Abstract
Hypertension and anemia are common in uremic patients. This article describes a 35-year-old uremic Taiwanese who was admitted to our hospital with refractory hypertension and refractory anemia following chronic hemodialysis for more than two years. He was diagnosed with Kimura's disease finally. Refractory hypertension and refractory anemia were noted over two years before an enlarged inguinal lymph node was observed. The symptoms lead to the diagnosis of Kimura's disease. Unlike most cases, refractory hypertension and refractory anemia were first noted before the inguinal mass and eosinophilia were presented. The inflammatory parameters increased when the disease was active. Steroid treatment was conducted, and the symptoms including hypertension and anemia promptly decreased. To the authors' knowledge, this case is for first one in which Kimura's disease has induced refractory hypertension and anemia in an ESRD patient and in which these symptoms rapidly subsided following steroid treatment. The activity of Kimura's disease is closely related refractory hypertension, suggesting that inflammation may be involved in refractory hypertension and anemia in a dialytic patient with Kimura's disease.
Introduction
Kimura's disease, described by Kimura in Japan in 1948, is a rare disease that frequently occurs in Asia. Takenaka et al. named the disease eosinophilic granuloma in 1976, but now most pathologists confer that this name refers to disease other than Kimura's. The main physical manifestation of this disorder is slowly enlarging subcutaneous masses, commonly in the head and neck area, usually associated with peripheral blood and tissue eosinophilia with significantly increased serum IgE concentrations.
Although Kimura's disease is not uncommon in Asia, Kimura's disease in chronic hemodialysis patients has been reported only once.Citation[[1]] The relationship between glomerular disease and Kimura's disease has been understood by nephrologists for years.Citation[[2]], Citation[[3]] Several reports state that Kimura's disease has frequently induced membranous glomerulonephritis, but no data clarifies the relationship between Kimura's disease and renal failure. Does hemodialysis increase the risk of Kimura's disease? No report has addressed the influence of Kimura's disease on refractory anemia and refractory hypertension. Recently, however, our patient presented, an unusual correlation of hypertension, anemia and activity with Kimura's disease.
Can a dialytic patient with Kimura's disease reasonably have refractory hypertension and anemia? When should the secondary causes of hypertension in dialytic patients be surveyed after overloading fluid has been removed? Chronic or micro-inflammatory processes are known to persist in dialysis patients and some relates with refractory anemia.Citation[[4]] Can inflammation make a patient's blood pressure hard to control? Some non-dialytic patients with more than one cause of anemia have been reported, when should we study these abnormal data in dialytic patients? A dialytic patient was diagnosed with Kimura's disease, which initially manifested as refractory hypertension and refractory anemia. After Kimura's disease was suppressed by steroid treatment, the patient's hypertension and anemia improved markedly.
Case Report
On January 30, 2001, a 35-year-old Taiwanese male was hospitalized for growing inguinal masses following two years of difficulty of out-patients-department in controlling hypertension. He had focal segmental glomerulosclerosis (FSGS) diagnosed by biopsy, since when he received steroid treatment for over ten years; FSGS progressed into ESRD two years ago, after which the patient received continuous ambulatory peritoneal dialysis (CAPD). CAPD has been replaced with Regular hemodialysis three times weekly for four hours each time since one year ago, because of poor self-hygiene. No previous medical history of eosinophilia, refractory hypertension, or anemia before hemodialysis was involved. Since 1999, high systolic blood pressure was recorded at 170–200 mmHg. Combined therapy for hypertension was prescribed stepwise including: Losarten (50) 1# bid, monoxidil (10) 1# bid, isordil (10) 1# tid labetalol (200) 2# bid and amlodipin1# bid, but systolic pressure remained above 170 mmHg over the past year. The anemic hemoglobin 7.0 g/dL was treated with a dose of over 400 IU/kg/month of EPO in the local hospital, but the patient's anemia never improved. Itching skin and eosinophilia were noted from eight months ago (July, 1998 local hospital record eosinophilia, 42.6%). An inguinal mass was noted two months ago, and it was growing slowly. The patient denied having asthma, rashes or symptoms related to a hypersensitive reaction.
On admission, he was well, but suffering intermittent dizziness and headache. His body temperature was 36.5°C with a normal heart rate and blood pressure at 176/90 mmHg. Physical examination revealed an apparently well man with an obvious swelling on the left of his inguinal area. A 3 × 3 cm, lobulated, non-tender, firm inguinal mass palpated. The overlying skin was not inflamed. Laboratory tests on admission revealed eosinophilia. He was anemic with hemoglobin at 7.8 gm/dL and an eosinophilia was found (WBC 8300/cm3; 60% segment form neutrophil, 19.5% eosinophil, 8% monocytes and 11.5% lymphocyte.) Blood chemistry was as follows: BUN/Cr 76/11.5 mg/dL, sodium 144 meq/L, K 4.5 meq/L, CO2 24.8 meq/L, and C-reactive protein 105 mg/dL. The Fe/TIBC level was 89/247 and the concentration of ferritin was 1361 ng/mL. A survey of secondary hypertension-including pheochromocytoma (MIBG: no evidence of pheochromocytoma) and hyperaldosteronism by renin-aldosterone RIA 35.1/27.6, revealed no evidence of secondary causes of hypertension. Chest X-rays revealed no active lesions.
After admission, an open biopsy of the inguinal swelling lymph node was conducted. Microscopic analysis of this material showed that the nodal architecture to be intact. Reactive follicular hyperplasia was present with prominent eosinophilia (). No evidence of a malignant change was found. Subsequently, the IgE concentration significantly rose to 1350 mg/L (normal 30–280 mg/L). Urine was normal. A hematologist was consulted to examine bone marrow, which showed eosinophilia (eosinophil 17.4%) but normal iron storage. (Grade 2, sideroblast decrease). A clinical diagnosis of Kimura's disease was made based on peripheral eosinophilia, histology, and absence of evidence of parasitic agents. On the ninth hospital day, treatment with prednisolone 50 mg dailyCitation[[5]] was begun. The treatment was continued for one month. The patient responded rapidly to steroid therapy. The pruritus subsided and eosinophilia fell to 0% in one week. The CRP level fell from 105 mg/dL to 6 mg/dL and Fe/TIBC increased from 89/247 to 105/211. Surprisingly, the patient's blood pressure and anemia began to respond to anti-hypertension agents and EPO treatment. He was discharged on the 15th hospital day. Pretreatment and three months of post treatment data are listed below. (See .)
Figure 1. Biopsy sample of inguinal mass showing intact follicular architecture with prominent infiltration by ecosinophils (hematoxylin and eosin, ×100).
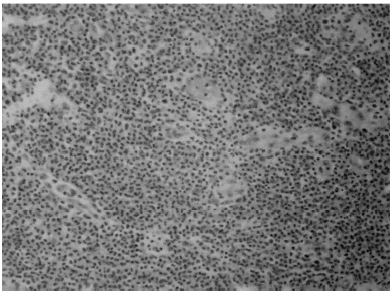
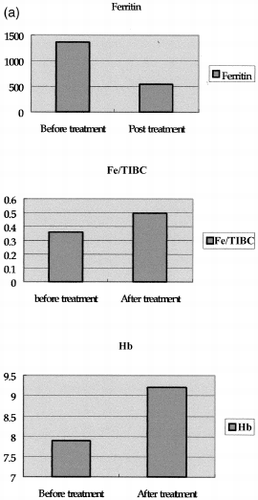
Figure 2. Compare before and post treatment data: Panel A. After treatment, the patient's hemoglobin (from 7.7 to 9.2) and saturation of Fe/TIBC (from 36 to 49.7%) increased. The reduced ferritin levels (from 1361 to 543) indicate that result from increased iron utilization; Panel B. CRP level reduced (from 105 to 6) after treatment and the mean arterial blood pressure (from 118.66 ± 6.9 mmHg to 97.63 ± 5.2 mmHg) was easily controlled. No significant difference in serum renin and aldosterone level. Panel C. IgE (from 1350 to 1020) and eosinophil (from 19.5 to 0%) decreased after steroid treatment implicated the activity of Kimura's disease decreased.
Following discharge, the patient took prednisolone 40 mg daily to control the disease. However, he unilaterally chose to stop taking the drug, due to a lake of symptoms one month later. Symptoms included refractory anemia (6.9 g/dL), refractory hypertension (systolic pressure above 180 mmHg), skin pruritus, eosinophilia (45% white cell count)), and recurring multiple lymph nodes over the head and in the neck area. The patient was readmitted to our hospital and CRP elevation was noted at 216 mg/dL. A large dose approximately 50 mg daily of steroids was used, as during the previous admission, and the same significant responses of hypertension and anemia occurred after five days following admission. The systolic blood pressure returned to below 130 mmHg and the patient was discharged after symptoms subsided on the 13th day. After discharge, he received regular hemodialysis three times per week at the local hemodialysis department. The hypertension was controlled at 141.4 ± 9.7/76 ± 6.3 mmHg using Norvasc 1# bid and trandate 1# bid and, the hemoglobin was 9.6 g/dL under EPO 20,000 U/month treatment, 2 months after discharge.
Discussion
Kimura's disease is a benign cause of painless localized lymphadenopathy and eosinophilia in Asians.Citation[[6]], Citation[[7]], Citation[[8]] However, the disease is rarely diagnosed in hemodialytic patients.Citation[[1]] The clinical triad of subcutaneous nodules found in the head or neck, prominent peripheral eosinophilia, and highly increased IgE concentrations, particularly when seen in an Asian, are highly suggestive of Kimura's disease. In hemodialytic patients, the symptomless eosinophilia may be persistent or intermittent without other apparent diseases, making the diagnosis of Kimura's disease more difficult.Citation[[1]] The patient discussed here had no Kimura's disease eight years ago, when FSGS was diagnosed. Eosinophilia was noted eight months ago, before the last admission, and was accompanied by refractory hypertension and refractory anemia. To the authors' knowledge, this patient represents the first reported case of Kimura's disease in a chronic dialysis patient who presented refractory hypertension and refractory anemia. While the clinical course of Kimura's disease is benign, hypertension and anemia, both hard to control, disturbed the patient. The difficult in controlling hypertension and anemia were noted more than two years before admission, even under adequate fluid control and drug treatment. However, the symptoms drastically subside following steroid treatment during the first admission. The same symptoms recurred after the steroids were discontinued. According to the CRP, eosinophil, and ferritin level, a close correlation exists between these parameters and the activity of Kimura's disease. During the first admission, a bone marrow study was performed while the disease was active, and revealed no iron overloading. No other etiologies of refractory anemia were noted in this patient. The elevation of CRP and ferritin may have been related to inflammation. These observations suggested a relationship between the activity of Kimura's disease and hypertension.
Can inflammation of Kimura's disease increase hypertension? Markedly relationships between inflammation and hypertension and inflammatory markers such as IL-6 and sICAM-1, have been reported in apparently health men.Citation[[9]] IL-6 also was proven to promote vascular smooth muscle cell proliferation betraying the early stages of hypertension and atherosclerosis. These mechanisms may partially explain the association between increased blood pressure and IL-6 level. Angiotensin II may play other important roles via blood pressure and an independent mechanism.Citation[[9]] The induction of hypertension by inflammation may also proceed by increasing the concentration of intrarenal angiotensin.
Some authors suggested that the production of renin is increased in angiolymphoid hyperplasia in the presence of eosinophilia; they have shown that Bowie staining and immunohistochemistry for human renin were positive in enlarged lymph nodes.Citation[[10]] This patient's serum level of renin-angiotensin II, however, was not markedly elevated, and the serum level did not represent local renin-angiotensin II activity. The proximal angiotensin II concentrations are in the nanomolar range, much higher than the circulating concentration. Angiotensin II and/or the precursors of angiotensin II are secreted in tubular lumen.Citation[[11]] Increased intrarenal angiotensin II accumulation occurs in various models of angiotensin II dependent hypertension, by receptor-mediated internalization in intracellular compartments, presumably endosomes, as well as by stimulating an angiotensinogen message.Citation[[11]], Citation[[12]] Like diabetic patients, these patients could have hypertension but their intrarenal angiotensin II levels are compartmentalized, which fact may not be determined by the circulating renin-angiotensin profile.Citation[[11]] Is it possible that compartmentalized angiotensin II levels results in our patient's hypertension? This report seems to be limited. For instance, the renin-angiotensin II staining in situ of this patient was not utilized to confirm our implication. A further examination should determine whether the staining of renin-angiotensin II increases in the lymph node tissue over normal population. Otherwise, for the patient in this report, steroid responsiveness supports the hypothesis that inflammation-induced cytokines may evoke the mechanisms of hypertension by a not yet verified pathway, and that anti-inflammatory agents may suppress this pathway.
Further more, this patient showed an increased hematocrit and a reduced EPO resistance after the activity of Kimura's disease was suppressed. Therefore, determining factors that explain the relationship between the marker of the iron index and the reduced EPO resistance may be important.Citation[[13]], Citation[[17]] A previous study claimed that serum ferritin and CRP both represent a positive acute phase protein.Citation[[13]], Citation[[14]] Many investigations have postulated an inflammatory block of erythropoieses.Citation[[14]], Citation[[15]] Thus, increments in CRP and ferritin level typically imply inflammation. Many reports have also considered these CRP and ferritin level as indirect indicators of inflammation.Citation[[14]], Citation[[15]] What is the implication of the presence of these parameters in our patient? Wizemann et al.Citation[[16]] utilized CRP as a marker of inflammation, indicating when CRP significantly decreased, the EPO resistance decreased and the anemia improved. The same results and observations are recorded in another dialysis study.Citation[[15]] Accordingly, the lower ferritin and CRP levels in our after steroid treatment may be due to improved inflammation and decreased activity of Kimura's disease.
In conclusion, a chronic hemodialysis patient who developed Kimura's disease has been discussed. The patient suffered refractory hypertension and refractory anemia while the disease was active, implying a strong correlation between Kimura's disease and these symptoms. The elevated parameters of inflammation (for example, CRP, ferritin) and dramatic responses to steroid treatment suggested the involvement of inflammation in inducing hypertension and, anemia during Kimura's disease. However, the mechanism must be detailed by further investigation. If the inferences presented here are true, then Kimura's disease should differentially diagnosed in dialytic patients with refractory hypertension, even when etiologies remain unknown.
References
- Chien-Te Lee, Chao-Cheng Huang King-Kwan Lam, Jun-Bor Chen. Kimura's disease in a chronic hemodialysis patient. Am. J. Nephro. 2001; 21: 47–50
- Yamada A., Mitsuhashi K., Miyakawa Y. Membranous glomerulonephritis associated with eosinophilic lymphfolliculosis of skin (Kimura's disease): report of a case and review of the literature. Clin. Nephrol. 1982; 18: 211–215
- Whenlan T.V., Maher J.F., Kragel P., Dysart N., Dannenhoffer R., Prager L. Nephrotic syndrome associated with Kimura's disease. Am. J. Kidney Dis. 1988; 11: 353–356
- Barany P., Jose C., Filho D., Bergstrom J. High C-reactive protein is a strong predictor of resistence to erythropoiectin in hemodialysis patients. Am. J. Kidney Dis. 1997; 29: 565–568
- Matsuda O., Makiguchi K., Ishibashi K. Long-term effects of steroid treatment on nephrotic syndrome associated with Kimura's disease and a review of the literature. Clinical Nephrology 1992; 119–124
- Kimura T., Yoshimura S., Ishikaura E. Unusual granulation combined with hyperplastic change of lymphatic tissue. Trans. Soc. Pathol. Jpn. 1948; 37: 179–180
- Motoi M., Horie Y., Akagi T. Kimura's disease: clinical, histological, and immunohistochemical studies. Acta. Med. Okayama 1992; 46: 449–455
- Irish J.C., Kain K., Keystone J.S., Gullane P.J. Kimura's disease: an unusual cause of head and neck masses. J. Otolaryngol. 1994; 23: 88–91
- Chae C.U., Lee R.T., Rifai N., Ridker P.M. Blood pressure and inflammation in apparently healthy men. Hypertension 2001; 38: 399–403
- Afernadez L., Oslen T.G., Barwick K.W. Renin in angiolymphoid hyperplasia with eosinophilia. Arch. Pathol. Lab. Med. 1986; 110: 1131–1135
- Mitchell K.D., Braam B., Navar L.G. Hypertensinogenic mechanisms mediated by renal actions of renin-angiotensin system. Hypertension 1992; 19(suppl 1)I18–I27, Review
- Navar L.G., Harrison-Bernard L.M. Intrarenal angiotensin II augmentation in angiotensin II dependent hypertension. Hypertens. Res. 2000; 23(4)291–301
- Kaysen G.A., Rathore V., Shearer G.C., Depner T.A. Mechanisms of hypoalbuminemia in hemodialysis patients. Kidney Int. 1995; 48(2)510–516
- Kaysen G.A., Stevenson F.T., Depner T.A. Determinants of albumin concentration in hemodialysis patients. Am. J. Kidney Dis. 1997; 29(5)658–668
- Lin C.L., Huang C.C. Improved iron utilization and reduced erythropoietin resistance by on-line hemodialfiltration. Blood Purification., in press
- Wierenga E.A., Backx B., Snoek M., Kapsenberg M.L. Relative contributions of human types 1 and 2 T-helper cell-derived eosinophilotrophic cytokines to development of eosinophilia. Blood 1993; 82: 1471–1479
- Carvill I. Iron status as measured by serum ferritin: the marker and its limitation. Am. J. Kidney Disp. 1999; 34: S12–S17

