Abstract
Background. Serum albumin level is an important prognostic marker in patients with chronic renal failure. However there are discrepancies in the methods of estimation of serum albumin. The objective of this study is to evaluate the magnitude of the discrepancy in the serum albumin levels as measured by Bromcresol Green (BCG) and Bromcresol Purple (BCP) dye methods in patients on hemodialysis (HD) and peritoneal dialysis (PD) and to ascertain the clinical determinants of the discrepancy (ΔSA = BCG-BCP; g/dL) in each of the modalities. Method. We measured serum and plasma albumin levels by BCG and BCP methods in 19 adult HD patients and 18 adult PD patients treated in the dialysis units of the University of Colorado Health Sciences Center. Similar measurements were performed in 10 normal adult subjects. In all groups, paired blood samples were taken to estimate the albumin in both serum and plasma. Nephelometry (NM) was subsequently performed on the serum of 13 of the HD patients, 14 of the PD patients, and each of the 10 normal subjects. Results. We found that for both the dye methods serum and plasma albumin levels are almost identical in each of the three subject groups. In the normal subjects serum albumin estimated by BCP is in good agreement with NM values but BCG overestimates the albumin levels. In the PD group the discrepancy between the BCG and BCP (ΔSA) is statistically significant with the BCG averaging 0.59 ± 0.12 g/dL more than the BCP. The BCG values are closer to those obtained by the “gold standard”, NM. In the HD group the ΔSA is significantly (p<0.001) less than in the PD group (0.34 ± 0.11 g/dL). As for PD, BCG values are closer to NM values. Increasing age, female gender, and higher dialysis adequacy are associated with higher ΔSA in the HD but not in the PD group. Utilizing linear regression analysis we developed equations for each dialysis modality to convert albumin measurements from one method to the other. Conclusion. We confirm that a discrepancy exists between the commonly used dye methods (BCG and BCP) for serum albumin estimation. This discrepancy is significantly lower in HD patients than in PD patients. Nephrologists should be aware of this discrepancy and appropriate corrections should be made during quality improvement analysis.
Introduction
Serum albumin is considered one of the most important prognostic markers in patients with renal failure on dialysis.Citation[[1]] In both HD and PD patients on chronic maintenance dialysis hypoalbuminemia is an intermediate marker for increased morbidity and mortality.Citation[[1]], Citation[[2]] Because of this, various regulatory agencies, like CMS, consider serum albumin as a marker for quality indicator of dialysis in U.S.Citation[[3]] Even in other countries, for example in the UK, the Renal Association Standards document asserts that the serum albumin should be within the normal range quoted by the local pathology laboratory.Citation[[4]]
The gold standard method for the measurement of serum albumin is nephelometry (NM). However, NM is a costly and tedious procedure. Therefore, serum albumin measurements are commonly performed by either of two dye methods: bromcresol green (BCG) or bromcresol purple (BCP). Introduced in 1965, BCG is the method most commonly used for serum albumin determinations in US laboratories. The advantage of BCG is the rapidity with which it can be calibrated.Citation[[5]], Citation[[6]] However, due to its nonspecific protein binding and, secondly, because of the interference by fibrinogen in heparinized samples (if measured monochromatically), BCG yields results greater than NM and BCP.Citation[[7]], Citation[[8]], Citation[[9]] In normal people, the albumin levels estimated by BCP are close to NM values, because of the protein specificity of BCP.Citation[[10]], Citation[[11]] Presently, roughly one third of US labs use the BCP method while two thirds use the BCG method for estimation of serum albumin.Citation[[12]]
The discrepancy between the various methods of albumin estimation is altered in patients with renal failure as compared to normal individuals. The few studies that have compared the results in HD and PD patients show that BCP underestimates the serum albumin measurements and the results obtained by BCG are comparable to NM.Citation[[8]], Citation[[11]], Citation[[13]] The studies have obtained various magnitudes of differences between BCG and BCP measurements in patients on HD ranging from 0.5 to 0.9.Citation[[11]], Citation[[12]], Citation[[13]] There is only one previous study in which this discrepancy was examined in PD patients.Citation[[13]] The authors of this study concluded that the discrepancy in PD patients is less than that in HD patients. Our current study was performed to compare the magnitude of the discrepancy between BCG and BCP (ΔSA) in patients undergoing HD and PD and to examine which demographic and clinical factors contribute to the ΔSA in each modality.
Materials and Methods
Patient Population
Nineteen adults undergoing HD and eighteen adults undergoing PD were studied. All the patients studied were treated in the dialysis units of the University Of Colorado Health Sciences Center. Ten healthy volunteers were also studied. Baseline demographic and clinical data was collected on all patients. As seen in the groups were well matched for age, gender, race, and for the percent of patients with ESRD due to diabetes mellitus. CAPD patients received a minimum of four 2 L exchanges daily; the hemodialysis patients received a minimum of 4 h of hemodialysis three times a week using a hollow fiber (1.7 m2) polysulfone dialyzer without reuse. The mean blood flow rate was 400 mL/min.
Table 1. Clinical characteristics of the study patients. Baseline demographic data is similar for the two groups
Details of Albumin Assays
At the time of their monthly blood draws in February 2001, each subject had two additional 3 mL vacutainers drawn to obtain serum and sodium–heparin plasma samples before dialysis. The serum and plasma samples were then simultaneously assayed on the same Hitachi917 chemistry analyzer to obtain albumin concentrations by the BCG and BCP methods on each serum and plasma specimen. The reference ranges for the BCG and BCP methods in our laboratory are 3.4–4.8 g/dL and 3.0–5.0 g/dL respectively. The coefficients of variation for the two assays are 2% and 2.8% respectively. Nephelometry was subsequently performed on samples from 13 of the HD patients and 14 of the PD patients. The reference range and coefficient of variation for nephelometry in our laboratory are 3.5–5.5 g/dL and 4.9% respectively.
Statistical Methods
Patient demographics are presented as mean ± SD. For univariate analysis, Student's t test and ANOVA were used for continuous variables. The Fisher's test or Chisquare test was used for categorical variables. Correlation analysis was employed to assess the relation between different variables.
Results
Difference Between Serum and Plasma Concentrations
For both of the dye methods and in each of the three patient groups (normal, HD, and PD) the serum and plasma levels of albumin are similar (). Therefore, all subsequent determinations will be reported for serum only.
Table 2. The albumin estimations in serum and plasma are not significantly different in any of the three groups for either of the two dye methods
Differences Between Various Methods in PD Patients
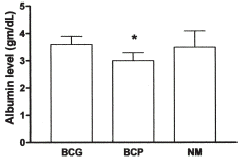
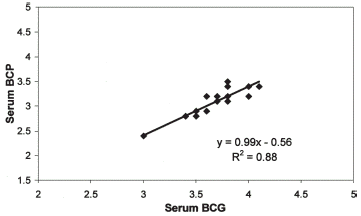
The results of albumin estimation by the three methods in the PD group are illustrated in . In PD patients the serum albumin concentration by BCP (3.02 ± 0.35 g/dL) is significantly less than the value obtained by either BCG (3.61 ± 0.33; p<0.001) or NM (3.47 ± 0.63; p<0.01). We performed linear regression to derive an equation whereby to convert BCG values to BCP. As seen in the results of serum albumin obtained by BCG can be converted to BCP using the equation BCP = 0.99*BCG−0.56.
Differences Between Various Methods in HD Patients
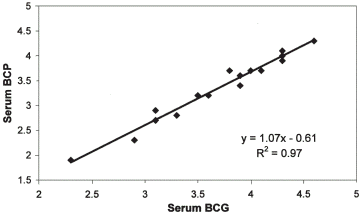
The findings in HD patients are illustrated in and . In contrast to the PD group, the results of the albumin estimations by the three methods in HD patients are not significantly different by ANOVA (BCG 3.71 ± 0.59; BCP 3.37 ± 0.64; NM 3.60 ± 0.51). As for PD, we performed linear regression analysis and derived the equation whereby to estimate albumin values obtained by BCG and convert to BCP. The equation for HD patients is BCP = 1.07*BCG−0.6.
Figure 3. Albumin estimation by various methods in patients on HD. The serum albumin is similar for each of the methods of measurement.
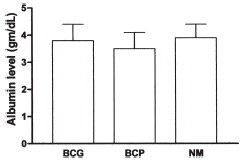
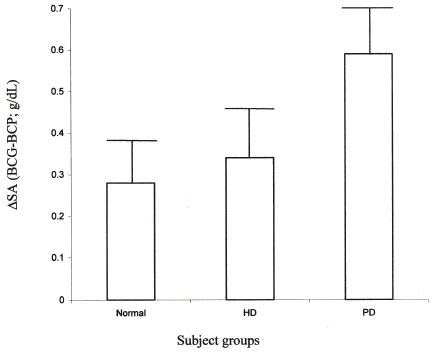
The ΔSA for the albumin measurements in the three groups of subjects studied is shown in . The ΔSA in HD patients is similar to that in normal subjects (0.34 ± 0.11 vs. 0.28 ± 0.10; p = NS). In contrast, the ΔSA in PD patients is significantly greater (0.59 ± 0.12; p<0.0001 vs. HD).
Determinants of the Discrepancy in Serum Albumin Measurements
We did a subgroup analysis in HD and PD patients to determine the demographic and clinical factors that affect ΔSA. In the HD patients ΔSA was higher in females than in males (0.42 ± 0.08 vs. 0.28 ± 0.09, p<0.05) and in patients older than 45 years compared to those younger (0.39 ± 0.09 vs. 0.27 ± 0.10, p<0.05). ΔSA in HD correlated positively with URR (r = 0.57, p<0.05) and age (r = 0.68, p<0.05). Interestingly, none of the above factors correlated with the ΔSA in the PD group.
Finally, we sought to determine whether the ΔSA is a function of serum albumin itself. This indeed proved to be the case as there was a negative correlation between ΔSA and the serum albumin measured by NM in both the PD group (r = −0.44, p<0.05) and the HD group (r = −0.29, p<0.05). Thus, in both HD and PD patients, as the level of the serum albumin in the blood decreases the ΔSA increases. This relation is stronger in the patients on PD as compared to HD.
Discussion
In normal subjects albumin estimation by BCP has been reported to be in good agreement with the estimation by nephelometry, while BCG tends to over-estimate the albumin level, especially at low albumin levels.Citation[[11]] In contrast, in patients with renal failure, albumin estimation by BCG is in good agreement with nephelometry values, while BCP tends to under-estimate the albumin level.Citation[[8]], Citation[[11]], Citation[[13]] The abnormal dye binding of BCP is postulated to be due to two reasons. First, uremic toxins, for example 3-carboxy-4-methyl-5-propyl-2-furanpropanic acid, may compete with the albumin for binding sites.Citation[[14]], Citation[[15]] Second, uremia may cause a change in the conformation of albumin itself, thereby decreasing the affinity of albumin for BCP.Citation[[8]]
The discrepancies between various methods of albumin estimation in patients with renal failure have been previously described.Citation[[8]], Citation[[11]], Citation[[13]], Citation[[16]], Citation[[17]] However, ours is the first study in which an attempt has been made to delineate the factors affecting the BCG-BCP discrepancy (ΔSA). In agreement with a previous study,Citation[[13]] we found that there is no significant difference between the serum and plasma values of albumin for either of the two-dye methods in any particular group of patients. In contrast, whereas those authors reported that the ΔSA is greater in HD patients, we found that the average ΔSA is greater in PD patients and that it is statistically significant only in PD patients. The exact reason for this phenomenon requires further investigation; most probably it is related to the difference in the clearance of uremic toxins by the specific dialysis modalities.
A very interesting finding of our study is that, for both dialysis modalities, the ΔSA is inversely proportional to the serum albumin level itself, as measured by nephelometry. The exact mechanism responsible for this phenomenon is unknown. It seems likely however, that as the serum albumin level increases the proportion of albumin bound to uremic toxins decreases thereby decreasing the magnitude of the ΔSA.
In the HD patients older age, female gender, and better dialysis adequacy are associated with a higher ΔSA. We speculate that a dialyzable toxin may preferentially bind to BCP, spuriously elevating the serum albumin measurements obtained by this method. In patients who are better dialyzed the interference by this substance will decrease causing the BCP to more closely approximate the true serum albumin and the ΔSA to increase due to the usual over-estimation of albumin by BCG. We recognize, however, that our findings regarding demographic variables are based on a univariate sub-group analysis and thus may be subject to confounding and interaction between these variables. In order to make independent associations with these variables our findings will need to be confirmed using multivariate analysis in a large group of patients.
The results of this study have important implications. Falsely high or falsely low albumin values will result in false interpretation of the patient prognosis. Inaccurate albumin measurements may also lead to inaccurate calcium measurements due to adjustments made for low serum albumin. This leads to under estimation of adjusted serum calcium with subsequent increases in vitamin D supplements and risks of metastatic calcification and, possibly, calciphylaxis. Other medical fields may be affected too, as albumin measurement may be an important part of assessment of liver disease, inflammatory bowel disease, systemic inflammation, and nutritional status.
DOQI considers a serum albumin concentration of more than 4 g/dL by BCG method as an outcome goal.Citation[[3]] As per these guidelines BCG is a better method than BCP as BCP tends to underestimate the serum albumin values by 19% when compared to BCG and NM methods. Conversion factors or exact practical guidelines were not issued to the one-third of US labs that utilize BCP.Citation[[3]], Citation[[12]] Larger studies need to be done for the estimation of conversion factor between the two dye methods. This will be immensely helpful for standardizing the results between various dialysis units. Efforts should be undertaken by CMS to have BCG as the preferred dye method to be used by all laboratories for albumin estimation in dialysis patients. Healthcare providers treating ESRD patients should be cognizant of this discrepancy in serum albumin values when reviewing serum albumin measurements in their patients with renal failure.
References
- Edmund G., Lowrie Nancy L., Lew. Death risk in hemodialysis patients: The predictive value of commonly measured variables and evaluation of death rate differences between facilities. Am. J. Kidney Dis. 1990; 15(5)458–482
- Avram M.M., Goldwasser P., Erroa M., Fein P.A. Predictors of survival in continuous ambulatory peritoneal dialysis patients: the importance of prealbumin and other nutritional metabolic markers. Am. J. Kidney Dis. 1994; 23(1)91–98
- CMS guidelines. NKF KIDOQL, update 2000
- The Renal Association/Royal College of Physicians. Treatment of Adult Patients with Renal Failure, 2nd Ed. London 1997
- Cocoran R.M., Duman S.M. Albumin determination by a modified bromcresol green method. Clini. Chem. 1977; 23: 765–766
- Webster D. The immediate reaction between bromcresol green and serum as a measure of albumin content. Clini. Chem. 1977; 23: 663–665
- Bas Leerink C., Eduard K.A. Winckers. Multilayer-film bromcresol green method for albumin measurement significantly inaccurate when albumin/Globulin Ratio is <0.8. Clin. Chem. 1991; 37: 766–768
- Wens F.E., Addison G.M., Postiethwaite R.J. Albumin analysis in serum of hemodialysis patients: discrepancies between bromcresol green and electro immunoassay. Ann. Clin. Biochem. 1985; 22: 304–309
- Hallbach J., Hoffman G.E., Gruder W.G. Overestimation of albuminin heparinized plasma. Clin. Chem. 1991; 37: 566–568
- Pinell A.E., Northam B.E. New automated dye binding method for serum albumin determination with bromcresol purple. Clini. Chem. 1978; 24: 80–86
- Maguire G.A., Price C.P. Bromcresol purple method for serum albumin gives falsely low values in with renal insufficiency. Clinica. Chimica Acta 1986; 155: 83–88
- Christopher R. Blagg, Raymond J. Leidtke, John D. Batjer, Betsy, Racoosin, Tom K. Sawyer, Martha J. Wick. Serum albumin concentration-related health care financing administration quality assurance criterion is method-dependent: revision is necessary. Am. J. Kidney Dis. 1993; 21: 138–144
- Regi Joseph, Laurel Tria, Robert T. Mossey, Alessandro G. Bellucci, Lionel U. Mailloux, Melchoire A. Vernace. Comparison of methods for measuring albumin in peritoneal dialysis and hemodialysis patients. Am. J. Kidney Dis. 1996; 27: 566–572
- Hisao Mabuchi, Hisamitsu Nakahashi. Underestimation of serum albumin by the bromcresol purple method and a major endogenous ligand in uremia. Clinica. Chemica. Acta 1987; 167: 89–96
- Hisao Mabuchi, Hisamitsu Nakahashi. Determination of 3-carboxy-4-methyl-5propyl-2-furanopropanoic acid, a major endogenous ligand substance in uremic serum, by high performance liquid chromatography with ultraviolet detection. Journal of Chromatography 1987; 415: 110–117
- Martha J. Wick, Katy Wilkens, Judy Moritz. Albumin testing methods differ: implications for the dialysis patient. Dialysis and Transplantation 1994; 23: 282–284
- Alison Carfay, Kieran Patel, Paul Whitaker, Paul Garrick, Graeme J, Grifths, Graham L. Warwick. Albumin as an out come measure in hemodialysis in patients: the effect of variation in assay method. Nephrol. Dial. Transplant. 2000; 15: 1819–1822