Abstract
A young male presented with oral ulceration for two years; swelling face and feet of seven days duration; diffuse goiter without signs of thyroid disease; normocytic normochromic anemia, thrombocytopenia, deranged renal functions, albuminuria of 2.5 g/24 h with active urinary sediment. ANA and anti-ds DNA were positive, sonography of abdomen suggested medical renal disease. Testing for HIV, HBV, VDRL, CRP, rheumatoid factor, p-ANCA and c-ANCA were negative. Thyroid hormone assays were normal. Kidney biopsy done to stage lupus nephritis did not show any evidence of lupus involvement but staining for SAA amyloid was positive. Subsequent biopsies from the liver and rectum also stained positive for amyloid. Diagnosis of “Systemic lupus erythematosus with renal and systemic secondary amyloidosis with euthyroid diffuse goiter” was made. The case is being reported and discussed because of the interesting and rare association between amyloidosis and systemic lupus erythematosus.
Introduction
Secondary amyloidosis is a well-recognized complication of chronic inflammatory, rheumatic, and malignant diseases. However, association of amyloidosis and systemic lupus erythematosus (SLE) appears to be rare.Citation[[1]], Citation[[2]], Citation[[3]], Citation[[4]] We report here an unusual case of secondary amyloidosis as cause of renal failure in a male patient of systemic lupus erythematosus.
Case Report
A 27-year-old male presented with swelling face and feet of seven days duration. On probing, the patient did reveal a history of recurrent oral ulcerations for two years. There was no history of oliguria, hematuria, pyuria, dysuria, or renal colic; no palpitations, dyspnea, orthopnea, cough, fever, chest pain, arthralgias, rashes, photosensitivity. There was no history suggestive of any endocrine disturbance, tuberculosis, diabetes, hypertension or any other chronic illness. Family history of connective tissue disorder, amyloidosis or endocrine disorder did not exist.
Physical examination revealed significant pallor, facial puffiness, oral ulcers, pedal edema, and diffuse goitre. Pulse was 98/min regular, BP-124/78 mmHg, temperature-37.2°C. Systemic examination did not reveal any abnormality. Respiratory, cardiovascular, per-abdomen, nervous system, and ENT examination were unremarkable. There was no clinical evidence of hypo- or hyperthyroidism.
Hematological investigations revealed hemoglobin of 7 g/dL, leucocyte count 8300/mm3, P60, L37, E3; platelet count—80,000/mm3 and ESR of 40 mm at the end of 1st h. Peripheral blood smear showed normocytic normochromic erythrocytes, with sparse platelets, no hemoparasites or atypical cells. Prothrombin time was deranged (INR = 1.5). Blood urea was 85 mg/dL, serum creatinine—4.5 mg/dL, serum electrolytes–sodium 136 meq/L, potassium 3.5 meq/L, calcium 8.5 mg/dL, phosphate 5.8 mg/dL; random blood sugar was 75 mg/dL, serum total proteins −3.6 g/dL, serum albumin 1.9 g/dL, globulin 2.6 g/dL, rest of the liver functions and lipid profile were normal. No evidence of hemolytic anemia was discernible.
Urine examination revealed dipstick positive proteinuria with 4–5 erythrocytes/HPF with erythrocyte casts, hyaline casts, and cylindroid casts; suggestive of proteinuria with marked active sediment. Quantitative estimation of proteinuria revealed 2.5 g/24 h urine specimen.
Chest radiograph and electrocardiogram were normal. Ultrasonography of the thyroid revealed enlarged bilateral lobes with normal outline and echogenicity. Thyroid hormone assays were normal. Ultrasonography of the abdomen revealed size of left kidney −10.6 × 4.8 cm, right kidney—9.3 × 4.1 cm, both kidneys were normal in shape, outline and position, showed increased echogenicity with loss of corticomedullary differentiation suggestive of medical renal disease. No other abnormality was detected on abdominal sonography and a guided biopsy was done of the left kidney.
Antinuclear antibody testing was positive in a titer of 1:640, and anti-ds DNA was also positive (value = 83.8 IU/mL). ELISA testing for HIV-1 and 2, HBsAg, Rheumatoid factor, VDRL, CRP were negative. p-ANCA and c-ANCA were negative.
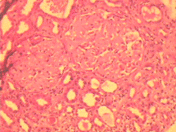
Kidney biopsy revealed eight glomeruli showing marked deposition of eosinophilic amorphous material (positive for amyloid stain) on the capillary wall and mesangium with near total replacement of normal glomerular cells. Tubular basement membrane and vessel walls also stained positive for amyloid with marked tubular atrophy (). Immunofluorescence microscopy was negative for IgA, IgG, IgM, and C3 components. Amyloid protein was positive for SAA protein on immune staining.
Figure 1. Photomicrograph of renal biopsy shows two glomeruli with deposition of eosinophilic amorphous material in the mesangium and the capillary wall. There is surrounding tubular atrophy and interstitial fibrosis (H&E X250).
Liver and rectal biopsies were done, which also stained positive for secondary amyloid deposition. Fine-needle aspiration cytology of the thyroid did not reveal any amyloid deposition.
A diagnosis of SLE with secondary systemic amyloidosis with renal amyloidosis with euthyroid goiter was made. The patient was put on salt restricted low potassium, low phosphate, protein-restricted diet and was started on conservative medical management. Colchicine was initiated at a dosage of 0.5 mg twice daily; however, patient did not tolerate it because of adverse gastrointestinal side effects.
Discussion
Systemic lupus erythematosus is a common autoimmune disorder presenting to the physician with myriad of features. It is primarily a disease of women, with a female: male ratio of 9:1. However, this sex ratio deviates from the usual female predominance in patients with amyloidosis. Approximately 40% of the reported cases of SLE complicated by secondary amyloidosis are men as is also our patient. This young adult male fulfilled the 1982 revised American Rheumatism Association criteria for diagnosis of SLECitation[[5]]; namely positive ANA, positive anti-ds DNA, thrombocytopenia, recurrent oral ulcers. In the presence of SLE and proteinuria, we had entertained a diagnosis of lupus nephritis, however, histopathological evaluation of the renal biopsy was illuminating though surprising; since no evidence of lupus nephritis was available. Overt clinical evidence of renal disease varies from 35–75% in SLE.Citation[[6]] Studies with kidney biopsy series even in absence of proteinuria or active urinary sediment have shown detectable abnormalities in almost all patients with SLE, especially if evaluated by immunofluorescence or electron microscopy.Citation[[7]] Apart from the well-defined and classified stages of lupus nephritis, unusual forms of renal involvement in SLE include—Crescentic lupus nephritis, interstitial nephritis, lupus vasculitis, glomerular thrombosis, and renal involvement in thrombotic thrombocytopenic purpura. Besides, co-existence of lupus nephritis and renal amyloidosis has been reported.Citation[[8]]
Association of SLE and amyloidosis is unique and rare. Secondary amyloidosis is not a classic complication of systemic lupus erythematosus and fewer than twenty cases have been reported in English literature to date.Citation[[9]] Most of the existing reports are in patients with long-standing SLECitation[[10]], Citation[[11]] or another well-known amyloid-associated disorder in addition to SLE.Citation[[12]]
Earlier reports of amyloidosis with SLE were in patients with long-standing SLE; however in this case, both entities were discovered at the same time, which makes this case more unusual. The general features of secondary amyloidosis complicating SLE were proteinuria with nephrotic syndrome, as in our patient.Citation[[9]] The delay between diagnosis of SLE and the onset of symptoms of secondary amyloidosis varied from 1 to 35 years.
In the reported cases, the clinical activity of SLE varied greatly, although CRP, when it was tested, was high in all cases. CRP was normal in our case, perhaps due to associated nephrotic syndrome. Steroids were not administered to our patient as corticosteroids and ACTH are known to accelerate amyloid formation.Citation[[13]] Symptomatic improvement has been observed with colchicine treatment in a case report.Citation[[14]] Our case did not tolerate colchicine due to severe gastrointestinal upset. Little, if any, information exists on the role of immunosuppressants and their efficacy in management of SLE complicated by systemic secondary amyloidosis. Successful management of SLE-associated renal and systemic amyloidosis still remains a therapeutic challenge.
References
- Huston D.P., Mc Adam K.P.W.J., Balow J.E. Amyloidosis in systemic lupus erythematosus. Am. J. Med. 1981; 70: 320–323
- Carstens P.H.B., Ogden L.L., Jr., Peak W.P. Renal amyloidosis associated with systemic lupus erythematosus. Am. J. Clin. Pathol. 1980; 74: 835–838
- Ellington K.T., Truong L., Olivero J.J. Renal amyloidosis in systemic lupus erythematosus. Am. J. Kid. Dis. 1993; 21: 676–678
- Ovellana C., Collado A., Hernandez M.V., Font J., Del Olmo J.A., Munoz Gomez J. When does amyloidosis complicate systemic lupus erythematosus?. Lupus 1995; 4: 415–417
- Tan E.M., Cohen A.S., Fries J.F. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982; 25: 1271–1277
- Hockberg M.C.S.L.E. A review of clinico-laboratory findings and immunogenetic markers in 150 patients with emphasis on demographic subsets. Medicine 1985; 64: 285–293
- Font J., Tomas A., Cervera R. Silent renal disease in SLE. Clin. Nephrol. 1987; 27: 283–288
- King R.W., Falls W.G., Jr. Renal amyloidosis: development in a case of systemic lupus erythematosus. Clin. Nephrol. 1976; 6: 497–499
- Guillaume Q., Francois B., Catherine M., Beatrice M., Francoise M. AA amyloidosis in systemic lupus erythematosus: an unusual complication. Nephrol. Dial. Transplant. 1998; 13: 1846–1848
- Terborg E.J., Janssen S., van Risswijk M.H. AA Amyloidosis associated with systemic lupus erythematosus. Rheumatol. Int. 1988; 8: 141–143
- Pettersson T., Toernroth T., Toetterman K.J. AA Amyloidosis in systemic lupus erythematosus. J. Rheumatol. 1987; 14: 835–838
- Webb S., Segura F., Cervantes F. Systemic lupus erythematosus and amyloidosis. Arthritis Rheum. 1979; 22: 554–556
- Teilum G. Cortisone-ascorbic acid interaction and pathogenesis of amyloidosis, mechanism of action of cortisone on mesenchymal tissue. Ann. Rheum. Dis. 1952; 11: 119–136
- Garcia-Tobaruela A., Gil A., Lavilla P. Hepatic amyloidosis associated with systemic lupus erythematosus. Lupus 1995; 4: 75–77