Abstract
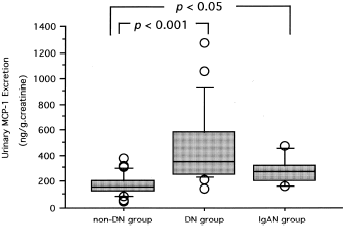
Monocyte chemoattractant protein-1 (MCP-1) is a chemokine that is produced mainly by tubular epithelial cells in kidney and contributes to renal interstitial inflammation and fibrosis. More recently, we have demonstrated that urinary MCP-1 excretion is increased in proportion to the degree of albuminuria (proteinuria) and positively correlated with urinary N-acetylglucosaminidase (NAG) levels in type 2 diabetic patients. Based on these findings, we have suggested that heavy proteinuria, itself, probably aggravates renal tubular damage and accelerates the disease progression in diabetic nephropathy by increasing the MCP-1 expression in renal tubuli. In the present study, to evaluate whether urinary MCP-1 excretion is increased in the proteinuric states not only in diabetic nephropathy but also in other renal diseases, we examined urinary MCP-1 levels in IgA nephropathy patients with macroalbuminuria (IgAN group; n = 6), and compared the results with the data obtained from type 2 diabetic patients with overt diabetic nephropathy (DN group; n = 23) and those without diabetic nephropathy (non-DN group; n = 27). Urinary MCP-1 excretion levels in non-DN, DN, IgAN groups were 157.2 (52.8–378.5), 346.1 (147.0–1276.7), and 274.4 (162.2–994.5) ng/g creatinine, median (range), respectively. Expectedly, urinary MCP-1 and NAG excretion levels in DN and IgAN groups were significantly elevated as compared with non-DN group. Therefore, we suggest that MCP-1 expression in renal tubuli is enhanced in proteinuric states, irrespective of the types of renal disease, and that increased MCP-1 expression probably contributes to renal tubular damage in proteinuric states.
Introduction
Recently, it has been considered that proteinuria, itself, is an independent mediator in the progression of proteinuric renal diseases.Citation[[1]], Citation[[2]], Citation[[3]], Citation[[4]] When glomeruli are damaged, plasma proteins are highly filtered through the damaged glomerular capillary into renal tubular fluid, and consequently urinary protein excretion is increased. When it occurs, renal tubular cells are excessively exposed to filtered plasma proteins. Since the filtered proteins are known to have intrinsic renal tubular toxicity,Citation[[5]], Citation[[6]], Citation[[7]], Citation[[8]] increased urinary protein excretion seems to contribute to the aggravation of renal tubulointerstitial lesions and the progression of renal diseases.
Monocyte chemoattractant protein-1 (MCP-1) is a chemokine that plays an important role in the recruitment of monocytes/macrophages into renal tubulointerstitium.Citation[[9]], Citation[[10]] It is known to be produced mainly by tubular epithelial cells in kidney,Citation[[11]] and to contribute to renal interstitial inflammation and fibrosis.Citation[[12]] Furthermore, protein overload in renal tubular cells is shown to upregulate MCP-1 gene and its protein.Citation[[13]], Citation[[14]] These lines of evidence collectively suggest that increased urinary protein excretion probably aggravates renal tubular damage by enhancing MCP-1 expression in tubular cells.
More recently, to assess whether MCP-1 is associated with renal tubular damage in diabetic nephropathy, we determined urinary MCP-1 levels in type 2 diabetic patients with different stages of nephropathy using an immunoradiometric assay (IRMA) method developed in our laboratory, and demonstrated a positive correlation between urinary excretion levels of MCP-1 and albumin.Citation[[15]] In addition, we also found a positive correlation between urinary excretion levels of MCP-1 and N-acetylglucosaminidase (NAG), which is widely used as a sensitive marker of renal tubular damage.Citation[[15]] Based on these results, we have suggested that MCP-1, which is probably produced in renal tubular cells and released into urine in proportion to the degree of proteinuria (albuminuria), is probably at work as a mediator of renal tubular damage in diabetic nephropathy.
Similar to diabetic nephropathy, urinary MCP-1 levels may be enhanced in the macroalbuminuric states and correlated with the degree of renal tubular damage also in other renal diseases. To evaluate this possibility, we determined urinary levels of MCP-1 and NAG in IgA nephropathy patients with macroalbuminuria, and compared the results with the data obtained from type 2 diabetic patients in the present study.
Patients and Methods
Study Population
Six IgA nephropathy patients with macroalbuminuria (IgAN group), 23 type 2 diabetic patients with overt diabetic nephropathy (DN group), and 27 type 2 diabetic patients without diabetic nephropathy (non-DN group) were recruited in this study. The diagnosis of IgA nephropathy was made by renal biopsy. In the type 2 diabetic patients, the diagnostic criteria for overt diabetic nephropathy were having macroalbuminuria (urinary albumin excretion [UAE]>300 mg/g creatinine). The type 2 diabetic patients with normoalbuminuria (UAE<30 mg/g creatinine) were regarded as being without diabetic nephropathy. The members of IgAN group did not have diabetes mellitus. Informed consent was obtained from all the study participants.
Measurements
Overnight urine samples were collected from all subjects to measure the urinary concentrations of MCP-1, NAG, and creatinine. Urine samples were stored at −85°C before testing. IRMA measured urinary MCP-1 concentrations as described previously.Citation[[15]] Urinary NAG concentrations were measured by the m-cresol purple method (Shionogi, Osaka, Japan). Urinary excretion levels of MCP-1 and NAG were expressed as the ratio of urinary concentrations of MCP-1 and NAG to grams of urinary creatinine.
Statistical Analysis
Data are presented as means ± SD or medians (range) or n. Comparisons of the data between non-DN and IgAN groups or between non-DN and DN groups were performed by unpaired t-test or Mann-Whitney U-test. A p-value of less than 0.05 was considered statistically significant.
Results
Clinical Characteristics
shows clinical characteristics of non-DN, DN, and IgAN groups. The three groups were matched with regard to age and body mass index. The two groups of non-DN and DN showed elevated levels with regard to HbA1c, while HbA1c levels of IgAN group were within a normal range. Serum creatinine and urinary NAG levels in DN and IgAN groups were significantly elevated as compared with non-DN group. Hypertension was observed in all the subjects in DN and IgAN groups.
Table 1. Clinical characteristics
Urinary MCP-1 Excretion Levels
Urinary MCP-1 excretion levels in non-DN, DN, IgAN groups were 157.2 (52.8–378.5), 346.1 (147.0–1276.7), and 274.4 (162.2–994.5) ng/g creatinine, median (range), respectively (). Expectedly, urinary MCP-1 excretion levels in DN and IgAN groups were significantly elevated as compared with non-DN group.
Discussion
The present study was conducted to evaluate whether urinary MCP-1 excretion is increased in the proteinuric states not only in diabetic nephropathy but also in other renal diseases. For this purpose, we examined urinary MCP-1 excretion levels in IgA nephropathy patients with macroalbuminuria. Since IgA nephropathy patients in this study did not have diabetes mellitus, their renal damage is considered to have been due to only IgA nephropathy. IgA nephropathy is a primary glomerular disease characterized histopathologically by predominant IgA mesangial deposits and clinically by hematuria and proteinuria.Citation[[16]], Citation[[17]] Similar to the patients with diabetic nephropathy, when the patients with IgA nephropathy exhibit proteinuria, the renal tubular cells are expected to be exposed to excessive proteins filtered through the damaged glomeruli.
In this study, we found that urinary MCP-1 excretion was significantly increased not only in type 2 diabetic patients with macroalbuminuria, i.e., with overt diabetic nephropathy, but also in IgA nephropathy patients with macroalbuminuria as compared with type 2 diabetic patients with normoalbuminuria, i.e., without diabetic nephropathy. Moreover, our study showed that urinary NAG levels were significantly elevated in the two patient group, which exhibited macroalbuminuria when compared with type 2 diabetic patients with normoalbuminuria. Considering these results together with the recent finding that protein overload in renal tubular cells upregulates MCP-1 gene and its protein,Citation[[13]], Citation[[14]] we suggest that MCP-1 is highly expressed in renal tubuli and excessively released into urine in proteinuric (macroalbuminuric) states, irrespective of the types of renal disease. In addition, we propose that increased MCP-1 expression probably contributes to renal tubular damage in proteinuric states.
Renal tubular epitherial cells are shown to secrete a wide variety of inflammatory mediators like complement components, cytokines, and chemokines.Citation[[18]] Based on the present results, an increase in urinary protein excretion, irrespective of the types of renal disease, is expected to lead to the overexpression of the inflammatory mediators in renal tubuli and aggravate renal tubular damage. Therefore, proteinuria, itself, seems to be a promoter in the progression of various proteinuric renal diseases.
References
- Remuzzi G. Abnormal protein traffic through the glomerular barrier induces proximal tubular cell dysfunction and causes renal injury. Curr. Opin. Nephrol. Hypertens. 1995; 4: 339–342
- Burton C., Harris K.P.G. The role of proteinuria in the progression of chronic renal failure. Am. J. Kidney Dis. 1996; 27: 765–775
- Remuzzi G., Ruggeneti P., Benigni A. Understanding the nature of renal disease progression. Kidney Int. 1997; 51: 2–15
- Remuzzi G. Nephropathic nature of proteinuria. Curr. Opin. Nephrol. Hypertens. 1999; 8: 655–663
- Kees-Folts D., Schreiner G.F. A lipid chemotactic factor associated with proteinuria and interstitial nephritis induced by protein overload. J. Am. Soc. Nephrol. 1991; 2: 548
- Howard R.L., Buddington B., Alfrey A.C. Urinary albumin excretion, transferrin and iron excretion in diabetic patients. Kidney Int. 1991; 40: 923–926
- Shore V.G., Forte T., Licht H., Lewis S.B. Serum and urinary lipoproteins in the human nephritic syndrome: evidence for renal catabolism of lipoproteins. Metabolism 1982; 31: 258–268
- Ito S., Fujita H., Narita T., Yaginuma T., Kawarada Y., Kawagoe M., Sugiyama T. Urinary copper excretion in type 2 diabetic patients with nephropathy. Nephron 2001; 88: 307–312
- Boucher A., Droz D., Adafer E., Noel L. Characterization of mononuclear cell subsets in renal cellular interstitial infiltrates. Kidney Int. 1986; 29: 1043–1049
- Danoff T.M. Chemokines in interstitial injury. Kidney Int. 1998; 53: 1807–1808
- Prodjosudjadi W., Gerritsma J.S.J., Klar-Mohamad N., Gerritsen A.F., Bruijn J.A., Daha M.R., Van Es L.A. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995; 48: 1477–1486, 1995
- Lloyd C.M., Minto A.W., Dorf M.E., Proudfoot A., Wells T.N.C., Salant D.J., GutierrezRamos J.C. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J. Exp. Med. 1997; 185: 1371–1380
- Wang Y., Chen J., Chen L., Tay Y., Rangan G.K., Harris D.C.H. Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J. Am. Soc. Nephrol. 1997; 8: 1537–1545
- Eddy A.A., Giachelli C.M. Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int. 1995; 47: 1546–1557
- Morii T., Fujita H., Narita T., Shimotomai T., Fujishima H., Yoshioka N., Imai H., Kakei M., Ito S. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J. Diabetes Complicat., in press
- Donadio J.V., Jr., Grande J.P. Immunoglobulin a nephropathy: a clinical perspective. J. Am. Soc. Nephrol. 1997; 8: 1324–1332
- Glassock R.J., Cohen A.H., Adler S.G. Primary glomerular diseases. The Kidney, 5th Ed., B.M. Brenner. W.B. Saunders Company, Pennsylvania 1996; 1392–1497
- van Kooten C., Langers A.M.J., Bruijn J.A., Daha M.R. Role of tubular cells in progressive renal disease. Kidney Blood Press. Res. 1999; 22: 53–61