Abstract
Introduction. Protein-bound dihydroxyphenylalanine (PB-DOPA) and its oxidation products are formed by free radical and oxidative attack on proteins. Hemodialysis and uremic toxins can activate leukocytes leading to overproduction of reactive oxygen species such as hydrogen peroxide and hypochlorous acid (HOCl) that increases protein oxidation. Methods. We have used a sensitive fluorometric method to measure PB-DOPA and its oxidation products in proteins after γ-irradiation and incubation with HOCl and in serum from hemodialysis patients and healthy controls. These PB-DOPA concentrations were compared with those measured by HPLC (PB-DOPAHPLC). Results. Fluorescent PB-DOPA increased linearly with increasing amounts of human serum and with increasing amounts of γ-irradiated bovine serum albumin. Concentrations of fluorescent PB-DOPA paralleled PB-DOPAHPLC levels but were approximately 60–70 times higher. Incubation of BSA and human serum albumin (HSA) with HOCl (39.4 mM) significantly (P<0.0001) increased fluorescent PB-DOPA by 5 fold and 10 fold respectively and PB-DOPAHPLC by 6-fold for both proteins. Fluorescent PB-DOPA concentration increased significantly (P<0.0001) by 16-fold in human serum incubated with HOCl (39.4 mM). Mean serum fluorescent PB-DOPA was significantly (P<0.0001) higher in 19 hemodialysis patients (57.7 ± 16.1 µM) compared with 21 healthy controls (33.5 ± 3.7 µM). Mean PB-DOPAHPLC was 4.45 ± 1.63 µM in the healthy controls and 12 hemodialysis patients had values within the range of values in these controls while five patients had values that were outside eight SDs of the mean for healthy subjects. Serum fluorescent PB-DOPA was not correlated significantly with PB-DOPAHPLC in these subjects. Conclusions. The results of this study suggest that fluorophores of the type, which are derived from DOPA can be reproducibly measured in delipidated serum protein and that HOCl can increase levels of these fluorophores-generating proteins and may potentially contribute to the high levels in serum from hemodialysis patients. This high level of fluorescent PB-DOPA compounds is only partially due to authentic PB-DOPA and might also be derived from other related protein oxidation products including those from DOPA oxidation.
Introduction
Uremic patients are often in a state of chronic inflammation and increased oxidative stressCitation[[1]], Citation[[2]], Citation[[3]] and these factors are thought to contribute to their high risk of cardiovascular disease.Citation[[4]] Neutrophils and monocytes are regularly activated by interaction with the dialysis membrane, by uremic toxins and endotoxins transferred from the dialysate to the blood.Citation[[5]], Citation[[6]] Activation of these cells leads to the generation of large amounts of reactive oxygen species including superoxide, hydrogen peroxide, singlet oxygen, and the hydroxyl radical and to the secretion of myeloperoxidase. Myeloperoxidase activity and hydrogen peroxide convert the chloride ion to hypochlorous acid, a potent oxidant, and bacteriocidal compound. Hypochlorous acid oxidizes proteins leading to a decrease in protein sulfhydryl groups and an increase in protein carbonyls.Citation[[7]], Citation[[8]], Citation[[9]] In addition, HOCl oxidizes serine to glycolaldehyde, which reacts with protein to form advanced glycation end (AGE) products in vitro.Citation[[10]] Concentrations of protein carbonyls and AGE products are abnormally high and protein sulfhydryl concentrations are abnormally low in patients with chronic renal failure.Citation[[7]], Citation[[11]]
Exposure of proteins to oxidative attack generates both oxidative and reductive species that are reactive and long-lived. Protein-bound 3,4-dihydroxyphenylalanine (PB-DOPA) is a major reducing species formed when hydroxyl radicals react with protein tyrosyl residues.Citation[[12]] Oxidation of proteins with HOCl also increases PB-DOPA to a very limited extent.Citation[[13]] In addition, amino acid oxidation products including DOPA and hydroperoxides are formed in proteins during glycoxidation reactions that lead to the formation of AGE products.Citation[[14]] It is thought that reactive oxygen species formed by redox cycling of PB-DOPA or by decomposition of amino acid hydroperoxides by metal ions may contribute to the induction of oxidative stress by AGE proteins.Citation[[14]]
Protein-bound dihydroxyphenylalanine is measured by high-pressure liquid chromatography (HPLC) of acid hydrolysates.Citation[[12]] This technically demanding method is not suitable for routine clinical use. A simpler, sensitive fluorometric assay for PB-DOPA and related products of radical-mediated protein oxidation that does not require protein hydrolysis has been developed.Citation[[15]] This method detects protein-bound o-benzoquinone derived from the oxidation of o-diphenols such as DOPA, by condensing them with ethylenediamine to form fluorescent derivatives. γ-Irradiation and treatment of proteins with tyrosinase increases PB-DOPA and its oxidation products and their characteristic fluorescence following condensation with ethylenediamine.Citation[[15]] The fluorometric DOPA assay has only been applied to pure proteins and has not been used for estimating protein-DOPA fluorescence in serum proteins. The present study describes a method for the fluorometric estimation of PB-DOPA and its oxidation products in human serum proteins, tests the method on protein-DOPA generated by γ-irradiation and HOCl treatment of proteins, and compares the values with concentrations of authentic protein-bound DOPA measured by HPLC (PB-DOPAHPLC) in serum from hemodialysis patients and healthy controls.
Methods
Materials
Sodium hypochlorite solution (1 M in 0.1 M sodium hydroxide was purchased from BDH and DOPA, ethylenediamine, ethylenediamine dihydrochloride, bovine serum albumin, and human serum albumin were obtained from Sigma-Aldrich Chemical Co.
Subjects
Nineteen patients (14 men and 5 women) aged 30–71 years (mean 48 years) with chronic renal failure receiving home hemodialysis with a cellulose acetate membrane (Baxter CA 150) were recruited from the Otago Regional Nephrology Service. Patients had been receiving dialysis for 4–270 months (mean 46 months). Exclusion criteria included clinical evidence of active infection, presence of vasculitis, treatment with immunosuppressive agents or a history of malignancies excepting minor skin cancers. The etiology of renal failure included glomerulonephritis (n = 8), reflux nephropathy (n = 4), atherosclerotic renal vascular disease (n = 4), diabetic nephropathy (n = 1), analgesic nephropathy (n = 1), and interstitial nephropathy (n = 1). All patients were receiving calcium carbonate and an angiotensin converting enzyme inhibitor drug and all but one were taking 1,25-dihydroxycholecalciferol. Other drugs prescribed to the patients included erythropoietin (n = 5), lipid-lowering therapy (n = 3), warfarin (n = 2), and aspirin (n = 2). Two patients smoked cigarettes. Patients had no evidence of malnutrition and were clinically stable. Twenty-one healthy subjects (8 men and 13 women) aged 23–75 years (mean 45 years) were recruited from the staff of the University of Otago and Dunedin Hospital. Informed consent was obtained from the subjects and the Otago Ethics Committee approved the study.
Methods
Fluorometric Measurement of DOPA and Related Compounds
Serum (0.1 mL) in duplicate was delipidated with 4 mL ethanol: diethyl ether (3:1 v/v).Citation[[16]] The mixture was vortexed briefly, left for 15 min at room temperature and then centrifuged at 1500 × g for 20 min at 4°C. The supernatant was discarded and the pellet was washed with 4 mL of ethanol diethyl ether (3:1). The pellet was dissolved in 1 mL distilled water. Chelex-treated phosphate buffered saline (PBS) (1 mL) was added followed by 2 M ethylenediamine dihydrochloride (100 µL) and ethylenediamine (140 µL). Aliquots (1 mL) of free DOPA solutions (0.98, 1.96, 2.93, 3.91, and 4.89 µM) in distilled water were used as standards and were treated the same as samples of delipidated serum protein solution. A blank of 1 mL distilled water was also prepared. Fresh standards were made up each day and the stock that was used to make the standards was replaced weekly and stored at 4°C in a brown glass container. A sample blank contained 1 mL of delipidated serum protein solution (from 0.1 mL serum) plus 1 mL of PBS and 240 µL distilled water. The tubes were heated for 2 h at 50°C and the fluorescence of their contents were read at excitation 430 nm and emission 512 nm with slit-widths set at 5 nm in an Aminco-Bowman spectrophotofluorimeter.Citation[[15]] The sample blank reading was subtracted from the readings for the samples and the difference was used to calculate the concentration of DOPA and related compounds from the standard DOPA curve.
HPLC Measurement of DOPA
PB-DOPAHPLC was measured by a modification of the method of Gieseg et al.Citation[[12]] An aliquot (20 µL) of serum or other protein solutions was added to Durham tubes with 980 µL distilled water. Trichloroacetic acid (140 µL of 72% w/v) was added to the tubes and the mixture was vortexed and placed on ice for 15 min. The tubes were then centrifuged at 8250 rpm for 15 min at 4°C and the supernatant was removed. Chloroform:methanol solution (2:1 v/v) was added to the pellet, the mixture was gently vortexed, centrifuged, and the supernatant was discarded. The pellet was then washed with 1 mL of diethyl ether. After brief drying of the pellet by inverting the tubes, the protein samples were freeze dried in a Speed Vac system (1 h). The tubes were placed in Pico-tag hydrolysis reaction vessels and 50 µL mercaptoacetic acid and 1 mL 6 M HCl containing 1% (w/v) phenol were added to each reaction vessel. The vessels were de-gassed with argon before they were evacuated with the Speed Vac system. The Pico-tag vessels were maintained at 110°C for 16 h to hydrolyze the protein. The remaining acid was removed during 2 h under vacuum. An aliquot (100 µL) of 0.1% trifluoroacetyl acid was added to each Durham tube, which were then vortexed and put on ice for 5 min. These tubes were vortexed again and the contents were poured into Eppendorf tubes and centrifuged at 15,000 rpm for 5 min at 4°C. An aliquot (20 µL) of the supernatant was injected onto a Phenomenex Aqua C18 125A, 250 × 4.6 mm, 5 µm HPLC column. The amount of DOPA was quantified using a 1 µM solution of DOPA as a standard. DOPA was detected by fluorescent emission at 320 nm with an excitation wavelength of 280 nm. The column was developed with a methanol at 1 mL/min. The mobile phase A was 5% methanol (v/v) plus 0.1% trifluoroacetyl acid, pH 2.5 and the mobile phase B was 100% methanol. The gradient program was 0 to 10% mobile phase B over 10 min, then an increase to 17% B over 4 min, followed by a wash with 50% B. The CV% for the method was 15%.
Laboratory Methods
Protein was measured by the Lowry method.Citation[[17]] Serum protein thiol concentration was measured spectrophotometricallyCitation[[18]] and a blank for each sample was included.
Statistical Analysis
Values are mean ± SD. Students' t-test was used to compare mean values of normally distributed data. The Mann-Whitney U-test was used to compare PB-DOPA values that were measured by HPLC, between hemodialysis patients and healthy controls. Pearson's product-moment correlation coefficients were used to test for relationships. Data with a nonnormal distribution was log-transformed before correlation coefficients were calculated. Two-sided significance tests were used and a P value of less than 0.05 was considered to be statistically significant.
Results
The DOPA standard solutions (concentrations 0.98, 1.96, 2.93, 3.91, and 4.89 µM) gave consistent and linear curves. A representative equation is [DOPA] µmol/L = 0.15 × Reading 430 nm/512 nm−0.02, r2 = 0.996. Readings of the standards at excitation 430 nm and emission 549 nm were on average 9% lower (n = 3 experiments) compared with the corresponding readings at excitation 430 nm and emission 512 nm.
The intra-assay and inter-assay coefficients of variation for the fluorometric DOPA assay were determined in aliquots of serum from one subject stored at −80°C. Seven samples were assay on three separate days. The mean intra-assay coefficient of variation was 5.7 ± 1.8% and the inter-assay coefficient of variation was 6%.
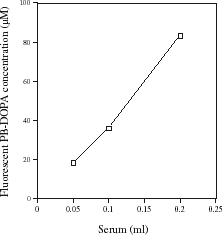
shows the effect of increasing the amount of serum and therefore serum protein on serum PB-DOPA measured by fluorometry. There was a linear increase in fluorescent PB-DOPA with increasing amounts of serum protein. When serum protein was heated with 0.1 mM borate buffer (pH 10.4) in the absence of ethylenediamine, values were not significantly different from serum blank values (data not shown).
Figure 1. The effect of the amount of serum and serum protein on serum protein-bound dihydroxyphenylalanine (PB-DOPA) concentration measured by fluorometry. The indicated volume of serum was delipidated and the precipitated, delipidated protein was redissolved in distilled water (1 mL) and assayed for PB-DOPA by fluorometry as described in the methods. Serum from one healthy individual was used in the experiment. Values are the mean of data from two experiments that differed by less than 10%.
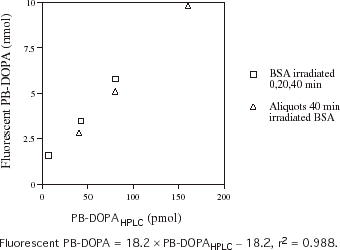
indicates the relationship between fluorometric and HPLC measurements of PB-DOPA in BSA that has been exposed to γ-irradiation for 0, 20, and 40 min and diluted BSA solution that had been irradiated for 40 min. There was a linear relationship between the amount of PB-DOPAHPLC and the amount of PB-DOPA and other moieties on proteins that react with ethylenediamine to give DOPA-like fluorescence, in the irradiated BSA samples. The amount of PB-DOPA like compounds estimated by fluorometry was 60–70 times higher than the amount of PB-DOPAHPLC.
Figure 2. The relationship between protein-bound dihydroxyphenylalanine (PB-DOPA) concentration measured by fluorescence and HPLC (PB-DOPAHPLC) in bovine serum albumin (BSA) that has been exposed to γ-irradiation. Aliquots of a solution (2 mg/mL) of bovine serum albumin (BSA) were irradiated at 7.8 gy/min for 20 min and 40 min. PB-DOPA was measured in these solutions and in nonirradiated BSA (2 mg/mL) by HPLC. Aliquots (0.2 mL) of each solution and 0.1, 0.2, and 0.4 mL of the solution that had been irradiated for 40 min were made up to 1 mL with distilled water and PBS (1 mL) was added. Fluorescent PB-DOPA was measured in these solutions and in an aliquot (0.2 mL) of nonirradiated BSA without delipidation.
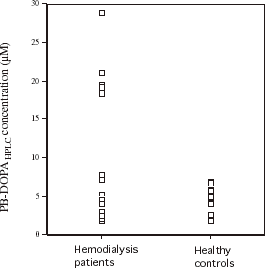
shows serum fluorometric PB-DOPA concentrations in 19 hemodialysis patients and 21 healthy subjects with a similar mean age. The mean serum DOPA concentration was significantly (P<0.0001) higher in the hemodialysis patients (57.7 ± 16.1 µM) compared with the healthy subjects (33.5 ± 3.7 µM) and only two hemodialysis patients had values within the range of values for the healthy subjects. The mean serum DOPA concentration expressed in µmol/g protein was also significantly (P<0.0001) higher in hemodialysis patients (0.61 ± 0.20 µmol/g) compared with healthy subjects (0.33 ± 0.03 µmol/g). shows authentic serum PB-DOPA concentration in these subjects, measured by HPLC. Values did not differ significantly (P = 0.58 between the hemodialysis patients and healthy subjects. The mean serum PB-DOPAHPLC in healthy subjects was 4.45 ± 1.63 µM. Serum PB-DOPAHPLC concentrations in 12 of the hemodialysis patients were within the range of values measured in the healthy subjects. Five hemodialysis patients had serum PB-DOPAHPLC concentrations that were outside eight SDs of the mean for healthy controls. Log-transformed concentrations of serum PB-DOPAHPLC concentration did not correlated significantly with the corresponding fluorometric measurement in hemodialysis patients (r = −0.023) and healthy subjects (r = −0.08). Serum protein thiol concentration was significantly (P = 0.006) lower in the hemodialysis patients (346 ± 65 µM, n = 19) compared with the healthy subjects (400 ± 55 µM, n = 21).
Figure 3. Serum fluorescent protein-bound dihydroxyphenylalanine (PB-DOPA) concentrations in 19 hemodialysis patients and 21 healthy subjects. Mean values are indicated by horizontal bars. Concentrations of serum fluorescent PB-DOPA were significantly (P<0.0001) higher in hemodialysis patients.
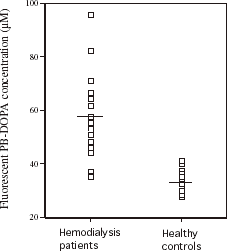
Figure 4. Serum protein-bound dihydroxyphenylalanine concentration measured by HPLC (PB-DOPAHPLC) in the 19 hemodialysis patients and 21 healthy subjects in .
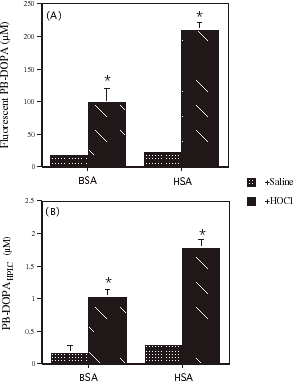
indicates the effect of incubating solutions of BSA and HSA with HOCl (39.4 mM) on fluorescent PB-DOPA concentration and PD-DOPAHPLC. The concentration of PB-DOPA and related products increased significantly and several fold in BSA and HSA during incubation with HOCl. The concentration of protein bound DOPAHPLC also increased significantly (P<0.001) and several-fold in solutions (2 mg/mL) of BSA and HSA incubated with HOCl (39.4 mM).
Figure 5. The effect of incubating bovine serum albumin (BSA) and human serum albumin (HSA) with HOCl on (A) fluorescent protein-bound dihydroxyphenylalanine (PB-DOPA) concentration and (B) PB-DOPA determined by HPLC (PB-DOPAHPLC). A solution of HOCl was prepared by diluting sodium hypochlorite solution (1 M in 0.1 M sodium hydroxide) with potassium phosphate buffer (100 mM). The absorption of the solution at 292 nm was determined and the concentration of HOCl (197 mM) was calculated using the appropriate molar absorption coefficient (ε = 350 M−1 cm−1). An aliquot (100 μL) of this HOCl solution or saline was added to 400 µL of BSA (8 mg/mL) and HSA (8 mg/mL) and the mixture was incubated for 30 min at room temperature. The final concentration of HOCl was 39.4 mM. Protein-bound DOPA concentration was measured in each solution by fluorometry. Values are mean ± SD, n = 3 separate experiments. The SD was too small to show for BSA incubated with saline. In a similar experiment, PB-DOPAHPLC was measured in solutions of BSA (2 mg/mL) and HSA (2 mg/mL) following incubation with HOCl (39.4 mM) for 30 min at room temperature. Values are mean ± SD (n = 3). * P<0.0001 compared with saline.
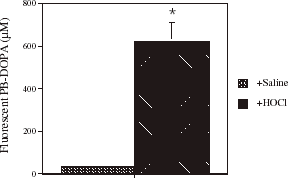
shows the effect of incubating human serum from healthy subjects with HOCl (39.4 mM) on fluorometric PB-DOPA concentration. Fluorometric PB-DOPA concentration increased significantly and 16-fold in serum incubated with HOCl.
Figure 6. The effect of incubating serum with HOCl on fluorescent serum protein-bound dihydroxyphenylalanine (PB-DOPA) concentration. Serum was obtained from a healthy individual and was incubated with HOCl (39.4 mM) as was described for BSA and HSA in . Values are mean ± SD, n = 3 separate experiments. The SD was too small to show for serum incubated with PBS. * P<0.0001 compared with saline.
Discussion
The present data indicate that fluorescent ethylenediamine derivatives of PB-DOPA and related products are reproducibly estimated in serum protein that has been delipidated with ethanol: diethyl ether. Incubation of serum with HOCl markedly increases the concentration of these compounds suggesting that HOCl generated by activated neutrophils may contribute to the abnormally high levels of fluorometric DOPA products we detected in hemodialysis patients. However, authentic serum PB-DOPA concentrations measured by HPLC are not abnormally elevated in the majority of hemodialysis patients suggesting that other DOPA-related compounds may be mainly responsible for the high levels of fluorescent compounds obtained by reacting serum protein from these patients with ethylenediamine.
Our data confirm the findings of a previous studyCitation[[15]] that the fluorescent intensity of PB-DOPA ethylenediamine derivatives of BSA parallels the linear increase in authentic PB-DOPA levels with increasing exposure of BSA to γ-irradiation. However, the absolute amount of DOPA-ethylenediamine fluorescent derivatives was 60–70 times higher than the corresponding amount of authentic PB-DOPA in the irradiated protein. The higher amount of fluorescent ethylenediamine derivatives may be due to the reaction of ethylenediamine with oxidation and addition products of PB-DOPA and differences in the fluorescent yield between free DOPA standards and PB-DOPA and related compounds in the fluorometric assay.Citation[[15]] Notwithstanding these differences in fluorescent yield, free DOPA standards provide a check on the performance of the assay. However, the units of PB-DOPA obtained using these standards are in some respects arbitrary.
The measurement of DOPA-ethylenediamine fluorophores in serum is reproducible and varies linearly with the amount of protein in the assay. Serum contains a variety of proteins and a previous study has reported that there is substantial variation in the fluorescent intensity of ethylenediamine derivatives from different proteins.Citation[[15]] Also, there are differences in the fluorescent wavelengths of DOPA-ethylenediamine derivatives among different proteins.Citation[[15]] However, Armstrong and Dean have pointed out that the widely used Lowry protein assay also has substantial variation in results for different proteins.Citation[[15]] Since albumin is the major protein in serum we have used wavelengths that are appropriate for measurement of DOPA-ethylenediamine fluorophores in albumin for the corresponding assay in serum.
Activation of circulating neutrophils and monocytes leads to increased secretion of reactive oxygen speciesCitation[[5]], Citation[[6]] and increased oxidative stress in hemodialysis patients.Citation[[1]], Citation[[2]], Citation[[3]] A number of these reactive oxygen species are capable of oxidizing proteinsCitation[[7]], Citation[[8]], Citation[[9]] and PB-DOPA may be formed as one of the products of oxidative attack on proteins.Citation[[12]] Hydroxyl radicals are released by activated neutrophils and increase PB-DOPA content in vitro.Citation[[12]] Incubation of BSA with HOCl also increases PB-DOPAHPLC.Citation[[13]] However, this increase in PB-DOPA was considerably smaller than the corresponding increase in the present study. The higher concentration of HOCl (39.3 mM vs. 0.75 mM) used to oxidize BSA may contribute to the greater increase in PB-DOPAHPLC in our data. Consistent with an increase in authentic PB-DOPA, ethylenediamine fluorophores also increased markedly following oxidation of BSA and HSA with HOCl in vitro. Thus, it is possible that increased generation of HOCl contributes to the abnormally high levels of serum PB-DOPA derived ethylenediamine fluorophores in hemodialysis patients in our data. However, the high levels of these fluorophores did not appear to be mainly due to increased levels of PB-DOPA. Serum PB-DOPAHPLC levels were not abnormally high in the majority of hemodialysis patients. Also, values from the fluorometric assay of PB-DOPA and related products were not correlated with PB-DOPAHPLC levels. It is conceivable that oxidation of PB-DOPA is increased and the products of this oxidation are detected by the fluorometric method and not by the HPLC method that measures DOPA but not its oxidation products. A previous study has indicated that oxidative attack can destroy PB-DOPA.Citation[[12]] Increased oxidative stressCitation[[1]], Citation[[2]], Citation[[3]] may accelerate oxidation of PB-DOPA as well as increasing its formation in hemodialysis patients. HOCl is a potent oxidant and may oxidize PB-DOPA to products that are detected by the fluorometric assay. HOCl oxidizes proteins resulting in a decrease in protein thiol levels and at higher concentrations an increase in protein carbonyl content.Citation[[7]], Citation[[8]], Citation[[9]] Whether this increase in protein carbonyls includes an increase in compounds that react with ethylenediamine to give DOPA fluorescence remains to be determined.
In conclusion, our data suggest that fluorophores generated by reaction of delipidated serum proteins with ethylenediamine may be a clinically useful index of serum protein oxidation. This assay may detect not only PB-DOPA but also other amino acid oxidation products. Increased production of HOCl and other reactive oxygen species may contribute to the abnormally high levels of fluorescent PB-DOPA products in serum from hemodialysis patients. Further studies of authentic PB-DOPA in hemodialysis patients are warranted to confirm the presence of abnormally high levels in some patients and to identify the factors responsible for these high levels of PB-DOPA that may increase oxidative stress and inflammatory activity.Citation[[14]]
References
- Witko-Sarsat V., Friedlander M., Capeillère-Blandin C., Nguyen-Khoa T., Nguyen A.T., Zingraff J., Jungers P., Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996; 49(5)1304–1313
- Mathur S., Devaraj S., Jialal I. Accelerated atherosclerosis, dyslipidemia, and oxidative stress in end-stage renal disease. Curr. Opin. Nephrol. Hypertens 2002; 11(2)141–147
- Miyata T., Fu M.-X., Kurokawa K., van Ypersele de Strihou C., Thorpe S.R., Baynes J.W. Autoxidation products of both carbohydrates and lipids are increased in uremic plasma: is there evidence of oxidative stress in uremia?. Kidney Int. 1998; 54(4)1290–1295
- Becker B.N., Himmelfarb J., Henrich W.L., Hakim R.M. Reassessing the cardiac risk profile in chronic hemodialysis patients: a hypothesis on the role of oxidant stress and other nontraditional cardiac risk factors. J. Am. Soc. Nephrol. 1997; 8(3)475–486
- Witko-Sarsat V., Friedlander M., Nguyen-Khoa T., Capeillère-Blandin C., Nguyen A.T., Canteloup S., Dayer J.-M., Jungers P., Drüeke T., Descamps-Latscha B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J. Immunol. 1998; 161(5)2524–2532
- Descamp-Latscha B., Herbelin A., Nguyen A.T., Roux-Lombard P., Zingraff J., Moynot A., Verger C., Dahmane D., de Groote D., Jungers P., Dayer J.M. Balance between IL-1β, TNF-α, and their specific inhibitors in chronic renal failure and maintenance dialysis: relationships with activation markers of T cells B, cells, and monocytes. J. Immunol. 1995; 154(2)882–892
- Himmelfarb J., McMonagle E., McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000; 58(6)2571–2578
- Hu M., Louie S., Cross C.E., Motchnik P., Halliwell B. Antioxidant protection against hypochlorous acid in human plasma. J. Lab. Clin. Med. 1993; 121(2)257–262
- Van der Vliet A., Hu M., O'Neill C.A., Cross C.E., Halliwell B. Interactions of human blood plasma with hydrogen peroxide and hypochlorous acid. J. Lab. Clin. Med. 1994; 124(5)701–707
- Anderson M.M., Requena J.R., Crowley J.R., Thorpe S.R., Heinecke J.W. The myeloperoxidase system of human phagocytes generates Nε-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J. Clin. Invest. 1999; 104(1)103–113
- Monnier V.M., Sell D.R., Miyata S., Nagaraj R.H., Odetti P., Lapolla A. Advanced Maillard reaction products as markers for tissue damage in diabetes and uraemia: relavance to diabetic nephropathy. Acta Diabetol. 1992; 29(3–4)130–135
- Gieseg S.P., Simpson J.A., Charlton T.S., Duncan M.W., Dean R.T. Protein-bound 3,4-dihydroxyphenylalanine is a major reductant formed during hydroxyl radical damage to proteins. Biochemistry 1993; 32(18)4780–4786
- Fu S., Davies M.J., Stocker R., Dean R.T. Evidence for roles of radicals in protein oxidation in advanced human atherosclerotic plaque. Biochem. J. 1998; 333(3)519–525
- Fu S., Fu M.-X., Baynes J.W., Thorpe S.R., Dean R.T. Presence of dopa and amino acid hydroperoxides in proteins modified with advanced glycation end products (AGEs): amino acid oxidation products as possible source of oxidative stress induced by AGE proteins. Biochem. J. 1998; 330(1)233–239
- Armstrong S.G., Dean R.T. A sensitive fluorometric assay for protein-bound DOPA and related products of radical-mediated protein oxidation. Redox Report 1995; 1(2)291–298
- Tsuchida M., Miura T., Mizutani K., Aibara K. Fluorescent substances in mouse and human sera as a parameter of in vivo lipid peroxidation. Biochim. Biophys. Acta 1985; 834(2)196–204
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951; 193(1)265–275
- Koster J.F., Biemond P., Swaak A.J.G. Intracellular and extracellular sulphydryl levels in rheumatoid arthritis. Ann. Rheum. Dis. 1986; 45(1)44–46