Abstract
Background: Besides possible bleeding complications a further problem in anticoagulation during continuous renal replacement therapy (CRRT) is the development of heparin‐induced thrombocytopenia type II (HIT II) where further anticoagulation with heparin is contraindicated. The application of continuous hirudin as alternative for heparin caused bleeding complications by comparable filter efficacy. Aim of this prospective‐controlled pilot study was to compare the efficacy and safety of intermittent hirudin and continuous heparin for anticoagulation during CRRT in critically ill patients. Methods: 26 patients receiving CRRT were randomly allocated to two groups: Heparin group (14 patients): continuous administration of 250 IU/h heparin, dose was adjusted in 125 IU/h steps with a targeted activated clotting time (ACT) of 180–210 s. Hirudin group (12 patients): initial bolus application of 2–2–5 µg/kg hirudin, dose was adjusted in 2 µg/kg bolus steps with a targeted ecarin clotting time (ECT) > 80 s. Observation time was 96 hours. Results: Measured filter run time was virtually longer for heparin. No bleeding complications were observed in the hirudin group, two bleeding complications in the heparin group. Conclusions: Intermittent hirudin can be used safely for anticoagulation in CRRT. However, the in tendency better filter survival for heparin elucidates the need for further investigations to find the right dosage equilibrium between filter clotting and bleeding complications.
Introduction
The incidence of Acute Renal Failure (ARF) varies in critically ill patients but it can be as high as 25%.Citation[1] Continuous renal replacement therapy (CRRT) has become the treatment of choice for ARF in the intensive care unit (ICU).Citation[2] Heparin is the most widely used substance for anticoagulation in CRRT.Citation[3] Besides possible bleeding, complications a further problem arises by the development of heparin‐induced thrombocytopenia type II (HIT II) where further anticoagulation with heparin is contraindicated.Citation[4], Citation[5] The overall incidence of HIT II has been reported to be as high as 10%.Citation[4] These and other adverse effects such as hypersensitivity reactions, skin necrosis, increase in liver enzymes and HIT II independent thrombocytopenia have led to the search for a safer and more effective anticoagulant.
Hirudin, a polypeptide made by recombinant technology with a molecular weight of 7000 Dalton, acts independently of cofactors and directly inhibits bound and unbound thrombin. Its elimination half‐life is 1–3 hours and the elimination pathway is > 90% unmetabolized through the kidneys. In the presence of renal failure, the half‐life of hirudin is considerably prolonged up to 100 times the normal half life.Citation[6] Hirudin is removed through hemofiltration membranes at a rate dependent on the sieving coefficient of the used membrane.Citation[7] In case of ARF, hirudin could persist at low levels even when it is not given anymore because of redistribution. In interaction with coagulation disorders due to other underlying diseases it could possibly lead to bleeding complications.Citation[8] As bleeding complications were mostly observed under continuous hirudin application,Citation[8], Citation[9] the aim of the present study was to evaluate the efficacy and safety of intermittent applied hirudin monitored by the ecarin clotting time (ECT) versus continuously applied heparin monitored by the activated clotting time (ACT) in a randomized, controlled, comparative trial performed in patients requiring CRRT.
Subjects and Methods
Patients
After ethical committee approval and written informed consent from a legal representative, 26 critically ill patients with ARF and indication for CRRT were enrolled in this prospective, controlled, single‐center, open‐labeled, randomized clinical trial. Exclusion criteria were age < 18 years, pregnancy, acute head injury, acute bleeding and HIT II. Enrolled patients were randomly (block randomization with a block size of 4 patients) allocated into two groups. Two patients were excluded from the study: one patient due to death in therapy‐refractory septic shock after enrollment and one patient due to hemodynamic instability with pending surgery. Therefore, 14 patients completed the study in the heparin group and 12 patients in the hirudin group. All patients were sedated, mechanically ventilated and given additional therapy according to the ICU standard protocol. ARF was defined as a urine output < 500 mL/24 hours in spite of adequate fluid resuscitation and/or an increase in creatinine (normal: < 115 µmol/L) and/or urea (normal: 2.3–7.6 mmol/L) of 2 times the normal values.Citation[10]
CRRT
A double‐lumen venous catheter (Large‐Bore catheter, 1.8 mm diameter, Arrow International, Reading PA, USA) in the jugular, subclavian or femoral vein was used for a vascular access. Continuous pump‐driven veno‐venous hemofiltration (CVVH) was performed using a Polyamide TM hemofilter (Polyflux 11 S, 1.1 m2 Gambro Dialysatoren, Hechingen, Germany) and a hemofiltration system (BM 11 + BM 14 equipment, Baxter, McGaw Park IL, USA). Pump‐driven blood flow in the extracorporeal circuit was maintained at 80–150 mL/min. Replacement solution was given by infusion after the filter (post‐dilution mode) at a rate of 1000–1500 mL/h). Anticoagulants were administered into the extracorporeal system before the hemofilter.
Groups
The heparin group received standard heparin continuously (Liquemin N™, Roche, Grenzach‐Wyhlen, Germany) with an initial dose of 250 IU/h. The anticoagulation therapy was monitored every four hours using the activated clotting time (ACT) (HemoTEG ACT, Englewood CL, USA). An ACT of 180–210 s was targeted, and subsequent heparin dose adjustments were made using steps of 125 IU/h.
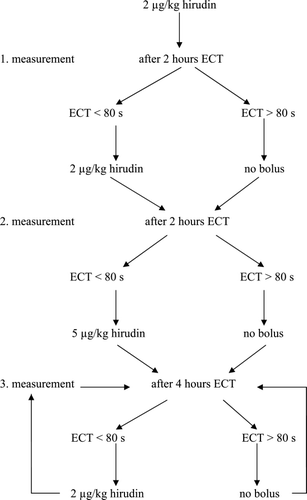
The hirudin group received hirudin bolus (Refludan™, Aventis Pharma, Bad Soden am Taunus, Germany). The extracorporeal system was rinsed with 3 liters of saline containing 100 µg of hirudin during the priming procedure. The anticoagulation therapy was monitored with the ECT (Thrombostat 2, Behnk Elektronik, Norderstedt, Germany). An ECT > 80 s was targeted. After inclusion, the patients received a hirudin bolus of 2 µg/kg. Dose management for hirudin boli is described in . The observation time was 96 hours.
ECT and ACT monitoring and dosing for the hirudin and heparin group, respectively, were chosen based on the results and discussion in our former study.Citation[8] However, the physician on duty could decide according to his clinical assumption of bleeding if the dose was adjusted or not.
Laboratory Measurements
ECT and ACT, hemoglobin (Hb) and platelet count (Technicon H3, Bayer Diagnostics, Fernwald, Germany), aPTT and PT (prothrombin time) (STA, Roche Diagnostics, Mannheim, Germany), hirudin levels (Hirudin‐Activity‐Assay, DADE Behring, Marburg, Germany, cut‐off 100 ng/mL) and blood gas analysis including electrolytes and lactate (ABL 500, Radiometer, Copenhagen, Denmark) was measured every four hours. Creatinine and urea (Hitachi 744 E, Roche Diagnostics, Mannheim, Germany) were determined on a daily basis.
Hemofiltration Efficacy
Hemofiltration efficacy regarding blood purification was determined as the comparison of the relative decrease in serum levels of creatinine and urea over the study time between the groups. The number of filter changes due to clotting (of the filter and/or clots in the circuit with the inability to further operate the circuit) was registered. Hemofiltration efficacy regarding filter patency was defined as filter run time of clotted circuits.
Bleeding Complications
A bleeding complication was defined as Hb decrease of 2 g/dL or more in the presence of estimated normovolemia and clinical signs of bleeding.
Statistical Analysis
Data were expressed as median and range. Overall values were defined as values over the whole study period. Kaplan‐Meier survival curves were plotted for comparison of filter run time in both groups and the Log Rank‐test was applied. Inter‐group statistical analysis was performed using the Mann‐Whitney‐U test, for dichotomous variables using the Pearson Chi‐Square and Fisher exact test, respectively. Intra‐group statistical analysis was performed with the Wilcoxon matched‐pairs signed rank sum test. For comparison of hemofiltration efficacy regarding blood purification longitudinal data analysis by Brunner was used. A p value of < 0.05 was considered statistically significant.
Results
Basic patient characteristics and renal function parameters did not differ between both groups (). 24 of the 26 patients were post surgery patients.
Table 1. Basic Patients' Characteristics and Indications for CRRT
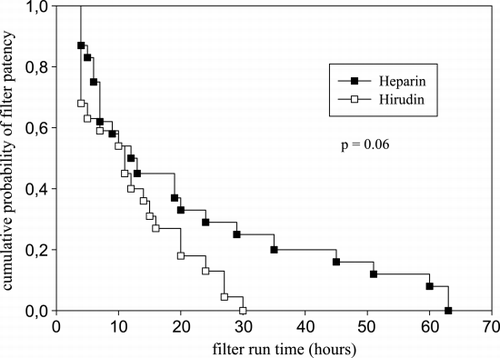
Filter run time was not significantly different between both groups (hirudin 11 (4–30) h vs. heparin 13 (4–63) h, p = 0.18). The survival curve for filter run time showed an in tendency longer survival for the heparin group (p = 0.06) ().
Figure 2. Filter run time Kaplan‐Meier survival curve. The difference in filter survival is not statistically different (Log Rank p = 0.06).
Baseline plasmatic and cellular coagulation parameters did not differ between groups (). The overall coagulation values showed a significant difference in the ECT values (p = 0.009) in the inter‐group statistical analysis (). In the hirudin group, a significant difference for ECT comparing baseline to overall values was found (p = 0.01) ().
Table 2. Basic and Overall Cellular and Plasmatic Coagulation Parameters
The dose for hirudin and hirudin plasma levels are shown in . Heparin was applied in a dose of 500 (125–1250) IU/h (8 (2–13) IU/kg/h). Hemofiltration efficacy regarding blood purification for the whole study period did not significantly differ between groups (p = 0.25 for urea; p = 0.95 for creatinine). Creatinine decrease from baseline to last value: heparin 25% (− 49%–75%) (p < 0.01), hirudin: 33% (− 18%–62%) (p = 0.01). Urea decrease from baseline to last value: heparin 37% (− 98–78%) (< 0.01), hirudin 26% (− 120%–53%) (p < 0.01).
Table 3. Dose and Hirudin Plasma Levels
No bleeding episodes were observed with hirudin. In two patients of the heparin group, bleeding complications occurred (heparin vs. hirudin, p < 0.01). shows heparin dose, units of packed red blood cells transfused, Hb levels, coagulation parameters, APACHE III and MODS score, norepinephrine requirements and lactate levels for the two heparin patients at the onset of bleeding.
Table 4. Laboratory and Clinical Parameters at Onset of Bleeding and Baseline Values in the 2 Patients with Bleeding Complcation
Outcome did not differ between both groups. The maximum APACHE III and MODS scores were comparable during ICU stay ().
Table 5. ICU Stay and Outcome
Discussion
Recently we showed that the continuous application of hirudin can be efficiently used in CRRT as far as filter patency and blood purification are concerned.Citation[8] However, significantly more bleeding complications were observed in the hirudin group.Citation[8] In this study we found that the intermittent application of hirudin was safe regarding bleeding complications and effective regarding blood purification. Filter survival in the hirudin group was virtually as effective as in the heparin group.
Filter patency, as far as comparison of filter run time is concerned, was not different between groups. However, in the Kaplan‐Meier survival curve, heparin shows in tendency a longer filter survival for the second half of the curve. The observed filter run times are in the lower range of those described in the literature for heparin and hirudin.Citation[8], Citation[9], Citation[11] One reason could be that in this study, 93% of the patients in the heparin group and 83% of the patients in the hirudin group had cardiac surgery with cardiopulmonary bypass (CPB). It has been described that CPB initiates an inflammatory response, which could hypothetically lead to pronounced filter clotting with shorter filter run times.Citation[12], Citation[13]
Comparing the second half of the filter survival curves for heparin and hirudin in this study with the former study, a marked difference with shorter survival for hirudin could be observed.Citation[8] The dose applied was lower in the present study: 46 (4–70) µg/kg/day (calculated over 96 hours observation time) as bolus administration, in comparison to our former study—96 (12–384) µg/kg/day (calculated over 96 hours observation time) as continuous administration.Citation[8] Correspondingly are the measured ECT values where the target of 80 s was not reached in the median, in comparison to our former study where the median ECT was > 80 s. The lower hirudin dosage, e.g., lower ECT levels, could probably have caused the in tendency shorter survival of the filter. However, the significant increase of ECT values in the hirudin group from baseline to overall values, shows nevertheless a marked hirudin effect. Hypofibrinogenemia and hypoprothrombinemia as other causes for ECT increase could be excluded.
We did not see any bleeding complications in the hirudin group. Kern et al. administered 1.5–9 mg/day hirudin as bolus doses and 240 µg/kg/d as continuous infusion with a targeted and achieved aPTT of 50–60 s.Citation[9] They observed severe bleeding complications in all patients in the continuous application form group and no bleeding complication during bolus management.Citation[9] Fischer administered 13–80 µg/kg/day hirudin as bolus administration and 500 µg/kg/day as continuous administration with an achieved aPTT of 44–100 s without bleeding complications.Citation[14] A reason for the missing bleeding complications in relation to the high dose given could be that all patients were medical patients with lower bleeding risk compared to surgical patients.Citation[14] In intermittent hemodialysis, some authors have seen bleeding complications and others have not, but a reduction in the dosage of the anticoagulant agent always led to cessation of bleeding events without reducing efficacy.Citation[6], Citation[15], Citation[16] A bolus administration seems to be safer regarding bleeding complications, minimizing possible accumulation.
In the present study, two bleeding complications were seen in the heparin group. However, the coagulation parameters of the heparin‐treated patients with bleeding complications showed marked thrombocytopenia in patient 2 and mild thrombocytopenia in patient 1 and high aPTT values for both patients, discording to measured ACT, at the beginning of bleeding. This underlines the problematic heparin therapy monitoring and the importance of short‐term dose adjustments according to the whole and very dynamic coagulation status of surgical patients with ARF when performing anticoagulation.Citation[8], Citation[17] Efficacy, i.e., urea and creatinine decrease over the whole study period, did not differ significantly between groups. The intra‐group decrease of urea and creatinine was significant in both groups. Successful urea and creatinine excretion was also observed in intermittent hemodialysis and in CRRT with continuous hirudin anticoagulation.Citation[8], Citation[16]
In conclusion, bolus hirudin application can be used safely for anticoagulation in CRRT in critically ill patients. However, filter survival was in tendency longer with continuous heparin than with intermittent hirudin, possibly caused by low hirudin dosage. Further studies with alternative anticoagulation regimes, e.g., combination of intermittent and continuous application, are required to find the difficult balance between bleeding and clotting complications.
References
- Guerin C., Girard R., Selli J. M., Perdrix J. P., Ayzac L. Initial versus delayed acute renal failure in the intensive care unit. A multicenter prospective epidemiological study. Rhone‐alpes area study group on acute renal failure. Am. J. Respir. Crit. Care Med. 2000; 161: 872–879, [PUBMED], [INFOTRIEVE], [CSA]
- Ronco C., Bellomo R., Homel P., Brendolan A., Dan M., Piccinni P., La Greca G. Effects of different doses in continuous veno‐venous hemofiltration on outcomes of acute renal failure: a prospective randomized trial. Lancet 2000; 356: 26–30, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kox W. J., Rohr U., Wauer H. Practical aspects of renal replacement therapy. Int. J. Artif. Organs 1996; 19(2)100–105, [PUBMED], [INFOTRIEVE], [CSA]
- Kaplan K. L., Francis C. W. Heparin‐induced thrombocytopenia. Blood Rev. 1999; 13: 1–7, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Warkentin T. E. Heparin‐induced thrombocytopenia: pathogenesis, frequency, avoidance and management. Drug Safety 1997; 17: 325–334, [PUBMED], [INFOTRIEVE], [CSA]
- Nowak G., Bucha E., Gödöck T., Thieler H., Markwardt F. Pharmacology of hirudin in renal impairment. Thromb. Res. 1992; 66: 707–715, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Frank R. D., Farber H., Stefanidis I., Lanzmich R., Kierdorf H. P. Hirudin elimination by hemofiltration: a comparative in vitro study of different membranes. Kidney Int. 1999; 56(S 72)41–45
- Vargas Hein O., von Heymann C., Lipps M., Ziemer S., Ronco C., Neumayer H. H., Morgera S., Welte M., Kox W. J., Spies C. Hirudin versus heparin for anticoagulation in continuous renal replacement therapy. Intensive Care Med. 2001; 27: 673–679, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kern H., Ziemer S., Kox W. J. Bleeding after intermittent or continuous r‐hirudin during CVVH. Intensive Care Med. 1999; 25: 1311–1314, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Vargas Hein O., Spies C., Kox W. J. Renal dysfunction in the perioperative period. Anesthesia, Pain, Intensive Care and Emergency Medicine (A.P.I.C.E.), A. Gullo. Springer Verlag, Milano 2001; 309–315
- Mehta R.L. Anticoagulation strategies for continuous renal replacement therapies: what works?. Am. J. Kidney Dis. 1996; 28(5)8–14, (S 3)
- Asimakopoulos G. Systemic inflammation and cardiac surgery: an update. Perfusion 2001; 16(5)353–360, [PUBMED], [INFOTRIEVE], [CSA]
- Schetz M. Anticoagulation in continuous renal replacement therapy. Contrib. Nephrol. 2001; 132: 283–303, [PUBMED], [INFOTRIEVE]
- Fischer K. G., Van de Loo A., Böhler J. Recombinant hirudin (lepirudin) as anticoagulant in intensive care patients treated with continuous hemodialysis. Kidney Int. 1999; 56(S 72)46–50
- Vanholder R. C., Camez A. A., Veys N. M., Soria J., Mirshahi M., Soria C., Ringoir S. Recombinant hirudin: a specific thrombin inhibiting anticoagulant for hemodialysis. Kidney Int. 1994; 45: 1754–1759, [PUBMED], [INFOTRIEVE]
- Van Wyk V., Badenhorst P. N., Luus H. G., Kotze H. F. A comparison between the use of recombinant hirudin and heparin during hemodialysis. Kidney Int. 1995; 48: 1338–1343, [PUBMED], [INFOTRIEVE]
- Murray D. J., William J., Pennell B., Kapalanski D., Weiler J. M., Olson J. Heparin detection by activated coagulation time: a comparison of the sensitivity of coagulation tests and heparin assays. J. Cardiothorac. Vasc. Anesth. 1997; 11(1)24–28, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]