Abstract
Objective. To investigate the anti proteinuric effect of pentoxifylline in diabetic patients, we prospectively studied in 25 hypertensive type 2 diabetic patients with persistent microalbuminuria and normal renal function the impact of combining pentoxifylline with an angiotensin converting enzyme inhibitor, lisinopril, on urinary albumin excretion and compared the results with those obtained in a control group of 25 type 2 diabetic patients treated with lisinopril only. Material and Methods. Fifty hypertensive type 2 diabetic patients with persistent microalbuminuria (31 males and 19 females, aged between 47–73 years) were randomly assigned to two groups. Group A received lisinopril 10 mg/day, while group B was given lisinopril 10 mg/day and pentoxifylline 600 mg/day for nine months. There were no significant differences between serum creatinine, HbA1c, blood pressure and urinary albumin excretion in both groups (p>0.05). Results. Serum creatinine, creatinine clearance, blood pressure, HbA1c levels did not change significantly during the study. Urinary albumin excretion decreased from 228 ± 28 to 148 ± 15 mg/day in group A (p<0.05). In group B urinary albumin excretion decreased from 219 ± 26 to 128 ± 12 mg/day (p<0.05). Pentoxifylline and lisinopril combination caused a significant additional reduction in urinary albumin excretion when compared to lisinopril regimen (p<0.05).
Conclusions. Our findings suggest that the combination of pentoxifylline with an angiotensin converting enzyme inhibitor in hypertensive type 2 diabetic patients with persistent microalbuminuria causes a significant reduction in urinary albumin excretion and this effect seems independent from blood pressure and glycemic control.
Introduction
In diabetic patients, the appearance of microalbuminuria is the first clinical sign of renal involvement and indicates generalized vascular dysfunction.Citation[[1]] Increased urinary albumin excretion (UAE) predicts overt proteinuria and increased mortality. It also predicts increased morbidity, especially hypertension and cardiovascular disease, in type 2 diabetic patients.Citation[[2]]
Reduction of UAE by antihypertensive therapy has been shown to delay the progression of incipient to overt diabetic nephropathy. Various studies have reported that treatment with angiotensin converting enzyme inhibitors (ACEI) lowers blood pressure and reduces albuminuria.Citation[[3]], Citation[[4]] Recently, pentoxifylline (PTF), an orally active hemorheological agent, has been shown to reduce UAE in early stages of diabetic nephropathy.Citation[[5]], Citation[[6]] We have prospectively studied the effect of combining PTF with an ACEI on UAE in type 2 diabetic patients with normal renal function and persistent microalbuminuria. The results were compared with those obtained in a control group of type 2 diabetic patients treated with ACEI only. The aim of the present study was to investigate the anti proteinuric of PTF administration on UAE in hypertensive type 2 diabetic patients.
Material and Methods
Fifty hypertensive type 2 diabetic patients (31 males and 19 females, aged between 47–73 years) with persistent microalbuminuria (UAE 30–300 mg/24 h or 20–200 µg/min found in at least three consecutive measurements) were included in this prospective study. Informed consent was obtained in all subjects. Before the start of the study, all antihypertensive treatment was withdrawn for at least three weeks. Patients with a history of myocardial infarction, cerebrovascular accident and malignant hypertension were excluded. After basal measurements, patients were randomly assigned to either a lisinopril treatment group (group A) or a PTF and lisinopril combination treatment group (group B). In group A, lisinopril 10 mg daily and in group B lisinopril 10 mg and PTF at an oral dose of 600 mg daily were initiated and continued for nine months. Patients did not change their medication during the study.
Laboratory evaluations, including hematology and serum levels of urea, creatinine, creatinine clearance, HbA1c and UAE were measured every month in each patient. Routine laboratory investigations were measured using conventional techniques. UAE was quantified by radioimmunoassay.
Statistical Analysis
Differences in parameters were compared using a two-sided, two-sample Student's t-test. All results are reported as mean ± SD. Statistical significance is assumed at p<0.05.
Results
There were no significant differences in the demographic and biochemical characteristics between two groups (). No significant differences between age, HbA1c, serum urea and creatinine levels were observed in both groups (p<0.05). Both groups had similar UAE and blood pressure levels at the beginning of the study. Serum urea and creatinine levels did not change during the study in either group (). Creatinine clearance did not change during the study. No significant changes in both systolic and diastolic blood pressure and HbA1c levels were recorded at the end of the study ().
Table 1. Demographic and biochemical parameters in both groups
Table 2. Blood pressure levels and laboratory parameters in both groups before and after treatment
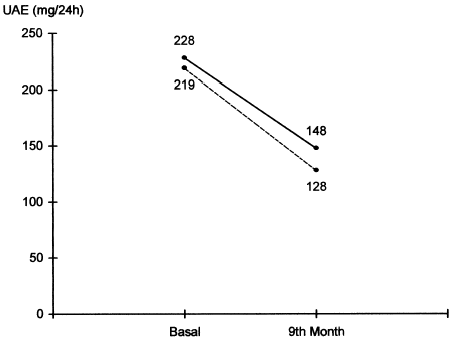
UAE decreased from 228 ± 28 mg/day to 148 ± 15 mg/day in Group A at the end of nine months (p<0.05; ). In Group B, UAE decreased from 219 ± 26 mg/day to 128 ± 12 mg/day at the end of the study (p<0.05; ). The reduction of UAE caused by PTF and lisinopril combination therapy was more prominent than that by lisinopril alone (p<0.05). In our study, UAE significantly decreased after combination of PTF with lisinopril.
Figure 1. Urinary albumin excretion of both groups (solid line: lisinopril, dotted line: lisinopril + pentoxifylline).
No serious side effects were observed during the study. Three patients developed dizziness, two patients complained of dyspepsia. No patient discontinued treatment because of side effects.
Discussion
The exact mechanism of the anti proteinuric effect of PTF is not clearly understood. PTF is a methylxanthine derivative with hemorheological properties and has favorable effects on microcirculatory blood flow.Citation[[7]] PTF strongly inhibits platelet aggregation by inhibition of phosphodiesterase and thromboxane synthesis and by increasing prostacyclin levels. The reduction of thromboxane levels has been associated with a decrease in UAE.Citation[[5]], Citation[[6]], Citation[[7]], Citation[[8]]
Diabetic hyperviscosity syndrome causes a reduction in peritubular vascular circulation, which leads to an increase in glomerular filtration pressure. Peritubular hemoconcentration causes glomerular injury by hyperfiltration and hyperviscosity. PTF reduces blood viscosity by increasing erythrocyte flexibility. This rheologic effect of PTF at the glomerulus causes a reduction of UAE by decreasing glomerular hydraulic pressure.Citation[[9]], Citation[[10]]
In diabetic nephropathy, mesangial cells produce several growth factors secondary to increased intraglomerular pressure. Among them, especially TNF-α has been shown to be cytotoxic to glomerular mesangial and epithelial cells. TNF-α increases glomerular permeability, induces glomerular epithelial cell proliferation and causes increased build up of collagen and basal membrane tissue, which causes significant glomerular injury.Citation[[10]], Citation[[11]] All these changes have been contributed to the progression of renal disease and proteinuria.
PTF is an immunomodulator and its administration has been shown to inhibit the production of TNF-α in animal models and humans by inhibiting the accumulation of TNF messenger RNA and the transcription of the TNF-α gene.Citation[[11]]
PTH may also reduce hyperfiltration and proteinuria through blockade of the adenosine receptors.Citation[[12]]
In early studies, PTF was reported to produce a reduction of both systolic and diastolic blood pressure and the normalization of blood pressure was associated with a reduction of UAE.Citation[[13]] Recent studies have shown that PTF does not have any significant effect on arterial blood pressure.Citation[[14]], Citation[[15]] In our study, PTF did not produce significant reduction in blood pressure suggesting that the anti proteinuric effect is not related to its renal hemodynamic actions.
We concluded that the addition of PTF to an ACEI in hypertensive type 2 diabetic patients causes further reduction in UAE and this effect seems to be independent from BP and glycemic control. Combination therapy of PTF and an ACEI appears to be a safe and potent approach for decreasing UAE and preventing progressive glomerular injury in the early phase of diabetic nephropathy.
References
- Deckert T., Feldt-Rasmussen B., Borch-Johnsen K., Jensen T., Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The steno hypothesis. Diabetologia 1989; 32(4)219–226
- Mogensen C.E. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes mellitus. N. Eng. J. Med. 1984; 310: 356–360
- Mathiesen E.R., Hommel E., Giese J., Parving H.H. Efficacy of captopril in postponing nephropathy in normotensive insulin-dependent diabetic patients with microalbuminuria. BMJ 1991; 303(6794)81–87
- Mogensen C.E. Renoprotective role of ACE inhibitors in diabetic nephropathy. Br. Heart J. 1994; S35–S45, Suppl.
- Tripathi K., Prakash J., Appaika D., Srivastava P.K. Pentoxifylline in management of proteinuria in diabetic nephropathy. Nephron 1993; 64(4)641–642
- Guerrero-Romero F., Rodriguez-Moran M., Paniagua-Sierra J.R., Garcia-Bulnes G., Salas-Ramirez M., Amato D. Pentoxifylline reduces proteinuria in insulin-dependent and non-dependent diabetic patients. Clin. Nephrol. 1995; 43(2)116–121
- Ward A., Clissold S.P. Pentoxifylline: a review of its pharmacokinetic properties, and its therapeutic efficacy. Drugs 1987; 34: 50–97
- Toth L., Szenasi P., Varsanyi M.N., Szilvasi I., Lehoczky E., Kammerer L., Romics L. Elevated levels of plasma and urine β-thromboglobulin or thromboxane-B2 as markers of real platelet hyperactivation in diabetic nephropathy. Hemostasis 1992; 22(6)334–339
- Ambrus J.L., Anain J.M., Anain S.M., Anain P.M., Anain J.M., Jr., Anain Stadler S., Mitchell P., Brobst J.A., Cobert B.L. Dose-response effects of pentoxifylline on erythrocyte filterability: clinical and animal model studies. Clin. Pharmacol. Ther. 1990; 48(1)50–57
- Ortiz A., Edigo J. Is there a role for specific anti-TNF strategies in glomerular diseases?. Nephrol. Dial. Transplant. 1995; 10(3)309–311
- Loftis L.L., Meals E.A., English B.K. Differential effects of pentoxifylline and interleukin-10 on production of tumor necrosis factor and inducible nitric oxide synthase by murine macrophages. J. Infect. Dis. 1997; 175: 1008–1011
- Angielski S., Jakubowski Z., Pawelczyk T., Piec G., Redlak M. Renal handling and metabolism of adenosine in diabetic rats. Contrib. Nephrol. 1989; 73: 52–57
- Solerte S.B., Adamo S., Viola C., Zambianchi E., Ferrari E. Pentoxifylline and arterial hypertension in diabetes mellitus: long-term results in randomized groups. Ric. Clin. Lab. 1985; 15(S1)515–526
- Frantz R.P., Edwards B.S., Olson L.J., Schwab M.K., Adams T.F., Textor S.C., Daly R.C., McGregor C.G., Rodeheffer R.J. Effects of pentoxifylline on renal function and blood pressure in cardiac transplant patients: a randomized trial. Transplantation 1997; 63(11)1607–1610
- Navarro J.F., Mora C., Rivero A., Gallego E., Chahin J., Macia M., Mendez M.L., Garcia J. Urinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effect of pentoxifylline administration. Am. J. Kidney Dis. 1999; 33(3)458–463