Abstract
Background. Recent report demonstrates that inadequate iron mobilization and defective iron utilization may cause recombinant erythropoieitin (rEPO) hyporesponsiveness in hemodialysis (HD) patients with iron overload. The effect of intravenous ascorbic acid (IVAA) in HD patients selected on the basis of iron overload and EPO resistance also has been proven. However, it is uncertain whether IVAA still works in diabetic ESRD patients with hyperferritinemia. Therefore, the aim of this study focusing on diabetic ESRD patients was to analyze the potential effect of low dose IVAA on improvement of anemia and erythropoiesis-related parameters when compared with control period. Patients and Method. This study consisted of 22 chronic hemodialysis patients with type II diabetes in a single dialysis unit. In studies of this type, all eligible patients are followed up, but the primary comparison is still between different sequentially treatment including control period and post-IVAA period in same patients. IVAA patients received ascorbic acid, 100 mg each administered intravenously three times per week for eight weeks of treatment and four months of post-treatment follow-up. Results. The demographic characteristics of 22 diabetic uremic patients show that mean age is 63.6 ± 10.2 years old. The ratio of sex (M/F) = 10/12. Mean duration of HD is 46.7 ± 33.2 months. As for the urea kinetic study between these two periods including KT/V, nPCR, and URR, there is no significantly different. As for anemia-related parameters, Hb and Hct increased significantly in post-IVAA period after 3 months compared with control period, while MCV did not increase significantly. Serum ferritin significantly decreased at study completion. The same situation is for iron. As for TS, it significantly increased at one month and further markedly increased at subsequent three months. Conclusion. This study has demonstrated that short-term low dose IVAA therapy can facilitate iron release from reticuloendothelial system but also increase iron utilization in diabetic hemodialysis patients with iron overload. Therefore, IVAA is a potential adjuvant therapy to treat erythropoeitin-hyporesponsive anemia in iron-overloaded patients.
Introduction
Having many underling causes, chronic anemia is a frequent complication of hemodialysis therapy (HD) in patients with chronic renal failure. With the advent of recombinant erythropoietin (rEPO), hyperferritinemia in HD patients is always related to previous multiple transfusion and overtreatment with intravenous iron if the inflammatory status, liver disease or malignancy is excluded. Some HD patients with iron overload or functional iron deficiency respond poorly to rEPO because of inadequate iron mobilization and defective iron utilization.Citation[[1]] Iron therapy in these patients perphase present a potential hazard, leading to iron overload and consequently to hemosiderosis. Ascorbic acid seems to provide a good way to improve iron mobilization from tissue stores and increase iron utilization.Citation[[2]], Citation[[3]] Tarng et al. have shown that the effect of intravenous ascorbic acid (IVAA) in HD patients selected on the basis of iron overload and EPO resistance.Citation[[4]]
The prevalence of type II diabetes in Taiwan population has steadily increased in recent years.Citation[[5]] Many reports have demonstrated the relationship between diabetes and oxidative stress.Citation[[6]] Oxidative stress has also been considered to be a common pathogenesis of EPO resistance.Citation[[7]] Ascorbic acid acts as a free oxygen radical scavenger to oxidative stress.Citation[[8]] Protective effects of treatment with vitamin C on experimental renal injury in diabetic have been reported.Citation[[9]] Few studies has been conducted and focused on these subgroups patients whether the administration of antioxidative agents such as ascorbic acid can improve EPO resistance and subsequently increase iron utilization. Therefore, we decided to test the hypothesis that supplementation of ascorbic acid in lower pharmacological dose can improve iron usage in diabetic HD patients.
The aim of this study focusing on diabetic ESRD patients was to analyze the potential effect of low dose IVAA on improvement of anemia and erythropoiesis-related parameters when compared with control group.
Patients and Methods
This study consisted of 22 chronic hemodialysis patients with type II diabetes in a single dialysis unit at Chang Gung Memorial Hospital in Taiwan. For exclusion of a selection bias, we conducted a prospective analysis of type II diabetic dialysis patients in a single dialysis unit. In studies of this type, all eligible patients are followed up, but the primary comparison is still between different sequentially treatment including control period and post-IVAA period in the same patients. IVAA patients received ascorbic acid, 100 mg each administered intravenously for 3 min postdialysis three times per week for eight weeks of treatment. The post-IVAA period mean four months of posttreatment follow-up. As for control period, before enrollment of IVAA period, 22 chronic diabetic uremic patients were not treated with ascorbic acid therapy for six months. Hyperferritinemia was related to previous multiple blood transfusions or overtreatment with intravenous iron. Inclusion criteria were time on HD for more than six months, duration of rEPO treatment for more than six months and serum ferritin and hematocrit (Hct) values that were stable for three consecutive months. Exclusion criteria were if any of the following event occurred in the preceding six months and during the study period: bleeding, blood transfusions, hemolysis, acute liver disease, and infections. Most patients routinely received multivitamins supplementation, but the supplements did not include vitamin C. Patients were dialyzed for 4 h three times per week using polysulfone hollow fiber (1.8 m2, polysulfone, Fresenius AG, Germany), blood flow of 250–300 mL/min, and dialysate flow of 500 mL/min.
For patients with iron therapy, if more than 100 mg of iron polysaccharide has been prescribed within one month, iron index measurement was delayed until the following month. For control and post-IVAA periods, KT/V, URR, and nPCR were measured once from predialysis and immediate postdialysis blood urea nitrogen levels using a formal single-compartment model of dialysis urea kinetics.Citation[[10]] Same policy of rEPO therapy and iron supplementation was applied to 22 diabetic dialysis patients during the course of two different treatments periods. The patients were administered two to three injections of rEPO weekly. The rEPO dose was titrated by 25% every 2 weeks individually and given as a bolus injection via venous bloodline at the end of dialysis to maintain a target Hct of 33%. During the course of rEPO treatment, those patients who do not need EPO treatment after arriving a target Hct are excluded in our study analysis.
Laboratory Measurements
Blood was drawn before HD. Hemoglobulin (Hb) and Hct were determined every 2 weeks. The erythropoiesis-related parameters were measured before dialysis at a regular monthly interval including red blood cell indices, hematocrit, iron indices, average monthly rEPO dosage, and serum albumin levels. Hemoglobin (g/dL) was measured by a colorimetric method. Iron indices such as serum iron, total iron binding capacity (TIBC), aluminum, and intact parathyroid hormone (iPTH) were measured every three months during control and post-IVAA periods. Blood counts and red blood cell indices were measured by standard automated counter methods. Serum iron and TIBC were measured by a colorimetic method (Katayam, Olympus AU550, Tokyo, Japan). Serum ferritin (ng/mL) was measured by a two-site immunoradiometric assay (IRMA-mat® Ferritin, Byk-Sangtec Diagnostica GmbH & Co.KG, Dietzenbach-Germany). Routine laboratory tests were conducted to determine urea, iPTH (Radioimmunoassay) and aluminum.
Statistical Analysis
The results in tables and figures are expressed as mean ± SD. Statistical analysis were performed with Student's pair t-test for difference of initial stage vs. end stage. Analysis of the difference between groups, using the mean changes in laboratory values for each individual patient was done by the Mann-Whitney ranked sum test. A p less than 0.05 for two-side tests is considered significant.
Results
Demographic Characteristics
summarizes the demographic characteristics of 22 diabetic uremic patients. Mean age is 63.6 ± 10.2 years old. The ratio of sex (M/F) = 10/12. Mean duration of HD is 46.7 ± 33.2 months.
Table 1. Characteristics of the diabetic hemodialysis patients in this study
Comparison of Baseline Parameters of Diabetic HD Patients Between Control and IVAA Groups
demonstrates matching comparison in the urea kinetic study between these two groups including KT/V (1.24 ± 0.24 vs. 1.23 ± 0.27), nPCR (1.13 ± 0.05 vs. 1.20 ± 0.24), and URR (68.9 ± 5.2 vs. 67.6 ± 4.9). The two groups were equivalent with regard to the values of serum aluminium (18.7 ± 1.9 vs. 20.8 ± 2.9), intact PTH (152.7 ± 60.4 vs. 160.4 ± 59.4), albumin (3.9 ± 0.6 vs. 3.8 ± 0.4), calcium (8.9 ± 2.8 vs. 9.1 ± 0.9) and phosphate (3.9 ± 1.6 vs. 4.2 ± 1.5).
Table 2. Baseline data of diabetic hemodialysis patients in control and IVAA period
Effect of IV Ascorbate Therapy
shows Hb, Hct, and MCV levels at the beginning, and at the end of the study. Hb and Hct increased significantly in IVAA period after 3 months compared with control period, while MCV did not increase significantly. As for control group, mean Hb, Hct, and MCV remained unchanged after three months. shows longitudinal comparison between control period and post-IVAA period. During IV ascorbate therapy, iron parameter values at −4, −1, +1, +4 months were serum ferritin of 741.9 ± 165.1, 810.5 ± 272.1, 746.5 ± 330.7, 590.3 ± 315.7 ug/L, serum iron of 68.8 ± 22.9, 69.7 ± 25.1, 68.5 ± 33.3, 84.2 ± 18.6 ug/dL and transferring saturation (TS) of 23.7 ± 7.9, 24.3 ± 6.8, 27.4 ± 9.6, 32.4 ± 11.7%, respectively ( and ). Serum ferritin significantly decreased at study completion. The same situation is for iron. As for TS, it significantly increased at one month and further markedly increased at subsequent three months.
Figure 1. Evolution of serum ferritin values in control period (−4 months and −1 month) and post-IVAA period (1 month and 4 months). “0”month in X axis mean the IVAA therapy for 8 weeks. *P < 0.05 as compared with previous control period.
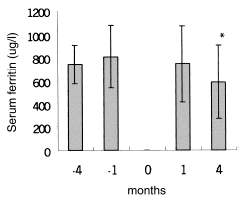
Figure 2. Evolution of serum iron values in control period (−4 months and −1 month) and post-IVAA period (1 month and 4 months). “0”month in X axis mean the IVAA therapy for 8 weeks. *P < 0.05 as compared with previous control period.
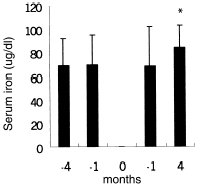
Figure 3. Evolution of transferrin saturation (TS) in control period (−4 months and −1 month) and post-IVAA period (1 month and 4 months). “0”month in X axis mean the IVAA therapy for 8 weeks. *P < 0.05 as compared with previous control period.
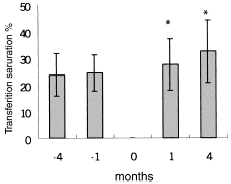
Table 3. Hemoglobulin, hematocrit, and MCV at the beginning, and at the end of study
Discussion
Our study shows that short duration treatment with IVAA in type II diabetic uremic patients significantly improve iron utilization, thus suggesting a potential beneficial effect in anemic end stage renal disease (ESRD) patients. Recent reports show that resistant anemia in patients with iron overload may be corrected by IVAA.Citation[[11]] As well known, vitamin C acts as a reducing agent leading to improve iron mobilization from reticuloendothelial system to transferrin with an increase of iron availability.Citation[[2]], Citation[[3]] To our interest, under the IVAA treatment, 22 diabetic uremic patients have significantly reduced the mean ferritin level and increased serum iron level. As for the mean TS, it also significantly increased. This finding implies relatively higher iron utilization exists in diabetic patients with iron overload. This is compatible with previous experience to patients that a lower ferritin and higher TS may indicate easy way to achieve target hematocrit. In addition, Tarng et al. also found that high erythrocyte ZPP levels in IVAA responding patients.Citation[[4]], Citation[[11]] Erythrocyte ZPP is a marker of endogenous iron-deficient erythropoiesis. The better correction of anemia was correlated with a significant reduction of erythrocyte ZPP in these patients.Citation[[4]] Thus, this is the reason why IVAA results in an enhanced erythropoietic response to rEPO and therefore a better correction of anemia.
There are other interesting findings in our studies. We prescribed a lower dose of IVAA to diabetic uremic patients. According to the statement of previous reports, a deficiency of ascorbic acid is related to inadequate dietary intake, loss through the dialyzer, and uremia-associated metabolic derangement.Citation[[12]], Citation[[13]] A daily dose of 100–200 mg is recommended in some reports, but the prescription actually varies between 100 and 1000 mg daily.Citation[[14]], Citation[[15]] However, excessive vitamin C treatment may worsen uremic-related oxalosis. Therefore, supplementation not less than 150 mg/day is still considered a safe dose.Citation[[16]] As for even lower dose of 100 mg three times per week, few reports using this treatment to treat uremic patients with iron overload. To answer the question whether the lower dose of IVAA can improve iron utilization in diabetic uremic patient, we designed this study. In our study, the dose of IVAA (300 mg weekly) is less than the recommended regimen. Following a short-term treatment, a satisfactory result provides us further evidence that this mode of treatment indeed has a significant correction of anemia on uremic patients with iron overload.
Further, diabetes mellitus currently accounts for the major cause of ESRD in Taiwan.Citation[[5]] Some studies have shown that oxidative stress has been considered to be a common pathogenesis of diabetic complications including severe anemia.Citation[[7]] In addition, diabetic itself also have been proven to be an independent mortality. Whether the administration of anti-oxidative agents can improve functional iron deficiency in diabetic ESRD patients? IVAA has also proven to reduce oxidative stress. However, it is still unclear whether it can improve iron utilization in uremic patients with diabetes. In our studies, we have found that IVAA therapy still plays an important role in improving iron utilization for diabetic uremic patients.
In our study, anemia was improved after the administration of IVAA and serum ferritin decreased simultaneously at the end of the study. Facing the potential hazards of iron overload, IVAA indeed provides an additional benefit for iron-overloaded diabetic HD patients. Some laboratory and clinical observations have raised concern about the safety of using IVAA. Some cardiac event has been reported in thalasemic child.Citation[[17]] However, similar finding was not noted in uremic patients with transfusional iron overload. In spite of cardiac events associated with IVAA may not be potential problems in uremic patients. We still reduce the dose of IVAA to 100 mg trice per week and follow up the cardiac function, particularly when vitamin C is being administered on a weekly basis.
In conclusion, this study has demonstrated that short-term low dose IVAA therapy can partially improve anemia in diabetic hemodialysis patients with iron overload. IVAA not only facilitate iron release from reticuloendothelial system but also increase iron utilization. Therefore, IVAA is a potential adjuvant therapy to treat erythropoeitin-hyporesponsive anemia in iron-overloaded patients.
References
- Sunder-Plassmann G., Horl W.H. Erythropoietin and iron. Clin. Nephrol. Mar. 1997; 47(3)141–157
- Bridges K.R., Hoffman K.E. The effects of ascorbic acid on the intracellular metabolism of iron and ferritin. J. Biol. Chem. Oct. 25 1986; 261(30)14273–14277
- Lipschitz D.A., Bothwell T.H., Seftel H.C., Wapnick A.A., Charlton R.W. The role of ascorbic acid in the metabolism of storage iron. Br. J. Hematol. Feb. 1971; 20(2)155–163
- Tarng D.C., Wei Y.H., Huang T.P., Kuo B.I., Yang W.C. Intravenous ascorbic acid as an adjuvant therapy for recombinant erythropoietin in hemodialysis patients with hyperferritinemia. Kidney Int. Jun. 1999; 55(6)2477–2486
- Wu M.S., Yu C.C., Yang C.W., Wu C.H., Haung J.Y., Hong J.J., Fan Chiang C.Y., Huang C.C., Leu M.L. Poor pre-dialysis glycemic control is a predictor of mortality in type II diabetic patients on maintenance hemodialysis. Nephrol. Dial. Transplant 1997; 12: 2105–2110
- Ha H., Kim K.H. Role of oxidative stress in the development of diabetic nephropathy. Kidney Int. Suppl. Sep. 1995; 51: S18–S21
- Eckardt K.U. Pathophysiology of renal anemia. Clin. Nephrol. Feb. 2000; 53(1 suppl)S2–S8
- Oostenbrug G.S., Mensink R.P., Hardeman M.R., De Vries T., Brouns F., Hornstra G. Exercise performance, red blood cell deformability, and lipid peroxidation: effects of fish oil and vitamin E. J. Appl. Physiol. Sep. 1997; 83(3)746–752
- Craven P.A., DeRubertis F.R., Kagan V.E., Melhem M., Studer R.K. Effects of supplementation with vitamin C or E on albuminuria, glomerular TGF-beta, and glomerular size in diabetes. J. Am. Soc. Nephrol. Sep. 1997; 8(9)1405–1414
- Kerr P.B., Argiles A., Flavier J.L. Comparison of hemodialysis and hemodiafiltration: a long-term longitudinal study. Kidney Int. 1992; 41: 1035–1040
- Tarng D.C., Huang T.P. A parallel, comparative study of intravenous iron versus intravenous ascorbic acid for erythropoietin-hyporesponsive anaemia in hemodialysis patients with iron overload. Nephrol. Dial. Transplant Nov. 1998; 13(11)2867–2872
- Sullivan J.F., Eisenstein A.B. Ascorbic acid depletion in patients undergoing chronic hemodialysis. Am. J. Clin. Nutr. Oct. 1970; 23(10)1339–1346
- Alkhunaizi A.M., Chan L. Secondary oxalosis: a cause of delayed recovery of renal function in the setting of acute renal failure. J. Am. Soc. Nephrol. Nov. 1996; 7(11)2320–2326
- Descombes E., Hanck A.B., Fellay G. Water soluble vitamins in chronic hemodialysis patients and need for supplementation. Kidney Int. Jun. 1993; 43(6)1319–1328
- Cotton A.B. Routines vitamin therapy for the dialysis therapy: current concerns. Contemp. Dial. Nephrol. 1995; 16: 26–27
- Costello J.F., Sadovnic M.J., Cottington E.M. Plasma oxalate levels rise in hemodialysis patients despite increased oxalate removal. J. Am. Soc. Nephrol. Jun. 1991; 1(12)1289–1298
- Nienhuis A.W., Delea C., Aamodt R., Bartter F., Anderson W.F. Evaluation of desferrioxamine and ascorbic acid for the treatment of chronic iron overload. Birth Defects Orig. Artic. Ser. 1976; 12(8)177–185