambert M.B., Tannenbaum J.S., Oliverio M., Schwab S.J. Tolerance of hemodialysis: a randomized prospective trial of high flux versus conventional high efficiency hemodialysis. J. Am. Soc. Nephrol. 1993; 4(2)148–154
- Salem M., Ivanovich P.T., Ing T.S., Dougirdas J.T. Adverse effects of dialyzers manifesting during the dialysis session. Nephrol. Dial. Transplant. 1994; 9(suppl 2)127–137
- Orofino L., Marcen R., Quereda C., Villefruela J.J., Sabater J., Matesanz R., Pascual J., Ortuno J. Epidemiology of symptomatic hypotension in hemodialysis: is cool dialysate beneficial for all patients? Am. J. Nephrol. 1990; 10: 177–180
- Cappelli G., DiFelice A., Perrone S., Ballestri M., Bonucchi D., Savazzi A.M., Guffreda A., Lusvarghi E. Which level of cytokine production is critical in hemodialysis? Nephrol. Dial. Transplant. 1998; 13(suppl 7)55–60