Abstract
Background. Ochratoxin A (OTA) is a nephrotoxic metabolite occurring in foodstuffs. In the last decade, OTA‐induced nephropathy in man and animals have been confirmed by previous literature. The correlation between OTA and the severity of CRI and nephrotic syndrome was also researched. Therefore, this study was designed to determine whether OTA also played an important role in renal insufficiency of patients with chronic renal diseases in Taiwan. Methods. The patients in this study were divided into nonnephrotic syndrome and nephrotic syndrome groups, first, to look for the relation between urine protein and OTA. And then these patients were also divided into six groups: (I) patients with chronic glomerulonephritis; (II) patients with chronic interstitial nephritis; (III) patients with diabetes mellitus; (IV) patients with hypertension; (V) patients with other diseases; (VI) patients with unknown reasons. For all groups, laboratory evaluation of kidney such as serum creatinine, urinary creatinine, creatinine clear rate, urinary protein, and urinary analysis were carried out coupled with determination of ochratoxin A level in urine. Results. Higher levels of OTA were found in patients with nephrotic syndrome. There was a significantly positive correlation (P < 0.001) between 24‐hr OTA and 24‐hr urine protein. On the other hand, the mean excretion of OTA in DM group (group III) was found significantly higher compared to the other groups (P < 0.05). Distinct differences (P < 0.01) were found especially when DM group was compared with patients with chronic glomerulonephritis (group I; P = 0.0019), patients with chronic interstitial nephritis (group II; P = 0.0032) and patients with hypertension (group IV; P = 0.0062). Conclusion. The results could lead to the conclusion that OTA could play an important role in proteinuria of patients with chronic renal diseases in Taiwan. And OTA may play a role in diabetes patients with nephropathy. Further longitudinal study is needed to clarify the role of OTA in diabetic nephropathy.
Introduction
Ochratoxins (OT) are secondary metabolites of Aspergillus and Penicillium strains, found on cereals, coffee and bread, as well as on all kinds of food commodities of animal origin in many countries.Citation[1], Citation[2] The most frequent is ochratoxin A (OTA), which is also the most toxic. It has been shown to be nephrotoxic, immunosuppressive, carcinogenic and teratogenic in all experimental animals tested so far.Citation[3] Ochratoxin A, this toxic substance produced by several species of Aspergillus, especially by Aspergillus ochraceus and Penicillium verrucosum, is nephrotoxic in pigs. This raises the possibility that OTA is also nephrotoxic in man.Citation[4] Short‐term and considerable quantities of inhalation or ingestion of OTA will cause acute renal failure.Citation[5] The pathological appearances of the kidney in many experimental animals were well analyzed by Krogh.Citation[6] Under the microscope the initial lesions are confined to the proximal tubules hyaline deposits in the free space of Bowman's capsule, atrophy of the glomeruli and in advanced stage interstitial cortical fibrosis and glomerular sclerosis.
In the last decade, OTA induced nephropathy in man and animals have been confirmed by previous literature.Citation[2], Citation[7] The occurrence of OTA in human sera from individuals with and without nephropathy has been studied in Canada, Tunisia, Algeria and Italy, but different results were reported.Citation[8] OTA contamination of grain, herb drugs and coffee was found recently.Citation[9] Hence, we performed this study to determine whether OTA also played an important role in renal insufficiency of patients with chronic renal diseases in Taiwan.
Materials and Methods
Selection of Patients
Ninety patients with abnormal renal function [serum creatinine level > 123.76 µmol/L (1.4 mg/dL)] who had been followed up for at least 1 year in the out patient department of our institution were examined for possible inclusion in our study. The age range of these patients is 20–80 years old.Citation[10]
Patients with other chronic diseases (e.g., diabetes mellitus, SLE, hypertension), which could cause nephropathy, were also included. However, patients with potential reversible renal insufficiency (for example, patients with malignant hypertension, urinary tract infection, hypercalcemia, drug‐induced nephrotoxicity), or patients with previous heavy OTA exposure were excluded.
For each patient, data was collected clarifying dietary habits as well as the clinical, biological, histopathological and radiological parameters denoting clinical and renal state. The data also comprised details of age, sex, height, body weight, and body surface area.Citation[11]
According to concentrations of protein in their urine and clinical symptoms, these patients were divided into nonnephrotic syndrome and nephrotic syndrome groups. Serum creatinine, urinary creatinine, urinary protein, and urinary analysis were performed just before OPD urine sampling for OTA quantification. Finally, we compare the mean of the variability and see if there is significantly statistic variability between nephrotic syndrome (24‐hr urine protein) and OTA. In this way, we can prove if OTA plays an important role in progression of renal insufficiency of patients suffered from renal diseases.
On the other hand, all patients were divided into six groups: (I) patients with chronic glomerulonephritis; (II) patients with chronic interstitial nephritis; (III) patients with diabetes mellitus; (IV) patients with hypertension; (V) patients with other diseases; (VI) patients with unknown reasons. Patients' characteristics were summarized in . We compared the mean excretions of 24‐hr OTA in theses groups, and looked for difference between groups.
Table 1. Patients' Characteristics and Concentrations of Ochratoxin A (OTA) in Human 24‐hr Urine from All Patients Groups.Footnotea
Determination of OTA
Sample and standard preparation: To 500 µl of urine or standard, 750 µl methanol, 125 µl ddH2O, 125 µl 10% NaOH were added and then filtrated through 0.22‐µm Millipore membrane. The OTA concentration was determined by HPLC.
HPLC analysis: urine and the standard were analyzed by HPLC using the procedures described by Li et al.Citation[12] The HPLC system included a Varian 210 Prostar pump, a Varian 363 fluorescence detector, a Varian 410 sample autoinjector, and a Varian C‐18 5‐µm column (Walnut Creek, USA).
Liquid chromatography conditions: The mobile phase was a gradient consisting of solvents A (distilled water acidified to pH 2.48 with H3PO4), and B (methanol–isopranol, 9:1, v/v) set at a flow rate of 0.6 ml/min at 40°C. The system was programmed to deliver an isocratic mixture containing 28% of B from 0 to 5 min, 28% to 45% of B at an incremental increase of 2.4%/min (5–12 min), 45% to 75% of B at an incremental increase of 3%/min (12–22 min), 75% to 90% of B at an incremental increase of 15%/min (22–23 min), 90% to 28% of B at an incremental decrease of 7.75%/min (23–31 min) for washing of the column and then equilibration with 28% of B and 72%of A for 8 min prior to the next analysis. Injection volume: 30 µl for urine sample and 10 µl for the standard; the compounds were detected using a Varian 363 fluorescence detector (excitation at 333 nm and emission at 450 nm). Analytical data were collected, stored and treated using the software Star 5.51 (Varian, USA). The quantification of OTA was achieved automatically by the computer according to the peak surface of one OTA standard (0.4 × 10− 3 mg/10 µl) injected sequentially with ochratoxin‐free methanol.
Biostatic Analysis
We compared quantity of OTA in the patient group with nephrotic syndrome and the patient group without nephrotic syndrome. The Student t‐test, chi‐square tests and ANOVA were used to measure differences between groups. The simple linear regression was used to see relation between urine protein and OTA. And the multiple linear regression analysis was used to adjust correlation between multiple variables and daily urinary protein excretion.
Results
Underlying Diseases vs. 24‐hr OTA
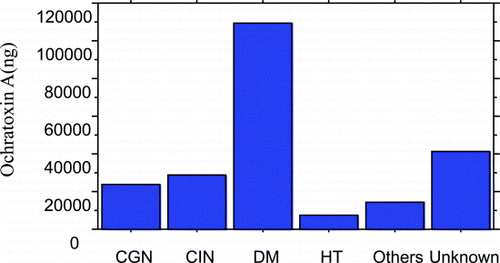
The mean excretions of urine 24‐hr OTA in the patients groups are presented in . The mean excretion of the toxin in DM group was found to be significantly higher compared to the other groups (P < 0.05) (; ). Distinct differences (P < 0.01) were found especially when DM group was compared with patients with chronic glomerulonephritis (P = 0.0019), patients with chronic interstitial nephritis (P = 0.0032) and patients with hypertension (P = 0.0062).
Table 2. Comparison of the Mean Ochratoxin A (OTA) Levels in Patient Group.Footnotea
Figure 1. Daily ochratoxin A excretion among different study groups. (View this art in color at www.dekker.com.)
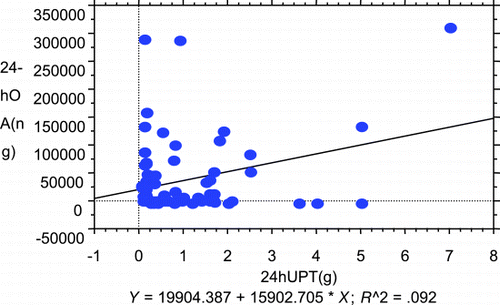
24‐hr OTA vs. 24‐hr UPT
According to , the excretions (ng) of 24‐hr OTA rose when 24‐hr urine protein (g) increased. There was a significantly positive correlation (Y = 19904.387 + 15902.705*X) between 24‐hr OTA and 24‐hr urine protein.
Figure 2. The correlation between daily ochratoxin excretion (ng) and daily urinary protein excretion (g) (24UPT) assessed by simple linear regression analysis (r = 0.303; P = 0.006, N = 85). (View this art in color at www.dekker.com.)
24‐hr UPT vs. 10 Factors Which May Influence 24‐hr UPT
There was a negative correlation between corrected Ccr and 24‐hr UPT (g) (). Significant correlation (P < 0.0001) showed higher 24‐hr UPT would cause lower Ccr. On the other hand, positive correlations were found when 24‐hr UPT was compared to BMI and daily OTA. The correlation with daily OTA (µg) was also powerful (P = 0.0009). For BMI, there was a significant but mild, weaker correlation (P = 0.0191) than Ccr and daily OTA. As for the other independent variables, no meaningful correlation was found.
Table 3. Multiple Linear Regression Analysis Coefficients: Daily Urinary Protein Excretion (24‐hr UPT) (g) vs. 10 Independent Variables
Discussion
In recent years, more and more studies have placed importance on ochratoxin A (OTA) due to its carcinogenic, immunosuppressiveCitation[13] and especially nephrotoxic potentials.Citation[4], Citation[5], Citation[8], Citation[11], Citation[14] Nephrotoxicity on experimental animals has been proven for a long time. However, such proof is still lacking in man. On the other hand, OTA has been identified in a number of animal feeds and foods, and seems to relate to climatic conditions, to methods of food preservation, and principal food in an area.Citation[15] And even large amount of OTA was also found in coffee and herb drugs. Therefore, we wanted to find out the correlation between OTA and nephropathy in Taiwan.
In many reported cases of nephropathy with undetermined etiology, such as cases of Balkan Endemic Nephropathy,Citation[16] and chronic interstitial nephritis in Tunisia,Citation[11] OTA had been suspected to be responsible for nephropathy. By comparing OTA concentration in serum, urine or tissues in different groups, Wafa E.W. et al.Citation[14] found that OTA could be correlated to the genesis of renal disease leading to ESRD or causing urothelial cancer.
In this study, we wanted to find the correlation of daily OTA excretion in urine and the amount of daily urine protein because the severity of proteinuria may exacerbate the progression of renal insufficiency.Citation[17] The statically significant correlation (P = 0.006 < 0.05) between 24‐hr urine protein and 24‐hr OTA revealed that the daily exposure of OTA increased when the amount of urine protein was high. It was difficult to decide whether OTA exacerbated proteinuria or proteinuria increased OTA secretion. Significant rise of proteinuria was also observed in the CIN‐OTA‐positive population.Citation[11] Causes of proteinuria may be glomerular wall defect or reabsorptive disorder of proximal tubule.Citation[18] In some experimental animals, OTA was found to produce degeneration of the proximal portion of renal tubules, hyaline deposits in the free space of Bowman's capsule, atrophy of the glomeruli and in advanced stages interstitial cortical fibrosis and glomerular sclerosis.Citation[6] Besides, renal biopsy of the patient who was suspected to inhale OTA, performed to verify acute tubular necrosis and minimal change glomerular lesion, showed basophilic degeneration of tubular epithelium with tubule–cyst appearance and fusion of podocyte pedicles.Citation[5] Therefore, it is probable that OTA‐aggravated proteinuria. In addition, Hagelberg et al.Citation[19] suggested that OTA excretion in humans, like the monkey, may rely on filtration for elimination of OTA. Although the patients with nephrotic syndrome have high levels of OTA in serum and urine in the previous literature,Citation[14] however, we first demonstrated a raised daily excretion of OTA related to an increased excretion of protein in urine. Our results also implied severe OTA exposure may lead to increase excretion of urine protein.
In the other part of our study, the excretions of OTA in DM group were significantly higher than other groups. The clinical implications of this finding are not clear. Whether OTA plays a role in diabetes patients with nephropathy is still unknown. Further longitudinal study is needed to clarify the role of OTA in diabetic nephropathy.
There was no statically significant correlation between OTA and severity of chronic renal insufficiency (CRI). The result differed from previous studiesCitation[8], Citation[11], Citation[14] about OTA nephropathy. In these studies,Citation[8], Citation[11], Citation[14] serum samples were used to detect concentrations of OTA. However, urine samples were used in the present study, so we just got amounts of excretions, not the concentrations of serum OTA. The different sampling method may explain the different results of our study. It is not clear whether serum levels or daily urine excretion of OTA indicates the total body burden of OTA or not. Because all patients ingest different amounts of OTA‐contaminated food everyday, daily food samples consumed by all patients could be collected to determine the OTA levelsCitation[8] and to clarify this problem.
To determine whether OTA plays an important role in progression of renal insufficiency of patients with chronic renal diseases, a long‐term follow‐up study is needed. This cross‐section study only revealed the relationship between renal function‐relating factors and OTA nephrotoxicity. In addition, complex components in Chinese herbs, e.g., aristolochia acids, may confuse OTA nephrotoxicity. Several nephrotoxic mycotoxins have been previously identified in food samples.Citation[11] The finding leads to a debate whether OTA alone can induce nephropathy in man or there are additive or synergistic effects from two or more mycotoxins. Therefore, further studies are needed to find a definite correlation between OTA concentration in food, biological samples, and the occurrence of progressive renal insufficiency in the patients with chronic renal diseases.
References
- Creppy E. E., Baudrimont I., Betbeder A. M. Prevention of nephrotoxicity of ochratoxin A, a food contaminant. Toxicol. Lett. 1995; 82/83: 869–877
- Godin M., Fillastre J. P., Simon P., Francois A., Roy F. L., Morin J. P. Is ochratoxin a nephrotoxic in human beings?. Adv. Nephrol. Necker Hosp. 1997; 26: 181–206, [PUBMED], [INFOTRIEVE], [CSA]
- Selected Mycotoxins: Ochratoxin, Trichothecenes, Ergot. Report of an Expert Committee. World Health Organization, Geneva 1990, (Environmental Health Criteria No. 105)
- Simon P., Godin M., Fillastre J. P. Ochratoxin A: a new environmental factor which is toxic for the kidney?. Nephrol. Dial. Transplant. 1996; 11(12)2389–2391, [PUBMED], [INFOTRIEVE], [CSA]
- DiPaolo N., Guarnieri A., Garosi G., Sacchi G., Mangiarotti A. M., DiPaolo M. Inhaled mycotoxins lead to acute renal failure. Nephrol. Dial. Transplant. 1994; 9(4)116–120
- Krogh P. Ochratoxins in food. Mycotoxin in Food, P. Krogh. Academic Press, New York 1987; 97–121
- Krogh P., Hald B., Plestina R., Ceovic S. Balkan (endemic) nephropathy and food‐borne ochratoxin A. Preliminary results of a survey of foodstuffs. Acta Pathol. Microbiol. Scand. 1977; 85(3)238–240
- Özçelik N., Koşar A., Soyal D. Ochratoxin A in human serum samples collected in Isparta‐Turkey from healthy individuals and individuals suffering from different urinary disorders. Toxicol. Lett. 2001; 121: 9–13, [CSA], [CROSSREF]
- Aziz1 N. H., Youssef Y. A., El‐Fouly M. Z., Moussa L. A. Contamination of some common medicinal plant samples and spices by fungi and their mycotoxins. Bot. Bull. Acad. Sin. 1998; 39: 279–285
- Lin J.‐L., Ho H.‐H., Yu C.‐C. Chelation therapy for patients with elevated body lead burden and progressive renal insufficiency. Ann. Intern. Med. 1999; 130: 7–9, [PUBMED], [INFOTRIEVE], [CSA]
- Maaroufi K., Achour A., Hammami M., May M. E.I., Betbeder A. M., Ellouz F., Creppy E. E., Bacha H. Ochratoxin A in human blood in relation to nephropathy in Tunisia. Human Exp. Toxicol. 1995; 14: 609–615, [CSA]
- Li S., Marquardt R. R., Frohlich A. A. Identification of ochratoxin and some of their metabolites in bile and urine of rats. Food Chem. Toxicol. 2000; 38: 141–152, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Petzinger E., Weidenbach A. Mycotoxins in the food chain: the role of ochratoxin. Livest. Prod. Sci. 2002; 76(3)245–250, [CROSSREF]
- Wafa E. W., Yahya R. S., Sobh M. A., El‐Baz M., El‐Gayar H. A.M., Betveder A. M., Creppy E. E. Human ochratoxicosis and nephropathy in Egypt: a preliminary study. Human Exp. Toxicol. 1998; 17(2)124–129, [CSA], [CROSSREF]
- Mantle P. G. Risk assessment and the importance of ochratoxin. Int. Biodeterior. Biodegrad. 2002; 50: 143–146, [CSA], [CROSSREF]
- Hall P. W. Balkan endemic nephropathy: more questions than answers. Nephron 1992; 62: 1–5, [PUBMED], [INFOTRIEVE]
- Malhotra A., Townsend R. R. Hypertension in parenchymal renal disease. Curr. Concepts Hypertens. 2001; 4(3)5
- Bradley M. D., Barry M. B. Alterations in renal and urinary tract function: azotemia and urinary abnormalities. Harrision's Principles of Internal Medicine15th Ed., E. Braunwald, A. S. Fauci, D. L. Kasper, S. L. Hauser, D. L. Longo, J. L. Jameson. McGraw‐Hill, USA 2001; Vol. 1: 262–267
- Hagelberg S., Hult K., Fuchs R. Toxicokinetics of ochratoxin A in several species and its plasma‐binding properties. Toxicology 1989; 9: 91–96