Abstract
Background. Coronary artery disease (CAD) is prevalent among endstage renal failure patients and remains the major cause of mortality following renal transplantation. Death with a functioning transplant institute remains the most common cause of kidney graft failure. In this study we attempt to evaluate the effectiveness of the clinical history and current screening techniques available in predicting posttransplant CAD and also assess the role of coronary angiography as a pretransplant screening technique. Methods. Clinical data of 190 renal transplant patients was analyzed. Any clinical history of cardiac disease and all preoperative cardiac screening data was recorded for each patient. The study endpoints were the subsequent development of myocardial infarction (MI), undergoing coronary artery bypass graft (CABG) or death. Results. Factors that were significantly associated with reaching a study end‐point included: age at transplant [Hazard Ratio (HR) 1.91, P < 0.001], history of heart failure (HR 8.22, P < 0.001), presence of CAD on coronary angiography (HR 5.55, P = 0.033), anterior Q wave on electrocardiograph {ECG} (HR 8.6, P < 0.001), carotid artery disease (HR 3.74, P = 0.030) and history of a cerebrovascular accident (HR of 4.32, P = 0.008). The screening techniques of exercise stress testing and echocardiography were not conclusive as predictive variables of outcome. Conclusion. Clinical history and ECG results are good, practical and low‐cost screening methods. In our study exercise stress testing and echocardiography were found to be of limited value. Coronary angiography is appropriate in certain high‐risk groups but not necessary as part of screening in all potential renal transplant recipients.
Introduction
Coronary artery disease (CAD) is common among patients with chronic renal disease and accounts for much of the morbidity and mortality in this patient population.Citation[1] The incidence of cardiac disease is considerably higher in this group of patients than in the general patient population.Citation[2] Although the long‐term survival of kidney transplants grafts has improved considerably over the past two decades, cardiovascular disease remains prevalent and is the leading cause of death in patients who receive a kidney transplant. Cardiovascular disease is also responsible for approximately 50% of the perioperative renal transplant deaths. The detection of CAD prior to placing a potential candidate on the renal transplant pool is a major goal of all renal transplant programs. The higher incidence of CAD in renal transplant recipients is due to an accumulation of classic cardiovascular risk factors before and after renal transplantation.Citation[2] These include standard risk factors such as male gender, advanced age, presence of diabetes mellitus, hyperlipidemia, smoking, hypertension and obesity. However, kidney transplant patients also have the added cardiac risk of the immunosuppressive therapies required to prevent rejection of the graft. Many of these immunosuppressive agents cause or exacerbate classic cardiac risk factors such as hypertension and hyperlipidemia.
In this study we assess the ability to predict the risk of cardiac outcomes using well‐recognized cardiac screening techniques. We looked at the preoperative use of electrocardiogram (ECG), echocardiogram (Echo), exercise stress test and coronary angiography and assess how accurately these techniques predict post‐transplant CAD and patient mortality.
Methods
All patients who received a cadaveric kidney transplant at Beaumont Hospital Renal Transplantation Department between the start of January 1992 and the end of December 1997 were potentially included. Beaumont Hospital is the only center performing renal transplantation in the Republic of Ireland. In this study we included all patients who were followed up at this center and therefore on whom all medical records were available. Data was derived retrospectively from the Beaumont Hospital renal database system and patients hospital charts. Patients were followed until recording of first cardiac event (myocardial infarction, heart bypass surgery), time of death was censored at the end of the study, which was September 2000.
Information was recorded on each patient using a computerized database system. In each case the pre‐operative data pertaining to the patients' cardiovascular status was noted. Information was recorded as documented in the patient's medical chart. We documented any clinical history of angina, myocardial infarction (MI), congestive cardiac failure (CCF) and/or coronary artery bypass grafting (CABG). We also recorded any history of a cerebrovascular accident (CVA), the presence of carotid artery disease and whether the patient had diabetes mellitus. For each patient, the results of screening for cardiac evaluations were recorded in the patient's medical records. Each potential renal transplant recipient had some or all of the following tests performed prior to placing them on the transplant waiting pool—ECG, Echo, stress test and coronary angiogram. The general policy in our unit is that all potential renal transplant recipients have at a minimum an ECG and an Echo performed. A stress test may also be carried out at the request of the physician attending the patient. A coronary angiogram is required in all patients who are greater than 40 years of age, all diabetic patients and any other candidate who is considered to be of high cardiac risk.
An ECG was recorded as “normal” or “abnormal” as documented in the clinical report given by an independent Cardiologist. We further graded each “abnormal” ECG depending on the finding—specifically documenting the presence of anterior Q waves, inferior Q waves, left ventricular hypertrophy, ST abnormality, T wave abnormality and/or the presence of any cardiac arrhythmia. When an Echo was performed, a report of “normal” or “abnormal” was documented. In the case of abnormal Echo, the presence of left ventricular hypertrophy and the measured ejection fraction were also noted. The cardiology unit in our hospital performed standard Bruce protocol treadmill exercise tests. These treadmill exercise tests were recorded as “positive,” “negative” or “inconclusive.” A stress test was considered positive if there was ST segment depression of 2 mm or greater, an arrhythmia was induced during the study or if there was an abnormal blood pressure response to exercise. Coronary angiogram studies were also reported as “positive” or “negative.” An angiogram was considered positive if there was at least 70% stenosis in any major coronary artery. Any posttransplant cardiac events were noted up to the end of the study period. We also documented the onset of diabetes mellitus and/or hyperlipidemia (defined as a total cholesterol > 5.5 umol/L) following renal transplant surgery.
Statistical analysis assessing the risk of an adverse outcome for selected variables was performed using Cox Proportional Hazards models. Kaplan Meier survivor functions were used to plot the graphs. Results of the modeling procedure were deemed significant for p values < 0.05. All of the statistical analysis was performed using Stata (V8 Stata Corp., Texas).
Results
During the study period, a total of 190 renal transplant recipients were followed up at Beaumont Hospital. There were 120 male patients and 70 female patients. The mean age of patients overall was 43 years with a male average of 42.8 (range 16–69) years and a female average of 44.2 (range 17–70) years. In 156 patients (82%) the kidney received was the first renal transplant for that patient. The remainder were re‐transplant patients including 30 (15%) who received a second graft, 3 (1.5%) a third, and 1 (0.5%) a fourth renal graft. Median follow‐up time was 5.2 years for the 190 patients overall.
Clinical History Findings
The clinical history was attained in all 190 patients from their medical records. There were 22 patients (11.5%) with documented angina prior to undergoing transplantation and 21 (11%) had already suffered a myocardial infarction. On reviewing the medical files 13 (6.5%) patients had documented CCF. There were 9 (4.7%) patients who had a prior CVA with 6 (3.1%) who had documented carotid artery disease. There were 25 (13%) patients who were diabetic at or prior to screening.
ECG Findings
All 190 patients had an ECG performed prior to renal transplantation. This was abnormal in 78 (41%) of cases—54 had left ventricular hypertrophy, 7 had anterior Q waves, 15 had inferior Q waves, 10 had ST abnormalities, 15 had T wave abnormalities and 13 had baseline arrhythmias.
Echocardiogram Findings
A total of 59 patients (31%) had an Echo performed, 35 of which had left ventricular hypertrophy and 13 of these also had an ejection fraction documented at less than 40%. Only 24 of the 59 patients who had an Echo test performed fitted the criteria for normal.
Treadmill Exercise Stress Test Results
An exercise stress test was performed in 47 patients (25%)—with 27 normal tests, 9 abnormal and 11 inconclusive.
Coronary Angiography Results
A total of 43 patients (23%) had a coronary angiogram performed pretransplant of which 24 were reported as “negative.” Following the angiogram study (which was positive in 19 patients) 8 patients required CABG and 2 required coronary angioplasty and stenting prior to going on the renal transplant waiting list. The remaining 9 patients with documented coronary artery disease on angiography were placed on the pool but were documented to be at higher than standard risk.
End Points Reached During Study Period
A total of 16 of 190 patients had an MI during the follow‐up period. Of these 16 patients, 11 had a prior documented history of CAD (MI or angina), 11 also had baseline ECG abnormalities, and 9 had Echo studies which were reported as abnormal in the pre‐transplant screening. Only 8 of these patients had a coronary angiogram as part of their screening tests of which 6 were positive and 2 were negative. During our follow‐up period, 4 of 190 patients required coronary bypass graft surgery. In 3 of these patients the presentation of IHD was posttransplantation and only 1 had a pretransplant record of heart disease. Interestingly, all 3 of these patients had normal screening ECG tests.
There were 20 patient deaths recorded during the study period. The cause of death as recorded in the patient death certificate was cardiac in 16 (80%) of these. Only 5 of these patients had a documented cardiac history prior to transplantation (3 of which had positive coronary angiogram studies but did not receive any intervention). In 12 of these 20 deaths (60% of cases), the baseline screening ECG was documented as abnormal.
Cox univariate proportional hazards tests were used to obtain outcome results for the primary study endpoints MI, CABG and death (see ).
Table 1. Univariate Cox Proportional Hazard Models
Variables that were highly significant in predicting adverse outcomes were a history of heart failure (P < 0.001), CVA (P = 0.008), age of recipient (P < 0.001) and time spent on dialysis (P = 0.010).
The risk for a negative outcome in patients with abnormal ECG (positive for anterior or inferior Q wave or ST changes) was found to be at 2.65 risk (P = 0.009) and the risk is even more highly significant (P < 0.001), if the ECG abnormality was anterior Q wave. illustrates the relative negative outcome for those who had an abnormal anterior Q wave result pretransplant.
Figure 1. Kaplan–Meier survivor functions illustrating effect of abnormal ECG anterior Q result on outcome CABG, MI and death.
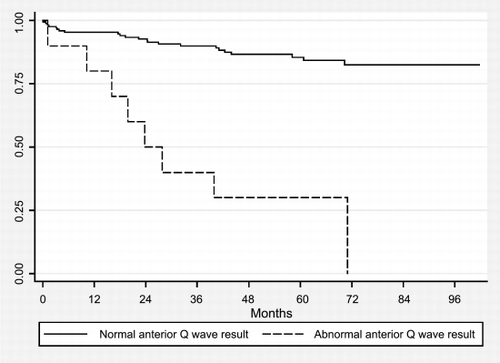
Stress tests were not found to be useful in predicting adverse outcome. Echo results were similarly inconclusive. Coronary angiography was only performed on selected patients (25%) who were considered to be at high‐risk and we found that patients with an abnormal angiogram have a 5.5 times (P = 0.033) greater risk of negative outcome in this patient group.
Pretransplant diabetes, while indicating a hazard ratio of twice that of other ESRD patients, was not found to be significant at the 5% level (P = 0.098). There were 6 new cases of new onset diabetes mellitus following renal transplantation and hyperlipidemia was documented in 73.5% of our renal transplant recipients postoperatively.
We subsequently performed a multifactorial model to test for independence of effect at the 5% level of significance. This model presented in shows variables that remain independently significant in the presence of other covariates. An interpretation of the model would be that an older person with a history of a cerebrovascular accident and with an abnormal finding on the ECG of an anterior Q wave would be of particular high risk of an adverse coronary event posttransplant.
Table 2. Multifactorial Cox Proportional Hazards Model with Independently Significant Variables
Discussion
The death rate of renal transplant recipients is recognized to be approximately 60 per 1000 patients' years.Citation[3] The 5‐year survival rate in our study group over this time period was 76% compared with data from the UNOS Renal Transplant Registry where the equivalent 5‐year survival for cadaver grafts was 82%.Citation[4] Over the past 2 decades the mortality associated with renal transplantation has decreased significantly due in part to better immunosuppressive regimes and also to a reduction in the incidence of infection‐related deaths. During this time however, there has been an increase in the proportion of cardiovascular disease‐related deaths.Citation[5] It is now accepted that 50–60% of renal transplant recipient deaths are directly attributable to CAD.Citation[6] This has led to an increased awareness for the need for screening for CAD prior to renal transplantation. The aim is to reduce posttransplant mortality in this group of patients. However, there are currently no absolute recommendations for precise screening tests that should be employed and in general these investigations are at the discretion of the attending transplant physician and transplant surgeon.
A detailed clinical history is vital in the cardiac risk assessment and a history of angina, MI, CVA, heart failure and carotid artery disease all significantly predicted an adverse outcome in our patients. A patient with a positive history of one of the above risk factors ought to be transferred to the high‐risk category for invasive screening.
An ECG is one of the basic routine screening tests performed on virtually all patients admitted to renal transplant programs. It is an efficient, cost‐effective and easily interpretable method of screening. In our study an abnormal ECG was independently predictive of a cardiac event. Left ventricular hypertrophy (LVH) and the measured ejection fraction were not accurate in prediction the survival outcome. LVH is a recognized risk factor for the development of CAD and the prevalence of a symptomatic LVH in patients with end‐stage renal disease (ESRD) is high at 50–75%. This is considerably higher than in the general population and varies directly with the level of blood pressure and inversely with the level of renal function and serum hematocrit.Citation[7], Citation[8]
The exercise stress test was also inconclusive in predicting a posttransplant cardiac event. This may be explained by the fact that not all the patients underwent a stress test and also that a stress test was not diagnostic in many cases because of the failure of patients to reach to the maximal heart rate due to advance age, poor mobility, anemia related to renal failure and fatigue. A study by Parfrey et al., which was aimed at evaluating the stress test as a screening tool for CAD, found that the sensitivity and specificity of stress testing is limited by the frequent inability to exercise to target heart rate.Citation[9] This group also compared the results of exercise stress testing versus Dobutamine Stress Echocardiogram (DSE) and reported the latter to be of higher sensitivity and specificity.Citation[9], Citation[10]
Coronary angiography has a definitive role as a screening test but is not routinely performed on all potential renal transplant recipients. Our current protocol is that we require coronary angiography studies on all patients aged greater than 40 years, all diabetic patients and any patient who is considered to be at particularly high risk for CAD. This would include patients who have any history suggestive of cardiovascular disease, hypertension or other conditions, which may place the potential candidate at a higher risk. While the side effects of coronary catheterization are well recognized, such as nephrotoxicity from the contrast material used, the risk appears to outweigh the benefit in terms of establishing cardiac risk preoperatively.
The incidence of silent ischemia in renal failure patients is high, particularly in diabetic patients.Citation[1] A randomized trial in diabetic transplant candidates showed that a strategy of routine coronary angiography screening and appropriate intervention (CABG or angioplasty) prior to transplantation significantly reduced the risk of acute MI and cardiac death compared to medical therapy.Citation[11] It is unclear however, whether screening would improve survival in lower‐risk patients. At our center we routinely perform coronary angiography in all our diabetic patients prior to placing them on the transplant waiting list.
Conclusion
Clinical history of cardiovascular disease and ECG testing are practical and low‐cost screening methods. They also allow the transferring of those patients with positive findings to a high‐risk group who warrant further invasive screening. In our study exercise stress test and echo were of limited value. Coronary angiography is necessary in certain high‐risk groups but not warranted for all potential transplant recipients.
References
- Murphy S. W., Foley R. N., Parfrey P. S. Screening and treatment for cardiovascular disease in patients with chronic renal disease. Am. J. Kidney Dis. November, 1998; 32(5, Suppl. 3)S184–S199, [PUBMED], [INFOTRIEVE], [CSA]
- Lewis M. S., Wilson R. A., Walker K., Stegeman‐Olsen J., Noreman D. J., Barry J. M., Bennett W. M. Factors in cardiac risk stratification of candidates for renal transplant. J. Cardiovasc. Risk Aug., 1999; 6(4)251–255, [PUBMED], [INFOTRIEVE], [CSA]
- Harper A. M., Rosendale J. D. The UNOS OPTN waiting list and donor registry. Clinical Transplantation 1996, P. I. Terasaki. UCLA Tissue Typing Laboratory, Los Angeles 1997; 69
- Cecka J. M. The UNOS scientific renal transplant registry. Clin. Transpl. 1998; 1–16, [CSA]
- Hill M. N., Grossman R. A., Feldman H. I., Hurwitz S., Dafoe D. C. Changes in causes of death after renal transplantation 1996–1987. Am. J. Kidney Dis. 1991; 17: 512, [PUBMED], [INFOTRIEVE]
- Lindholm A., Albrechtsen D., Frodin, Tufveson G., Persson N. H., Lundgren G. Ischaemic heart disease—major cause of death and graft loss after transplantation in Scandinavia. Transplantation 1995; 60: 451, [PUBMED], [INFOTRIEVE], [CSA]
- Parfery P. S., Foley R. N., Harnett J. D., Kent G. M., Murray D. C., Barre P. E. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol. Dial. Transplant. 1996; 11: 1277–1285, [CSA]
- Foley R. N., Parfery P. S., Harnett J. D., Kent G. M., Martin C. J., Murray D. C., Barre P. E. Clinical and echocardiographic disease in patients starting end stage renal disease therapy: prevalence, association and prognosis. Kidney Int. 1995; 47: 186–192, [PUBMED], [INFOTRIEVE]
- Murphy S. W., Parfrey P. S. Screening for cardiovascular disease in dialysis patients. Curr. Opin. Nephrol. Hypertens. 1996; 5: 532–540, [PUBMED], [INFOTRIEVE], [CSA]
- Manske C. L., Wang Y., Rector T., Wilson R. F., White C. W. Coronary revascularization in insulin‐dependent diabetic patients with chronic renal failure. Lancet 1992; 340: 998–1002, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kasiske B. L., Ramos E. L., Gaston R. S., Bia M. J., Danovitch G. M., Bowen P. A., Lundin P. A., Murphy K. J. The evaluation of renal transplant candidates: clinical practice guidelines. J. Am. Soc. Nephrol. 1995; 6: 1–34, [PUBMED], [INFOTRIEVE], [CSA]