Abstract
Regular administration of recombinant human erythropoietin (rHuEPO) is frequently associated with a rise in arterial blood pressure in hemodialysis (HD) patients. The aim of this study was to examine the effects of rHuEPO on plasma endothelin (ET)-1 and nitric oxide products (NOx) concentration in HD patients. Fifteen patients on maintenance HD with hematocrit of less than 25% were included in the present study. All patients received 3,000 units of rHuEPO intravenously three times a week at the end of each HD session. Plasma levels of ET-1, NOx, thromboxane B2 (TXB2), prostacyclin (6-keto-PGF1α), and cyclic guanosine 3′,5′-monophosphate (cGMP) were measured before, 2, and 4 weeks after rHuEPO treatment. Plasma concentrations of ET-1, TXB2, and 6-keto-PGF1α were measured by radioimmunoassay. Plasma NOx was measured by highperformance liquid chromatography. An rHuEPO-induced increase in mean arterial blood pressure of over 6 mmHg occurred in 7 patients (hypertensive group), whereas the elevation of mean arterial blood pressure was less than 5 mmHg in 8 patients (nonhypertensive group). Plasma ET-1 levels were elevated in all HD patients. Elevated plasma ET-1 levels remained unchanged after rHuEPO treatment in the hypertensive group, whereas the increase in plasma ET-1 levels was attenuated in the nonhypertensive group. Plasma NOx concentrations were also increased in all HD patients. This increase in plasma NOx levels was lessened in the hypertensive group after rHuEPO administration; however, plasma NOx levels remained increased in the nonhypertensive group. Changes in mean arterial blood pressure were significantly correlated with changes in plasma ET-1/NOx ratio. Plasma levels of TXB2, 6-keto-PGF1α, and cGMP were unchanged after rHuEPO administration in the hypertensive and nonhypertensive groups. These results suggest that an increase in ET-1/NOx ratio in blood, probably occurring in vascular endothelial cells, may be associated with rHuEPO-induced hypertension in HD patients.
Introduction
Anemia in hemodialysis patients is responsible for numerous complications that contribute substantially to morbidity and mortality in these patients. The partial correction of anemia with recombinant human erythropoietin (rHuEPO) has improved their quality of life, including improvements in maximal exercise capacity and cognitive function.Citation[[1]] Since the introduction of rHuEPO therapy for the treatment of anemia in hemodialysis patients in 1989, numerous complications have manifested.Citation[[2]], Citation[[3]] Hypertension is a major complication of rHuEPO therapy occurring in at least a third of the hemodialysis population.Citation[[1]], Citation[[4]], Citation[[5]], Citation[[6]] Although the pathogenesis of rHuEPO-induced hypertension has not been fully elucidated, an increase in peripheral vascular resistance has been postulated as one of the most important factors.Citation[[7]]
Endothelin (ET)-1 is a naturally occurring potent vasoconstrictive peptide produced by vascular endothelial cells.Citation[[8]] An increase in plasma ET-1 levels has been reported in patients with chronic renal failure.Citation[[9]], Citation[[10]], Citation[[11]] In animal models, ET-1 infusion results in sustained elevation in mean blood pressure.Citation[[12]] The fact that endothelial cells have erythropoietin receptors suggests a possible link between ET-1 and erythropoietin-associated hypertension.Citation[[13]]
Nitric oxide (NOx), identified as an endothelium-derived relaxant factor, can be another possible factor that contributes to hypertension after rHuEPO treatment. Vaziri et al.Citation[[14]] have demonstrated that erythropoietin-induced hypertension may be causally related to impaired vasodilatory response to NOx in rats.
The present study was designed to examine the effect of rHuEPO treatment on plasma ET-1 and NOx levels in patients with chronic renal failure to assess whether rHuEPO exerts some action on ET-1 and NOx release by endothelial cells. To this end, plasma levels of ET-1, NOx, thromboxane (TXB2), prostacyclin (6-keto-PGF1α), and cyclic guanosine 3′,5′-monophosphate (cGMP) were measured in patients on maintenance hemodialysis during a 4-week rHuEPO treatment.
Patients and Methods
Fifteen hemodialysis patients (7 men and 8 women; mean age, 58.2 years) with hematocrits of less than 25% were recruited from Koto Hospital and Mizue Dialysis Center. The underlying diseases included 9 cases of diabetic nephropathy and 6 cases of chronic glomerulonephritis. All patients received 4 h hemodialysis three times weekly. Average hemodialysis duration was 54.5 months (8 months to 21 years). None of the patients had been given rHuEPO for at least 1 month before enrollment. Thirteen patients who had received antihypertensive drugs when they were enrolled in this study were allowed to be given the same hypertensive drugs at the same dose during the study period. Prehemodialysis mean arterial blood pressure was measured on three occasions after the patients were in the supine position for 15 min. Mean arterial blood pressure was measured by standard sphygmomanometry. Each patient signed a written consent form before participating in this study. The study protocol was approved by the Human Research Ethics Committee for Koto Hospital.
The rHuEPO (Epoetin β Chugai Pharmaceutical, Tokyo, Japan) was administered via the venous access at a dose of 3,000 units, three times a week at the end of each dialysis session. The patients were divided into hypertensive and nonhypertensive groups according to the change in blood pressure after rHuEPO administration. The hypertensive group comprised 7 patients whose mean arterial blood pressure rose from 111 ± 10.5 mmHg before administration of rHuEPO to 120 ± 10.4 mmHg at 4 weeks after administration, whereas the nonhypertensive group included 8 patients whose mean arterial blood pressure declined slightly from 116 ± 14 mmHg to 110 ± 16 mmHg during the same period.
Blood samples were serially collected before, 2, and 4 weeks after rHuEPO administration to determine blood cell counts, and plasma ET-1, NOx, TXB2, 6-keto-PGF1α, and cGMP concentrations. Plasma ET-1, TXB2, 6-keto-PGF1α, and cGMP were measured by radioimmunoassay. Nitrate (NO3) and nitrite (NO2) concentration in plasma was determined with high-performance liquid chromatography.Citation[[15]] NO3 in plasma was reduced by the cadmium column to NO2, which reacted with a Griess reagent to form a purple azo dye. NO2 in plasma needed no reduction and so reacted with the Griess reagent when the cadmium column was bypassed. The absorbance of the product dye at 540 nm was determined by a flow-through UV spectrophotometer (Model SPD-10A, Shimazu, Tokyo, Japan).
Results are expressed as mean ± SE. Paired t-test, unpaired t-test, and Post-hoc test were utilized to compare differences between groups. The difference was considered statistically significant when p<0.05.
Results
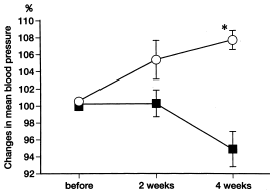
Since mean arterial blood pressure measurement was different from one subject to the next, the average blood pressure before rHuEPO treatment was taken as 100, and the mean arterial blood pressure at 4 weeks after treatment was expressed relative to the pretreatment mean arterial blood pressure. In the hypertensive group, the percent mean arterial blood pressure showed an 8% rise, whereas in the nonhypertensive group, the percent mean arterial blood pressure showed a 6% fall (p<0.01) ().
Figure 1. Percent changes in mean arterial blood pressure in hypertensive group (open circles, n = 7) and nonhypertensive group (closed squares, n = 8) patients undergoing hemodialysis. *p<0.01 hypertensive group vs. nonhypertensive group.
The hematocrit in hypertensive group patients rose from 24.1 ± 0.5% at pretreatment by rHuEPO to 25.6 ± 0.5% at 2 weeks after treatment and to 27.9 ± 0.7% at 4 weeks after treatment (p<0.01). In nonhypertensive group patients, the hematocrit also rose from 23.4 ± 0.6% before treatment to 24.7 ± 0.9% at 2 weeks after treatment and to 27.3 ± 1.2% at 4 weeks after treatment (p<0.01). No significant difference was observed between the two groups.
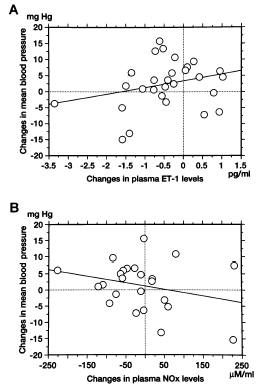
Plasma ET-1 levels were elevated in hemodialysis patients compared to that in healthy subjects (p<0.01). Plasma ET-1 levels were 3.51 ± 0.40 pg/mL in the hypertensive group and 4.09 ± 0.27 pg/mL in the nonhypertensive group. There was no significant difference in plasma ET-1 concentrations between the hypertensive and nonhypertensive groups. Administration of rHuEPO resulted in little change in plasma ET-1 concentrations in the hypertensive group. Plasma ET-1 levels at 2 and 4 weeks after rHuEPO administration were 3.24 ± 0.33 pg/mL and 3.30 ± 0.26 pg/mL, respectively. By contrast, a significant fall in plasma ET-1 levels was seen at 4 weeks after rHuEPO treatment in the nonhypertensive group. Plasma ET-1 concentration at 2 and 4 weeks after treatment was 3.90 ± 0.26 pg/mL and 2.98 ± 0.43 pg/mL, respectively (p<0.05) (). A significant relation between changes in plasma ET-1 levels and changes in mean arterial blood pressure was not observed after rHuEPO treatment (A).
Figure 2. Effects of recombinant human erythropoietin administration on plasma endothelin-1 levels in (□) hypertensive group (n = 7) and (▪) non-hypertensive group (n = 8) hemodialysis patients. Each column represents the mean ± SE. ET: endothelin.
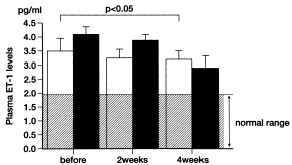
Figure 3. Effects of recombinant human erythropoietin administration on plasma nitric oxide levels in (□) hypertensive group (n = 7) and (▪) nonhypertensive group (n = 8) hemodialysis patients. Each column represents the mean ± SE. NOx: nitric oxide product.
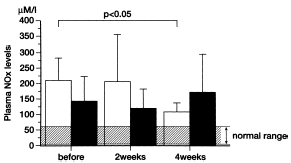
Plasma NOx levels in chronic renal failure patients on hemodialysis were significantly higher than those of healthy subjects (p<0.01). Plasma NOx concentration was 209.8 ± 33.3 µM/mL in the hypertensive group and 144.0 ± 30.4 µM/mL in the nonhypertensive group. There was no significant difference between the two groups. Administration of rHuEPO in the hypertensive patients decreased plasma NOx levels significantly to 206.0 ± 67.4 µM/mL at 2 weeks and 105.6 ± 13.7 µM/mL at 4 weeks (p<0.05, ). However, in the nonhypertensive group, serum NOx levels showed no significant changes after rHuEPO administration. Serum NOx level was 118.7 ± 23.0 µM/mL at 2 weeks and 168.4 ± 47.3 µM/mL at 4 weeks after treatment (). No significant correlation was found between changes in plasma NOx levels and changes in mean arterial blood pressure (B).
Figure 4. Relation of changes in plasma endothelin-1 (A) or plasma nitric oxide (B) to changes in mean arterial blood pressure. Neither changes in plasma endothelin-1 levels (p = 0.154) nor changes in plasma nitric oxide product levels (p = 0.21) are related significantly to changes in mean arterial blood pressure. ET-1: endothelin-1, NOx: nitric acid product.
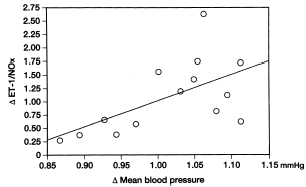
The ET-1/NOx ratio increased significantly from 0.032 ± 0.015 to 0.047 ± 0.007 in the hypertensive group at 4 weeks after rHuEPO administration (p<0.05). In the nonhypertensive group, however, ET-1/NOx ratio decreased significantly from 0.031 ± 0.006 to 0.020 ± 0.005 at 4 weeks after rHuEPO administration (p<0.05). There was a significant correlation between changes in mean arterial blood pressure and plasma ET-1/NOx ratio in hemodialysis patients treated with rHuEPO ().
Figure 5. Correlation between changes in plasma endothelin/nitric oxide product ratio and changes in mean arterial blood pressure in erythropoietin-induced hypertension. The relative change in mean arterial blood pressure after recombinant human erythropoietin administration was significantly related (p<0.05) to the relative change in plasma endothelin/nitric oxide ratio.
Pretreatment plasma cGMP concentration in hemodialysis patients was 23.5 ± 2.06 pmol/mL in the hypertensive group and 36.6 ± 6.88 pmol/mL in the nonhypertensive group; cGMP concentration in both groups was significantly higher than that in the healthy subjects (p<0.01). After rHuEPO treatment, plasma cGMP concentrations were not changed significantly either in the hypertensive group (33.5 ± 7.5 pmol/mL) or in the nonhypertensive group (27.2 ± 7.2 pmol/mL). Plasma thromboxane (TXB2) concentrations also did not change significantly either in the hypertensive group (14.6 ± 3.4 pg/mL) or in the nonhypertensive group (15.1 ± 8.2 pg/mL) with the administration of rHuEPO. Plasma 6-keto-PGF1α levels before rHuEPO administration were 6.1 ± 0.9 pg/mL in the hypertensive group patients, and 7.6 ± 2.0 pg/mL in the nonhypertensive group patients. There was no significant difference between the two groups. After administration of rHuEPO, plasma 6-keto-PGF1 α levels in the hypertensive group (8.3 ± 1.7 pg/mL) were not different significantly from those in the nonhypertensive group (8.1 ± 1.5 pg/mL).
Discussion
Although administration of rHuEPO is very effective in treating renal anemia in hemodialysis patients, hypertension is one of the most serious complications associated with rHuEPO treatment. A number of studies have been done in an attempt to elucidate the mechanism whereby administration of rHuEPO leads to hypertension in patients on hemodialysis. However, the details of the mechanism remain unclear. Some proposed mechanisms include volume expansion, enhanced tissue reninangiotensin activity,Citation[[16]] and direct vasopressor action of rHuEPO.Citation[[17]] It has been known that ET-1, NOx, and prostanoids are released from vascular endothelial cells and directly affect the contraction or dilatation of vascular smooth muscle cells.
The data for the effects of rHuEPO on ET-1 production are contradictory. Carlini et al.Citation[[18]] have shown a significant increase in plasma ET-1 in hemodialysis patients treated with intravenous rHuEPO for several months. They have also observed a significant increase in ET-1 release by cultured endothelial cells after incubation with 200 U/mL of rHuEPO.Citation[[19]] Takahashi et al.Citation[[20]] reported that plasma ET-1 concentration was increased in hemodialysis patients whose mean arterial blood pressure was raised by 10 mmHg or more after administration of rHuEPO, whereas serum ET-1 concentration tended to be lower in patients whose increase in mean arterial blood pressure was less than 10 mmHg. Bode-Boeger et al.Citation[[21]] subsequently reported that incubation with rHuEPO increased the release of ET-1 and TXB2 and decreased prostacyclin (6-kto-PGF1α) formation in human umbilical veins. However, bovine aortic endothelial cells incubated in the presence of rHuEPO were reported to show no alteration in ET-1 mRNA expression.Citation[[22]] Zhou et al.Citation[[23]] found no significant difference in plasma ET-1 concentration between uremic rats treated with rHuEPO for 6 weeks and those receiving placebo injections. Brunet et al.Citation[[24]] reported no changes in plasma ET levels by rHuEPO administration and suggested the possibility that the enhanced production of ET-1 stimulated by rHuEPO may be transient. Similarly, Hand et al.Citation[[25]] reported no significant increase in plasma ET-1 after a 12-week course of rHuEPO administration in hemodialysis patients. Lai et al.Citation[[26]] showed a significant increase in blood pressure with both intraperitoneal and subcutaneous administration of rHuEPO for 8 weeks but found no significant change in plasma ET-1 levels. In the present study, no significant increase in plasma ET-1 levels after rHuEPO administration was seen in the hypertensive group.
Nitric oxide products is an endogenous modulator with diverse biological functions. In isolated human endothelial cells, rHuEPO down-regulates endothelial NOx production and depresses endothelial NO synthase expression in isolated human endothelial cells.Citation[[27]] In normal rats, chronic rHuEPO administration increased blood pressure, hematocrit, and urinary excretion of NOx.Citation[[28]], Citation[[29]] Moreau et al.Citation[[30]] showed the important role of NOx release in opposing the action of vasopressors on blood vessel tone, which appears more important in uremic rats treated with rHuEPO. Kim et al.Citation[[31]] reported that rHuEPO did not affect plasma NOx levels and renal inducible NO synthase expression, whereas it decreased urine NOx levels in chronic renal failure rats. Our data showed no significant correlation between plasma NOx levels and mean arterial blood pressure after rHuEPO treatment in hemodialysis patients.
We observed a significant correlation between changes in plasma ET-1/NOx ratio and changes in mean arterial blood pressure in hemodialysis patients with rHuEPO treatment. Several factors affect the transcription of prepro ET-1 and NO-synthase genes in opposite directions, and nuclear factor-κB plays a key role in these regulatory mechanisms.Citation[[32]] ET-1 and NOx interplay seems to have a great relevance in the physiological regulation of vascular tone and mean arterial blood pressure. An imbalance between ET-1 and NOx systems may underlie the mechanisms involved in the pathogenesis of erythropoietin-induced hypertension.
References
- Eschbach J.W., Kelly M.R., Haley N.R., Abels R.I., Adamson J.W. Treatment of the anemia of progressive renal failure with recombinant human erythropoietin. N. Engl. J. Med. 1989; 321: 158–163
- Eschbach J.W., Egrie J.C., Downing M.R., Browne J.K., Adamson J.W. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N. Engl. J. Med. 1987; 316: 73–78
- Winearls C.G., Oliver D.O., Pippard M.J., Reid C., Downing M.R., Cotes P.M. Effect of human erythropoietin derived from recombinant DNA on the anemia of patients maintained by chronic hemodialysis. Lancet 1986; 22: 1175–1177
- Sundal E., Kaeser U. Correction of anemia of chronic renal failure with recombinant human erythropoietin: safety and efficacy of one year's treatment in a european multicenter study of 150 hemodialysis-dependent patients. Nephrol. Dial. Transplant. 1989; 4: 979–987
- Raine A.E., Roger S.D. Effects of erythropoietin on blood pressure. Am. J. Kidney Dis. 1991; 18(4 suppl. 1)76–83
- Abraham P.A., Macres M.G. Blood pressure in hemodialysis patients during amelioration of anemia with erythropoietin. J. Am. Soc. Nephrol. 1991; 2: 927–936
- London G.M., Zins B., Pannier B., Naret C., Berthelot J.M., Jacqot C., Safar M., Drueke T.B. Vascular changes in hemodialysis patients in response to recombinant human erythropoietin. Kidney Int. 1989; 36: 878–882
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332: 411–415
- Warrens A.N., Cassidy M.J., Takahashi K., Ghatei M.A., Bloom S.R. Endothelin in renal failure. Nephrol. Dial. Transplant. 1990; 5: 418–422
- Saito Y., Kazuwa N., Shirakami G., Mukoyama M., Arai H., Hosoda K., Suga S., Ogawa Y., Imura H. Endothelin in patients with chronic renal failure. J. Cardiovasc. Pharmacol. 1991; 17(suppl. 7)S437–S439
- Deray G., Carayon A., Maistre G., Benhmida M., Masson F., Barthelemy C., Petitclerc G., Jacobs C. Endothelin in chronic renal failure. Nephrol. Dial. Transplant. 1992; 7: 300–305
- Mortensen L.H., Pawloski C.M., Kanagy N.L., Fink G.D. Chronic hypertension produced by infusion of endothelin in rats. Hypertension 1990; 15: 729–733
- Anagnostou A., Lee E.S., Kessimian N., Levinson R., Steiner M. Erytheopoietin has a mitigenic and positive chemotactic effect on endothelial cells. Proc. Natl. Acad. Sci. USA 1990; 87: 5978–5982
- Vaziri N.D., Zhou X.J., Smith J., Oveisi F., Baldwin K., Purdy R.E. In vivo and in vitro pressor effects of erythropoietin in rats. Am. J. Physiol. 1995; 269: F838–F845
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and (15N) nitrate in biological fluids. Anal. Biochem. 1982; 126: 131–138
- Eggena P., Willsey P., Jamgotchian N., Truckenbrod L., Hu M.S., Barrett J.D., Eggena M.P., Clegg K., Nakhoul F., Lee D.B.N. Influence of recombinant human erythropoietin on blood pressure and tissue renin-angiotensin systems. Am. J. Physiol. 1991; 261: E642–E646
- Heidenreich S., Rahn K.H., Zidek W. Direct vasopressor effect of recombinant human erythropoietin on renal resistance vessels. Kidney Int. 1991; 39: 259–265
- Carlini R.G., Obialo C.I., Rothstein M. Intravenous erythropoietin (rHuEPO) administration increases plasma endothelin and blood pressure in hemodialysis patients. Am. J. Hypertens. 1993; 6: 103–107
- Carlini R.G., Dusso A.S., Obialo C.I., Alvarez U.M., Rothstein M. Recombinant human erythropoietin (rHuEPO) increases endothelin-1 release by endothelial cells. Kidney Int. 1993; 43: 1010–1014
- Takahashi K., Totsune K., Imai Y., Sone M., Nozuki M., Murakami O., Sekino H., Mouri T. Plasma concentrations of immunoreactive-endothelin in patients with chronic renal failure treated with recombinant human erythropoietin. Clin. Sci. 1993; 84: 47–50
- Bode-Boger S.M., Boger R.H., Kuhn M., Radermacher J., Frolich J.C. Recombinant human erythropoietin enhances vasoconstrictor tone via endothelin-1 and constrictor prostanoids. Kidney Int. 1996; 50: 1255–1261
- Lopez Ongil S.L., Saura M., Lamas S., Rodriguez Puyol M., Rodorigez Puyol D. Recombinant human erythropoietin does not regulate the expression of endothelin-1 and constitutive nitric oxide synthase in vascular endothelial cells. Exp. Nephrol. 1996; 4: 37–42
- Zhou X.J., Pandian D., Wang X.Q., Vaziri N.D. Erythropoietin-induced hypertension in rat is not mediated by alterations of plasma endothelin, vasopressin, or arial natriuretic peptide levels. J. Am. Soc. Nephrol. 1997; 8: 901–905
- Brunet P., Lorec A.M., Leonetti F., Roubicek C., Jaber K., Roux F., Berland Y. Plasma endothelin in haemodialysis patients treated with recombinant human erythropoietin. Nephrol. Dial. Transplant. 1994; 9: 650–654
- Hand M.F., Haynes W.G., Johnstone H.A., Anderton J.L., Webb D.J. Erythropoietin enhances vascular responsiveness to norepinephrine in renal failure. Kidney Int. 1995; 48: 806–813
- Lai K.N., Lui S.F., Leung J.C., Law E., Nicholls M.G. Effect of subcutaneous and intraperitoneal administration of recombinant human erythropoietin on blood pressure and vasoactive hormones in patients on continuous ambulatory peritoneal dialysis. Nephron 1991; 57: 394–400
- Wang X.Q., Vaziri N.D. Erythropoietin depresses nitric oxide synthase expression by human endothelial cells. Hypertension 1999; 33: 894–899
- Del Castillo D., Raij L., Shultz P.J., Tolin J.P. The pressor effect of recombinant human erythropoietin is not due to deceased activity of the endogenous nitric oxide system. Nephrol. Dial. Transplant. 1995; 10: 505–508
- Tsukahara H., Hiraoka M., Hori C., Hata I., Okada T., Gejyo F., Sudo M. Chronic erythropoietin treatment enhances endogenous nitric oxide production in rats. Scand. J. Clin. Lab. Invest. 1997; 57: 487–493
- Moreau C., Lariviere R., Kingma I., Grose J.H., Lebel M. Chronic nitric oxide inhibition aggravates hypertension in erythropoietin-treated renal failure rats. Clin. Exp. Hypertens. 2000; 22: 663–674
- Kim S.W., Lee J., Kang D.G., Jung K., Kim N.H., Suh S.P., Choi K.C., Kang Y.J. Erythropoietin does not affect nitric oxide system in rats with chronic renal failure. J. Korean Med. Sci. 2000; 15: 183–188
- Rossi G.P., Seccia T.M., Nussdorfer G.G. Reciprocal regulation of endothelin-1 and nitric oxide: relevance in the physiology and pathology of the cardiovascular system. Int. Rev. Cytol. 2001; 209: 241–272