Abstract
Background. Transforming growth factor-β1 (TGF-β1), the major fibrogenic growth factor, is implicated in the pathogenesis of renal scarring in experimental and clinical nephropathies as well as in chronic allograft nephropathy. In this study we examined the pattern of changes of TGF-β1 excretion in the urine and the sites of TGF-β1 expression in the kidney of transplanted patients during the early post-transplantation period. Methods. Eighteen renal allograft recipients were included in the study. In all patients urinary TGF-β1 levels were determined by ELISA in sequential measurements during the first two postoperative months and compared to that of 14 healthy subjects. The renal expression of TGF-β1 protein was studied in 4 patients that underwent a biopsy of the transplanted kidney at the same period. All patients were treated with prednisolone, cyclosporin, and mycophenolate mofetil. Results. Urinary TGF-β1 levels were increased during the first postoperative days. Although they were gradually reduced during the first two post-operative months, they remained significantly higher compared to those of normal subjects (580 ± 148 ng/24 h vs. 310 ± 140 ng/24 h p<0.01). The decline of urinary TGF-β1 excretion followed that of serum creatinine. TGF-β1 protein expression was identified within the cytoplasm of tubular epithelial cells of transplanted patients. Conclusions. Elevated urinary TGF-β1 levels are observed during the early post-transplantation period in renal allograft recipients and are maintained high even after restoration of renal function to normal.
Introduction
Transforming growth factor-β (TGF-β) represents a group of 25-kD proteins, that are actively involved in the development and differentiation of various tissues.Citation[[1]], Citation[[2]] TGF-β1, the most important isoform in humans, is implicated in the pathogenesis of renal scarring in experimental and clinical nephropathies.Citation[[3]], Citation[[4]], Citation[[5]], Citation[[6]] It is involved in the stimulation of synthesis of extracellular matrix (ECM) components and blockade of their degradation.Citation[[2]], Citation[[3]] Recent evidence has shown that TGF-β1 is involved in the development of chronic renal allograft nephropathy and cyclosporin nephrotoxicityCitation[[7]] and it also induces plasminogen activator inhibitor (PAI-1), which leads to inhibition of fibrinolysis and accumulation of extracellular matrix components in the renal allograft.Citation[[8]] An upregulation of TGF-β1 expression within tubular epithelial cells has been described in both experimental animals and humans with chronic allograft nephropathy and/or cyclosporin nephrotoxicity.Citation[[9]], Citation[[10]], Citation[[11]] In such patients urinary TGF-β1 excretion is also elevated.Citation[[8]] Although the contribution of TGF-β1 in the development of renal allograft fibrosis is established, it is not known whether it is related to any changes observed during the early post-transplantation period. The purpose of this study was to evaluate the pattern of urinary TGF-β1 changes and its localization in the renal tissue of transplanted patients, during the early post-transplantation period.
Patients and Methods
Patients
Eighteen renal allograft recipients (12 males and 6 females), 42 ± 9 years old, were included in the study and examined over a follow up period of two months postoperatively. Thirteen patients received a cadaveric and five received a living related graft. The mean time of cold ischemia was 12 ± 4 h and of warm ischemia 60 ± 10 min. All patients were treated with prednisolone (1 mg/kg/day gradually reduced to 20 mg/day), cyclosporine-A (CyA) (4–5 mg/kg/day, targeting to C0 blood levels of 200 ng/mL) and mycophenolate mofetil 2 g/day. None of them developed acute cellular rejection. Urinary TGF-β1 levels were determined in sequential measurements (1st, 8th, 15th, 30th, and 60th day postoperatively) in all patients and compared to those of normal subjects. In addition, TGF-β1 protein expression was studied immunohistochemically in the renal tissue from 4 patients that underwent a renal biopsy because of delayed graft function.
Determination of TGF-β1 Levels in the Urine
Urinary TGF-β1 was determined in the 18 transplanted patients and on 14 healthy subjects (control group). The concentration of TGF-β1 was measured by EIA, according to Honkanen et al.Citation[[12]] Microtitre plates (Costar, USA) were coated with 0.1 µg/well monoclonal mouse anti TGF-β s antibodies (Genzyme Co., USA) in 0.05 M Na2CO3 buffer pH 9.0, by incubating overnight at 4°C. The wells were washed with phosphate buffered saline (PBS), 0.05% Tween 20. One hundred microliters of standard dilutions (R&D Systems, UK) and undiluted samples were acid-activated (1 N HCl, for 2 h at room temperature) and neutralized (1.2 N NaOH/0.5 M HEPES). One hundred microliters of neutralized samples were incubated in the wells overnight at 4°C. The TGF-β1 bound into the wells was then detected with a rabbit polyclonal anti-TGF-β1 (R&D Systems, UK) labeled with horseradish peroxidase (200 µL, 1.5 h at room temperature). Peroxidase activity was determined using tetramethylbenzidine (TMB) as substrate (R&D Systems, UK). Plasma TGF-β1 concentration was calculated in ng/mL. The intra-assay and inter-assay coefficients of variation (CV) were 7.4 and 6.3% respectively. The recovery of TGF-β1 standards (50 and 100 pg/mL) ranged from 83 to 112%. TGF-β1 urinary excretion was calculated in ng/24 h.
Immunohistochemistry for TGF-β1 Protein
Immunohistochemistry for detection of TGF-β1 protein was performed in kidney sections from 4 transplanted patients that underwent a renal biopsy because of delayed graft function and in renal tissue (with normal parenchyma) from 4 patients who underwent nephrectomy for renal cell carcinoma that were used as controls.
TGF-β1 was detected immunohistochemically on kidney biopsy sections. Briefly, formalin fixed paraffin-embedded kidney sections (4 µm) were cut and mounted on gelatinized slides. De-waxed and hydrated sections were processed in a microwave oven for 3 cycles 5 min each at 450 W in citrate buffer pH 6.0. After cooling, sections were then treated with Protein Blocking Agent to reduce the nonspecific binding of antibodies. Slides were washed in PBS and incubated with a polyclonal anti-TGF-β1 antibody (1:50) (Santa Cruz, USA) for 1 h at 37°C, in a humid atmosphere. Thereafter slides were washed in PBS and incubated for 10 min with a polyvalent biotinylated secondary antibody (Kwik kit, IMMUNON™, USA). Slides were washed in PBS, incubated with alkaline phosphatase reagent for 10 min and washed again with PBS. The visualization of the antigen-antibody complex was achieved by incubating slides in nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP) chromogen solution for 10–20 min, until a satisfactory color development. Finally, slides were washed in water, counterstained with hematoxylin for 2 min and mounted with resin mounting medium. All steps, except that of the primary antibody, were performed at room temperature. Control sections were incubated with nonimmune rabbit antiserum or processed after the omission of the primary antibody.
Statistical Analysis
The results were expressed as means ± SD. Student's t-test for unpaired and paired data were used for comparison of TGF-β1 levels in the urine of patients and healthy subjects and between the first and last measurement of TGF-β1 urinary levels in transplanted patients, respectively. Linear regression analysis was used for correlation of urinary TGF-β1 levels with creatinine clearance and CyA levels. A p value <0.05 was considered to be significant.
Results
General Observations
Early function of the renal allograft was observed in 13 (72%) whereas delayed graft function was observed in 5 patients (28%), that remained in hemodialysis for 11 ± 5 days. In 4 patients with delayed graft function, renal biopsy was performed and showed acute tubular necrosis without any evidence of cyclosporin nephrotoxicity. The mean time to renal function improvement (Clcr<50 mL/min) was 9 ± 4 days whereas the mean creatinine clearance two months after transplantation was 85 ± mL/min.
Urinary TGF-β1 Excretion
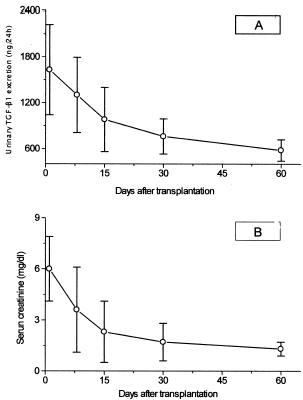
Urinary TGF-β1 levels were measured in 24 h urine collections at a regular basis during the first 60 days after transplantation. The excreted amount of TGF-β1 in the urine was increased during the first few days and it was gradually reduced over the follow up period of 60 days (from 1630 ± 590 ng/24 h to 580 ± 148 ng/24 h, p<0.05). (A). The pattern of urinary TGF-β1 excretion decline was rather similar to that of serum creatinine (from 6.0 ± 1.9 mg /dL to 1.3 ± 0.3 mg /dL, p<0.05) (B). Although, urinary TGF-β1 levels of transplanted patients were gradually reduced they remained higher compared to those of normal subjects (580 ± 148 ng/24 h vs. 310 ± 140, p<0.01). No correlation of urinary TGF-β1 levels with serum creatinine, creatinine clearance, and CyA levels was observed.
Classical Histology and Immunohistochemistry
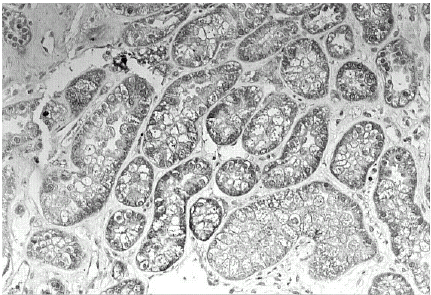
In the renal biopsies of patients examined no evidence of rejection or cyclosporin toxicity was found. In these sections, TGF-β1 was localized within the cytoplasm of tubular epithelial cells (). Glomerular and/or interstitial TGF-β1 expression was not identified. Control sections were negative for TGF-β1 protein.
Discussion
The pattern of urinary TGF-β1 levels during the early post-transplantation period was examined in this study. In the first two weeks, urinary TGF-β1 excretion was elevated and was then gradually reduced following a pattern similar to that of serum creatinine. However, urinary TGF-β1 levels of renal allograft recipients remained higher than normal even after restoration of renal function.
Upregulation and increased urinary excretion of TGF-β1 in patients with chronic allograft nephropathy, lead to accumulation of extracellular matrix in the graft, due to PAI-1 activation.Citation[[8]] Synthesis and expression of TGF-β1 in the transplanted kidney is also induced by a direct effect of CyA.Citation[[13]] In this study, increased urinary TGF-β1 excretion was observed during the first two weeks, postoperatively. At that time, ischemia/reperfusion injury is still ongoing in the graft. Since no evidence of CyA nephrotoxicity was found, in the examined biopsies, the increased urinary TGF-β1 excretion is probably related to ischemia/reperfusion injury, which follows the reintroduction of oxygen free radicals, due to anaerobic metabolism.Citation[[14]]
TGF-β1 was identified within the cytoplasm of tubular epithelial cells of transplanted patients. This finding suggests an upregulation of TGF-β1, during the period of early allograft dysfunction. The ischemia/reperfusion injury may cause upregulation and exposure of histocompatibility antigens and adhesion molecules.Citation[[14]], Citation[[15]] Nitric oxide, produced by the nitric oxide synthase enzymes, may provide the link between injury and immune activation.Citation[[14]], Citation[[16]] Activation of inflammatory cytokines and growth factors such as TGF-β1 may facilitate the development of low-grade inflammation that in turn facilitates acute and chronic immune injury.Citation[[14]], Citation[[15]] Thus, the initial injury followed by inflammation and immune response leads to further injury of the graft.Citation[[14]]
TGF-β1 expression within tubular epithelial cells and its upregulation in the renal allograft has been described in experimental animals with chronic rejectionCitation[[9]] and in patients with chronic allograft nephropathy and/or cyclosporin toxicity.Citation[[10]], Citation[[11]], Citation[[16]] The increased expression of TGF-β1 within tubular epithelial cells along with the elevated urinary TGF-β1 excretion, over the early post-transplantation period, might represent local activation of tubular epithelial cells.Citation[[17]] Whether these findings have any predictive value for the development of late allograft dysfunction is under investigation. The elevated urinary TGF-β1 levels suggest that a low-grade activation is probably ongoing in the transplanted kidney, even after restoration of renal function to normal. This might be related to various factors implicated in the development of chronic allograft nephropathy.Citation[[18]]
In conclusion, increased urinary TGF-β1 excretion and TGF-β1 tubular expression are observed in renal allograft recipients during the early post-transplantation period. Further research is necessary in order to clarify the importance of this issue in the clinical outcome of renal transplanted patients.
References
- Roberts A.B., Sporn M.B. Physiological action and clinical applications of transforming growth factor-β. Growth Factors 1993; 8(1)1–9
- Border W.A., Noble N.A. Transforming growth factor-β in tissue fibrosis. N. Engl. J. Med. 1994; 331(19)1286–1291
- Lawrence A.D. Transforming growth factor-β: an overview. Kidney Int. 1995; 47(suppl. 49)S19–S23
- Yamamoto T., Noble N.A., Miller D.A., Border W.A. Sustained expression of TGF-β underlies development of progressive kidney fibrosis. Kidney Int. 1994; 45(3)916–927
- Yamamoto T., Noble N.A., Cohen A.H., Nast C.C., Hishiba A., Gold L.I., Border W.A. Expression of TGF-β in human glomerular disease. Kidney Int. 1996; 49(2)461–469
- Goumenos D.S., Tsamandas A.C., Oldroyd S., Sotsiou F., Tsakas S., Petropoulou C., Bonikos D., El Nahas A.M., Vlachojannis J.G. Transforming growth factor-β1 and myofibroblasts: a potential pathway towards renal scarring in human glomerular disease. Nephron 2001; 87(3)240–248
- Campistol J.M., Iñigo P., Larios S., Bescos M., Oppenheimer F. Role of transforming growth factor-β1 in the progression of chronic allograft nephropathy. Nephrol. Dial. Transpl. 2001; 16(Suppl 1)S114–S116
- Grandaliano G., Di Paolo S., Monno R., Stallone G., Ranieri E., Pontrelli P., Gesualdo L., Schena F.P. Protease-activated receptor 1 and plasminogen activator inhibitor 1 expression in chronic allograft nephropathy. Transplantation 2001; 72(8)1437–1443
- Shihab F.S., Tanner A.M., Shao Y., Weffer M.I. Expression of TGF-β1 and matrix proteins is elevated in rats with chronic rejection. Kidney Int. 1996; 50(6)1904–1913
- Horvath L.Z., Friess H., Schilling M., Borisch B., Deflorin J., Gold L., Korc M., Büchler M.W. Altered expression of transforming growth factor-βs in chronic renal rejection. Kidney Int. 1996; 50(2)489–498
- Pankewycz O.G., Miao L., Isaacs R., Guan J., Pruett T., Haussman G., Sturgill B.C. Increased renal tubular expression of transforming growth factor beta in human allografts correlates with cyclosporine toxicity. Kidney Int. 1996; 50(5)1634–1640
- Honkanen E., Teppo A.M., Tornroth T., Groop P.H., Groonhagen-Riska C. Urinary transforming growth factor-β1 in membranous glomerulonephritis. Nephrol. Dial. Transplant. 1997; 12(12)2562–2568
- Khanna A., Kapur S., Sharma V., Li B., Suthanthiran M. In vivo hyperexpression of transforming growth factor-β1 in mice: stimulation by cyclosporine. Transplantation 1997; 63(7)1037–1039
- Amend W.J.C., Vincenti F., Tomlanovich S.J. The first two post-transplantation months. Handbook of Kidney Transplantation, 3rd Ed., G.M. Danovitch. Lippincott, Williams and Wilkins, Philadelphia 2001; 163–181
- Shoskes D.A., Halloran P.F. Delayed graft function in renal transplantation: etiology, management, and long-term significance. J. Urol. 1996; 155(6)1831–1840
- Smith S.D., Wheeler M.A., Lorber M.I., Weiss R.M. Temporal changes of cytokines and nitric oxide products in urine from renal transplant patients. Kidney Int. 2000; 58(2)829–837
- Mohamed M.A.S., Robertson H., Booth T.A., Balupuri S., Kirby J.A., Talbot D. TGF-β expression in renal transplant biopsies. Transplantation 2000; 69(5)1002–1005
- Massy Z.A., Guijarro C., Wiederkehr M.R., Ma J.Z., Kasiske B.L. Chronic renal allograft rejection: immunologic and nonimmunologic risk factors. Kidney Int. 1996; 49(2)518–524