Abstract
The major complications of myelodysplastic syndromes are related to cytopenia and evolution to acute myeloid leukemia. Bleeding episodes in MDS, although relatively uncommon, are often related to thrombocytopenia. Bleeding may be exacerbated by platelet dysfunction, which is also found frequently. Furthermore, the major hemostatic problem underlying hyperleukocytosis, as evident in patients with MDS on blast crisis, appears to be hemorrhage rather than thrombosis. Acute thromboembolism, which causes occlusion of blood supply and organ infarction, has rarely been observed in patients with MDS. Recently, we encountered an elderly female patient, who had chronic myelomonocytic leukemia with marked myelodysplasia, terminating in blast crisis and bilateral renal infarction. This complication rapidly led to oliguric acute renal failure and mortality.
Introduction
Myelodysplastic syndrome (MDS) refers to a heterogenous group of bone marrow disorders characterized by clonal hematopoiesis, progressive bone marrow failure, and a propensity to transform into acute myeloid leukemia (AML).Citation[[1]], Citation[[2]] The major complications of MDS are related to cytopenia and the progression to AML. MDS generally presents because of one or more cytopenias, such as recurrent bacterial infections from neutropenia, easy bruising or abnormal bleeding from thrombocytopenia, or excessive tiredness from anemia.Citation[[1]], Citation[[2]]
Bleeding episodes in MDS, although relatively rare, are often frequently related to thrombocytopenia.Citation[[3]] Bleeding may be worsened by platelet dysfunction, which is also frequent.Citation[[3]] Meanwhile, acute thromboembolism, which leads to occlusion of blood supply and organ infarction, has rarely been described in patients with MDS. Recently, we encountered an elderly patient suffering chronic myelomonocytic leukemia (CMMoL) with marked myelodysplasia. CMMoL is one of the five categories included in the French-American-British classification of MDS, and is distinguished by the distinctive features of peripheral blood monocytosis, below 5% of blasts in the peripheral blood and up to 20% blasts in the bone marrow.Citation[[1]], Citation[[2]] Blast crisis and acute infarction of bilateral kidneys develop in the late stage of the disease. The above patient died despite aggressive treatment. The case of this patient has inspired us to review the relationship between MDS and renal infarction.
Case Report
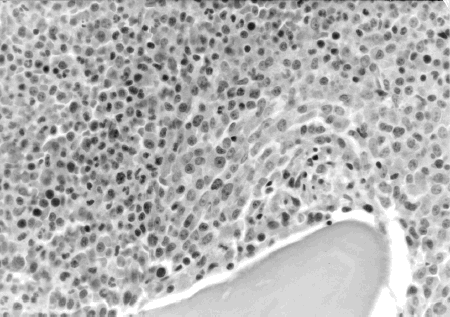
A 72-year-old Taiwanese housewife was first admitted to Chang Gung Memorial Hospital in April 1998 suffering weakness, weight loss, and fever. Initial hemogram found hemoglobin 9.3 mg/dL, hematocrit 28.7 mg/dL, and platelets 94,000/mm3. The white blood cell (WBC) count was 22,500/mm3 with 47.5% neutrophil, 0.5% myeloblast, 2.5% metamyelocyte, 26.0% lymphocyte, 0.5% eosinophil, 0.5% basophil, and 22.5% monocyte. Meanwhile, bone marrow aspiration found MDS with dysplastic changes of erythroid, myeloid, and megakaryocytic lineages, together with 11.4% of myeloblasts. A bone marrow biopsy revealed hypercellular marrow with 95–100% cellularity, and the myeloid to erythroid ratio was significantly increased (). Consequently, a diagnosis of CMMoL was established. Blood urea nitrogen (BUN) was 20.0 mg/dL, and creatinine (CRE) was 0.7 mg/dL at the moment. The patient was prescribed hydroxyurea 50 mg daily and followed regularly at our out patient clinic. The patient remained reasonably well until June 1999 when intermittent spiking fever developed. Under the impression of sepsis, the patient was admitted to Chang Gung Memorial Hospital for further assessment and management.
Figure 1. Bone marrow biopsy showed hypercellular marrow with 95–100% cellularity. The myeloid to erythroid ratio is markedly increased.
Upon admission, physical examination revealed an ill-looking woman with high fever. Body temperature was 39.6°C, blood pressure was 140/90 mm Hg, and pulse rate was 90 beats per minute. The patient was alert but pale. The spleen was palpable, three fingerbreadths below the left costal margin. The lungs were clear, heartbeat regular, and the liver was not enlarged. No edema was noted on the legs, and no obvious wounds were found.
Laboratory investigations disclosed the following values. Hemogram showed hemoglobin 7.9 mg/dL, hematocrit 24.0 mg/dL, and platelets 239,000/mm3. Meanwhile, the WBC count was 36,400/mm3 with 79.0% neutrophil, 1.5% myeloblast, 0.5% metamyelocyte, 2.0% atypical lymphocyte, 4.0% lymphocyte, 0.5% eosinophil, and 12.5% monocyte. Serum biochemical tests revealed albumin 2.6 gm/L, total protein 8.4 gm/L, aspartate aminotransferase 12 U/L, alanine aminotransferase 10 U/L, uric acid 12.1 mg/L, BUN 19.0 mg/dL, CRE 1.8 mg/dL, sodium 130.8 meq/L, potassium (K) 4.7 meq/L, calcium 8.0 mg/dL, and lactate dehydrogenase (LDH) 208 U/L. Furthermore, urinalysis showed mild proteinuria (25 mg%) without hematuria and pyuria. Renal sonography showed bilateral normal-sized kidneys with normal echogenicity. Finally, repeated bone marrow aspiration showed increased cellularity and dysplasia of erythroid, myeloid, and megakaryocytic lineages, along with a heavy infiltration of myeloblast (24.2%). Consequently, a diagnosis of CMMoL in acute blast crisis was established.
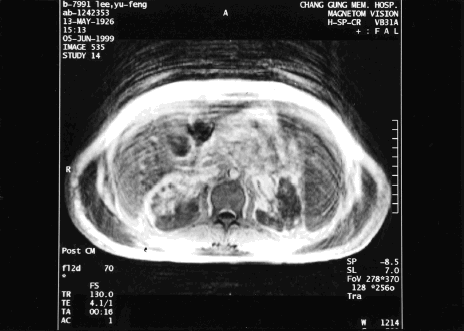
The fever persisted despite treatment with intravenous wide-spectrum antibiotics. Initially cefixime and oxacillin were used, followed by aztreonam, oxacillin, and metronidazole. All microbiological cultures were negative, including blood culture, urine culture, and sputum culture. The WBC count quickly rose to 121,000/mm3 with 63.1% neutrophil, 1.8% myeloblast, 8.0% myelocyte, 1.0% metamyelocyte, 7.8% lymphocyte, 0.5% eosinophil, 0.3% basophil, and 17.5% monocyte. Meanwhile, urine suddenly reduced (200 mL/day) when serum CRE increased to 5.6 mg/dL. Meanwhile, metabolic acidosis appeared with tachypnea and respiratory distress. Abdominal magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) were conducted to identify the renal vascular lesions. MRI and MRA suggested infarction in both kidneys. Meanwhile, the involvement of the left renal artery was more serious, and the left renal artery was completely obliterated. Partial occlusion of the right renal artery with preserved upper polar artery and capsular eatery was also found ( and ). The patient became increasingly confused. Surgical embolectomy was suggested, but the patient was too weak to undergo such invasive surgery. Arterial blood gas displayed increasingly severe metabolic acidosis, with serum bicarbonate level as low as 14.2 meq/L. Continuous veno-venous hemofiltration was performed to treat the progressive oliguric acute renal failure. However, despite these efforts the condition of the patient still deteriorated into coma, shock, and respiratory failure. The patient expired on the 9th hospital day without responding to resuscitation.
Figure 2. Magnetic resonance imaging and magnetic resonance angiography showed infarction in bilateral kidneys. The involvement of left renal artery was more severe with complete obliteration of left renal artery. Partial occlusion of right renal artery with preserved upper polar artery and capsular eatery was also found.
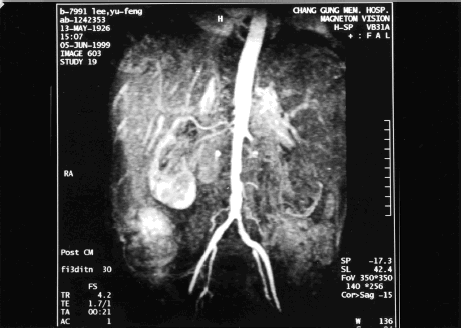
Figure 3. Magnetic resonance imaging and magnetic resonance angiography showed infarction in bilateral kidneys. The involvement of left renal artery was more severe with complete obliteration of left renal artery. Partial occlusion of right renal artery with preserved upper polar artery and capsular eatery was also found.
Discussion
The patient was diagnosed with CMMoL in April 1998, and remained relatively healthy for one year. Acute blast crisis and renal infarction developed abruptly and rapidly resulted in mortality. Hemorrhagic complications in patients with either MDS or AML have been repeatedly described in the medical literature.Citation[[4]], Citation[[5]] Bleeding in MDS is often related to thrombocytopenia.Citation[[3]] However, since MDS is a stem cell disorder, it is possible that platelet function abnormalities may also play a role in the condition.Citation[[3]] Mittlelman and Zeidman in 2000 analyzed 68 patients from the literature and concluded that platelet aggregation defects are quite common in MDS.Citation[[3]] MDS patients had impaired platelet aggregation caused by epinephrine (75%), arachidonic acid (54%), ADP (46%), collagen (43%), and ristocetin (22%). Despite the relatively high incidence of platelet dysfunction, bleeding in MDS patients is uncommon, and bleeding episodes in those patients who do bleed recurrently are generally mild.Citation[[3]]
White blood cell count rapidly rose to 121,000/mm3 with many immature myeloid cells, a phenomenon called hyperleukocytosis, during the second admission. The hyperleukocytosis that can be observed in AML may cause leukostasis, a life-threatening complication caused by leukemic cells turning to sludge and obstructing blood capillaries. Cellular hyperviscosity caused by extreme leukocyte count elevation may rapidly cause multiple organ failure and death.Citation[[6]] Pulmonary and cerebral leukostasis are well-known early complications of AML, particularly in patients with initial hyperleukocytosis and in the myelomonocytic subtypes of AML.Citation[[7]] Parenchymal hemorrhage in these organs generally results from bleeding diathesis owing to severe thrombocytopenia, disseminated intravascular coagulation, hyperfibrinolysis, or a combination of these, and is rare in their absence.Citation[[7]] Consequently, the major hemostatic problem underlying hyperleukocytosis, as seen in patients with either MDS on blast crisis or acute leukemia, appears to be hemorrhage rather than thrombosis. On the other hand, acute thromboembolism that leads to infarction of the bilateral kidneys, as occurred in the subject patient following blastic transformation, is very unusual.
A thrombotic tendency occasionally occurs in patients with acute leukemia. This complication is thought to be due to a hypercoagulable state in which thrombocytes, antihemophilic globulin, fibrinogen, and other coagulation factors are increased.Citation[[8]] Interestingly, disseminated zygomycosis in the lung and the spinal vasculature that causing transverse spinal infarction had been described in a patient with MDS.Citation[[9]] In summary, some CMMoL patients are also likely to have these coagulation complications during the acute blast crisis phase, although these coagulation factors were not measured in the subject patient.
Clinically recognized acute renal occlusion causing renal infarction is mostly caused by either arterial embolism or trauma.Citation[[10]] Treatment of the acute renal artery or segmental branch occlusion must be ultimately directed at patient survival and the preservation of renal function. Therapeutic options include: systemic anticoagulation with dialysis as necessary, intraarterial thrombolytic therapy, and surgical embolectomy.Citation[[10]] However, the above therapeutic modalities could not be applied to the subject patient owing to her weak medical condition. The subject patient deteriorated rapidly into coma, shock, and respiratory failure, and eventually expired after failing to respond to resuscitation.
References
- Dansey R. Myelodysplasia. Curr. Opin. Oncol. 2000; 12: 13–21
- Lowenthal R.M., Marsden K.A. Myelodysplastic syndromes. Int. J. Hematol. 1997; 65: 319–338
- Mittelman M., Zeidman A. Platelet function in myelodysplastic syndromes. Int. J. Hematol. 2000; 71: 95–98
- Barbui T., Cortelazzo S., Viero P., Buelli M., Bassan R. Infection and hemorrhage in elderly acute myeloblastic leukemia and primary myelodysplasia. Hematol. Oncol. 1993; 11: 15–18
- Törnebohm E., Lockner D., Paul C. A retrospective analysis of bleeding complications in 438 patients with acute leukemia during the years 1972–1991. Eur. J. Haematol. 1993; 50: 160–167
- Stucki A., Rivier A.S., Gikic M., Monai N., Schapira M., Spertini O. Endothelial cell activation by myeloblasts: molecular mechanisms of leukostasis and leukemic cell dissemination. Blood 2001; 97: 2121–2129
- Würthner J.U., Köhler G., Behringer D., Lindemann A., Mertelsmann R., Lübbert M. Leukostasis followed by hemorrhage complicating the initiation of chemorherapy in patients with acute myeloid leukemia and hyperleukocytosis. Cancer 1999; 85: 368–374
- Rosner F., Dobbs J.V., Ritz N.D., Lee S.L. Disturbance of hemostasis in acute myeloblastic leukemia. Acta Hemat. 1970; 43: 65–72
- Machida U., Kami M., Uozaki H., Makimura K., Yamaguchi H., Hirai H. Subacute spinal cord infarction due to zygomatic thrombosis in a patient with myelodysplastic syndrome. Hematologica 2000; 85: 1004–1006
- Braun D.R., Sawczuk I.S., Axelrod S.A. Idiopathic renal infarction. J. Urol. 1994; 45: 142–145