Abstract
The toxic effect of chloroquine (CQ) has been attributed to oxidative stress with the consequences of lipid peroxidation. This study investigates the effects of α‐lipoic acid (LA) on CQ‐induced nephrotoxicity in rats. A single oral administration of CQ (970 mg/kg)‐induced nephrotoxicity, manifested biochemically by a significant increase in serum creatinine and blood urea nitrogen concentrations. In addition, renal tissue from CQ‐treated rats showed a significant increase in lipid peroxides measured as thiobarbituric acid reactive substances and hydroperoxides, along with significant decrease in nonenzymic antioxidants (vitamin C, vitamin E, and reduced glutathione) and enzymic antioxidants (superoxide dismutase, catalase, glutathione peroxidase, and glutathione‐S‐transferase) levels. Oral administration of LA (10, 30, or 100 mg/kg) in different doses for 10 days produced a significant protection against nephrotoxicity induced by CQ. Treatment with LA markedly reduced the elevated lipid peroxidation, restored the depleted renal antioxidant defense system. LA at 100 mg /kg was effective when compared with other doses (10 and 30 mg/kg). This was accompanied by the histopathological observations in kidney tissue. The results suggest that LA ameliorate the lipid peroxidation and the loss of cellular antioxidants, thereby protecting the CQ‐induced oxidative damage in kidney.
Introduction
Chloroquine (CQ) is the 4‐aminoquinoline drug widely used for both treatment and prophylaxis of malaria.Citation[1] In addition to antimalarial action, CQ has been used as a therapeutic agent for the treatment of amoebic, liver abscess, reheumatoid arthritis, and systemic or discoid lupus erythematosus. CQ also acts as an antiviral agent by inhibiting viral replication.Citation[2], Citation[3]
CQ accumulates in various organs of the body and is eliminated slowly. CQ causes increased lipid peroxidation and decreased antioxidant enzyme activities in rat retinaCitation[4] and liver.Citation[5] CQ has been shown to alter kidney structure and also impair the kidney function resulting from inappropriate retention of Na+ and Cl− in renal tubules and alterations in renally active hormones.Citation[6] It is possible that CQ may act directly or indirectly and alter the antioxidant status that makes certain organs more susceptible to the effects of oxidative stress.Citation[5]
Thiols play a pivotal role in protecting cells against lipid peroxidation. α‐Lipoic acid, a disulfide derivative of octanoic acid, is known to act as a cofactor in multienzyme dehydrogenase complexes that catalyze the oxidative decarboxylation of α‐keto acids. LA has been proved as a novel biological antioxidant and therapeutic agent in prevention or treatment of pathological conditions mediated via oxidative stress.Citation[7], Citation[8] The antioxidant role of LA has been implicated in hepatitis, diabetes, atherosclerosis, urolithiasis, and heavy metal poisoning.Citation[9] In addition, LA has been reported to protect the renal tissue from several toxicants, including gentamicin‐,Citation[10] cisplatin‐,Citation[11] lead‐,Citation[12] and adriamycinCitation[13]‐induced damages.
Because CQ is the most commonly ingested drug in the malaria endemic world, there is a need to study its toxic effects on the organs and the use of antioxidants to protect its deteriorative effects. In this interest, this study was carried out to investigate the possible role of LA in protection against CQ‐induced peroxidative damage in renal tissue.
Materials and Methods
Chemicals
1‐Chioro‐2, 4‐dinitrobenzene (CDNB), 5,5′‐dithiobis‐2‐nitrobenzoicacid, reduced glutathione (GSH), bovine serum albumin (BSA), thiobarbituric acid, nicotinamide adenine dinucleotide phosphate, xylenol orange, and butylated hydroxy toluene (BHT) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The rest of the chemicals used were of analytical grade.
Animals
Female albino Wistar rats, body weight of 180 to 200 g bred in Central Animal House, Rajah Muthiah Medical College, Annamalai University, were used in this study. The animals were fed on a pellet diet (Hindustan Lever Ltd., Mumbai, India) and water ad libitum. The animals were maintained in accordance with the guidelines of the National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India, and the study approved by the ethical committee at Annamalai University.
Experimental Protocol
Rats were randomly assigned to six groups of six rats in each group:
Group 1: Control rats received only the vehicle (groundnut oil 1 mL kg− 1 day− 1) orally by using an intragastric tube for 10 days.
Group 2: Rats in this group orally received LA (100 mg kg− 1 day− 1) dissolved in groundnut oil by using an intragastric tube for 10 days.
Group 3: Rats orally received single administration of CQ (970 mg kg− 1 day− 1) suspended in 1 mL salineCitation[14], Citation[15] by using an intragastric tube at the end of the ninth day.
Group 4: Rats orally received LA (10 mg kg− 1 day− 1) dissolved in groundnut oil for 7 days before the single oral administration of CQ and treatment with LA followed for 3 more days.
Group 5: Rats orally received LA (30 mg kg− 1 day− 1) dissolved in groundnut oil for 7 days before the single oral administration of CQ and treatment with LA followed for 3 more days.
Group 6: Rats orally received LA (100 mg kg− 1 day− 1) dissolved in groundnut oil for 7 days and then a single oral administration of CQ and LA for 3 more days.
Blood and Tissue Sampling
After the experimental period, animals in different groups were sacrificed. The rats were killed by decapitation after inducing anesthesia [pentobarbitone sodium (60 mg/kg)], and blood was collected for serum. Kidneys were rinsed in chilled saline and homogenized in 0.1 M, pH 7.4 phosphate buffer, and used for the biochemical analysis.
Determination of Kidney Function
Creatinine present in serum directly reacts with alkaline picrate, resulting in the formation of a red color, and the intensity is measured at 505 nm/green filter.Citation[16] Blood urea nitrogen was measured by using the diacetyl monoxime method,Citation[17] the determinants of renal function.
Estimation of Lipid Peroxidation
Lipid peroxidation was measured as thiobarbituric acid reactive substances (TBARS) and hydroperoxides by the method of Nichans and SamuelsonCitation[18] and Jiang et al.,Citation[19] respectively. In brief, 0.1 mL of tissue homogenate (Tris‐Hcl buffer, pH 7.5) was treated with 2 mL of (1:1:1 ratio) TBA‐TCA‐HCl reagent [thiobarbituric acid 0.37%, 0.25N HCl, and 15% Trichloroacetic Acid (TCA)] and placed in water bath for 15 min, cooled. The absorbance of clear supernatant was measured against reference blank at 535 nm.
Hydroperoxides were expressed as mM dL− 1. To 0.1 mL homogenate, 0.9 mL of Fox reagent (88 mg of BHT, 7.6 mg of xylenol orange, and 0.8 mg of ammonium iron sulphate were added to 90 mL of methanol and 10 mL of 250 mM sulphuric acid) was added and incubated at 37°C for 30 min. The color that developed was read at 560 nm.
Determination of Non‐enzymic Antioxidants in Kidney
Reduced GSH was determined by the method of Ellman.Citation[20] One milliliter of supernatant was treated with 0.5 mL of Ellmans reagent and 3 mL of phosphate buffer (0.2 M, pH 8.0). The absorbance was read at 412 nm. Ascorbic acid (vitamin C) concentration was measured by Omaye et al.,Citation[21] using a method in which the ascorbate is oxidized by copper to form dehydroascorbic acid, which reacts with 2,4 dinitrophenol hydrazine to form bis 2,4 dinitrophenol hydrazine. This undergoes further rearrangement to form a product with absorption maximum at 530 nm.
Lipids were extracted from tissues using a chloroform‐methanol mixture (2:1 v/v), and the extract (0.1 mL) was used for the estimation of vitamin E by the Desai method.Citation[22] This method involves the reduction of ferric ion to ferrous ion by vitamin E and formed a red‐colored complex with 2,2′ bipyridyl. An intense red‐colored layer obtained on addition of 4 mL butanol was read at 520 nm.
Assay of Antioxidant Enzymes
Superoxide dismutase (SOD) activity was determined by the modified method of Nicotinamideadenin edinucleotide (NADH) phenazinemethosulphate–nitroblue tetrazolium (NBT) formazan inhibition reaction spectrophotometrically at 560 nm.Citation[23] A single unit of enzyme was expressed as 50% inhibition of NBT reduction/min/mg protein. Catalase (CAT) was assayed colorimetrically as described by SinhaCitation[24] using dichromate–acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in 1:3 ratio). The intensity was measured at 620 nm, and the amount of hydrogen peroxide hydrolyzed was calculated for the catalase activity.
Glutathione peroxidase (GPx) activity was measured by the method described by Rotruck et al.Citation[25] The activity was expressed on the basis of inhibition of GSH. The glutathione‐S‐transferase (GST) activity was determined spectrophotometrically by the method of Habig et al.Citation[26] in which 1‐chloro‐2, 4‐dinitrobenzene used as substrate. The activity of GST is expressed as mmoles of GSH–CDNB conjugate formed/min/mg protein using an extinction coefficient of 9.6 mM− 1 cm− 1.
Protein was determined by the method of Lowry et al.Citation[27] using BSA as standard, at 660 nm.
Histopathological Examination
The kidneys from all the groups were excised immediately after sacrifice. Tissues were fixed in 10% formalin in phosphate buffer (pH 7.0) for 24 hr at room temperature for light microscopy. Tissues were embedded in paraffin wax and sections were cut at 5 mm slices and were stained with hematoxylin‐eosin and observed under light microscope.
Statistical Analysis
The results were expressed as mean ± SD. The difference between control and treated groups were tested for significance using one‐way analysis of variance followed by Duncan's Multiple Range Test, taking p < .05 as significant.
Results
The hepatotoxic dose of CQ also caused abnormal renal function, which was manifested by significantly increased levels of serum creatinine and blood urea nitrogen when compared with control groups (). Upon administration of LA, the levels of these kidney function markers were significantly decreased in a dose‐related manner. LA at 100 mg/kg body weight was more effective when compared with other doses of LA (10 and 30 mg/kg).
Table 1. Effects of α‐Lipoic Acid on Serum Creatinine and Blood Urea of Normal and Experimental Rats
depicts the levels of lipid peroxidation measured as TBARS and hydroperoxides and the levels of nonenzymic antioxidants in kidney of normal and experimental animals. Lipid peroxidation level was significantly increased, while the levels of nonenzymic antioxidants—namely, vitamin C, vitamin E, and GSH—were significantly decreased in CQ‐treated rats. Treatment with LA significantly decreased the levels of lipid peroxidation, whereas the levels of nonenzymic antioxidants were significantly increased.
Table 2. Changes in the Levels of Lipid Peroxidation and Nonenzymatic Antioxidant in Kidney of Control and Experimental Animals
The changes in the activities of enzymic antioxidants—namely, SOD, CAT, GPx, and GST—in renal tissue of control and experimental animals are shown in . A significant decrease in the activities of enzymic antioxidants was noted after single administration of CQ. Upon administration of LA, the activities of enzymic antioxidants were significantly reversed to near normal.
Table 3. Changes in the Activities of Kidney SOD, CAT, GPx, and GST in Control and Experimental Rats
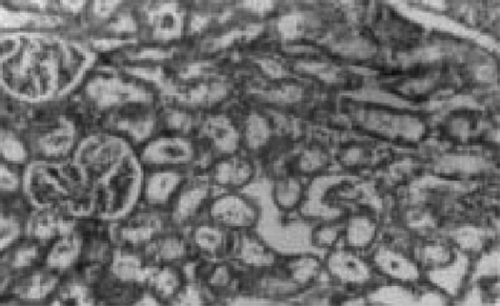
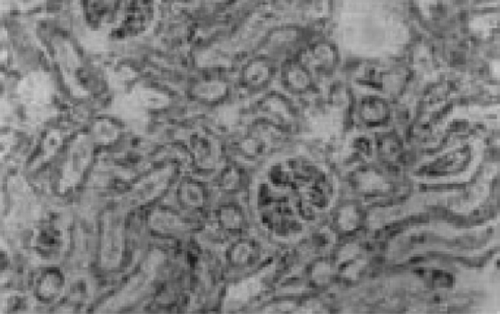
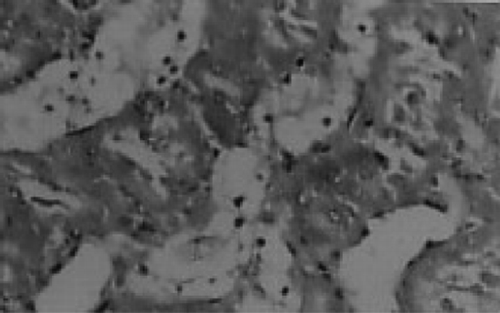
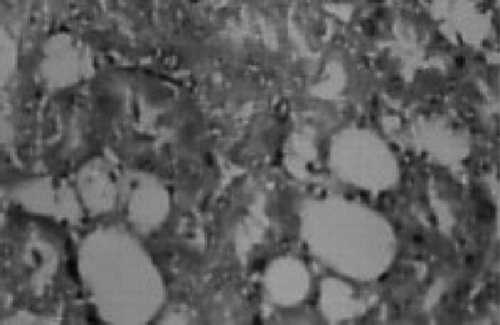
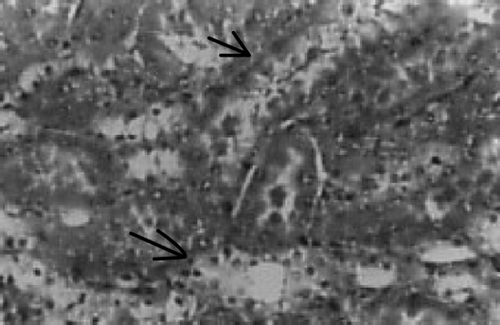
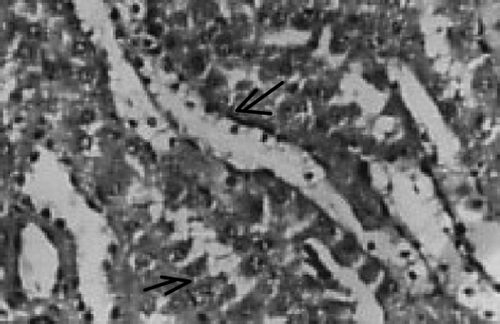
There were no renal histological alterations observed in control () or LA () alone treated groups. The cloudy swelling of tubules with hyaline casts in tubular lumen were shown in CQ‐treated rats ( and ). The cloudy swelling of tubules with hyaline casts in tubular lumen were shown in CQ treated rats (, and ). Treatment with LA reduced the CQ‐induced damage as shown in and .
Discussion
The kidney is easily susceptible to damage from drugs or toxins because of its larger perfusion and the increased concentration of excreted compounds that occur in renal tubular cells during absorption and secretion.Citation[28] The determination of serum creatinine and urea is of great value in helping to ascertain the renal function. The serum creatinine is less influenced by extrarenal factors than serum urea and is a most accurate test.Citation[29] In this study, the increased levels of serum creatinine and urea clearly indicate the abnormalities in renal function of CQ‐treated rats. Administration of LA restores the renal function by preserving the structural integrity of renal cells against CQ challenge, evidenced by significantly lowered levels of serum creatinine and urea. The membrane‐stabilizing effect of LA has already been reported.Citation[30], Citation[31]
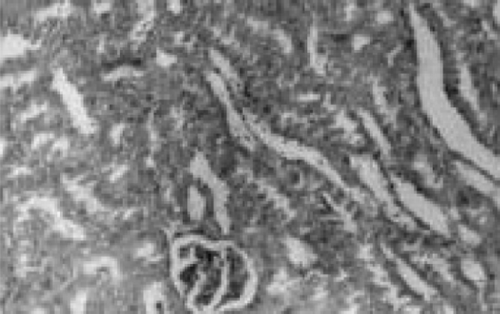
Figure 7. Chloroquine + lipoic acid. Mild parenchymal inflammation with less cloudy swelling (→). HX10.
Lipid peroxidation of membrane lipids has been implicated as a possible mechanism of acute oxidative stress‐induced lethal injury.Citation[32] The measurement of lipid peroxidation is a convenient method to monitor the oxidative damage.Citation[33] The oxidative stress caused by CQ leads to excessive formation of free radicals and enhanced lipid peroxidation resulting in renal injury. Treatment with LA significantly reduced the level of lipid peroxidation indices in CQ‐treated rats by their ability to scavenge a variety of free radicals produced during the course of lipid peroxidation.Citation[10], Citation[34] A smaller torsional angle in the cyclic structure of LA results a comparatively higher electron density of disulphide bridge, which enhances the free radical scavenging effect.Citation[11]
The remarkable depletion of vitamin C, vitamin E, and GSH in kidney tissue of CQ‐treated rats represents increased utilization due to oxidative stress. It is well established that vitamin C, vitamin E, and GSH are closely interlinked to each other and play an excellent role in preventing cells from oxidative threats.Citation[10], Citation[35] The depletion of nonenzymic antioxidants in CQ‐treated rats has already been reported.Citation[15]
The increased levels of nonenzymic antioxidant administration of LA could be due to the capability of LA to replenish the active forms of vitamin C, vitamin E, and GSH by reduction of their radicals via the redox cycle.Citation[10] LA has already been reported to stimulate the GSH biosynthesis by facilitating the transport of cystine, the rate‐limiting factor in GSH synthesis.Citation[36] This might be the basis of increased levels of nonenzymic antioxidants upon treatment with LA in CQ‐administrated rats.
SOD, CAT, and GPx are among the endogenous antioxidant enzymes that play a pivotal role in reduction of lipid peroxides; scavenge superoxide radicals and hydrogen peroxide thereby interrupt the propagation of the lipid peroxidation reaction.Citation[37], Citation[38] GST has the capability to detoxify various endogenous and exogenous substances, especially halogenated aromatics by conjugation with glutathione.Citation[39] The reactive oxygen radicals can themselves reduce the activity of enzymes.Citation[40] The decreased activities of SOD, CAT, GPx, and GST in renal tissues of CQ‐treated rats may be due to decreased synthesis of enzymes or inhibition of enzyme activity by enhanced lipid peroxidation. In addition, therapeutic doses of CQ were shown to decrease protein turnover in human.Citation[41]
The decreased activities of enzymic antioxidant lead to accumulation of superoxide, peroxide, and hydroxyl radicals, which are effectively cleared by lipoate.Citation[30] LA has higher singlet oxygen‐quenching capacity than other sulfur compounds and also prevents the propagation of lipid peroxidation.Citation[10] In addition, LA is known to improve overall protein synthesis, repair oxidized proteins, and also protect them from further peroxidative damage.Citation[10], Citation[42] This might be responsible for increased activities of SOD, CAT, GPx, and GST on administration of LA in rats treated with CQ.
The histopathological observations in CQ‐treated rat is further evidence for CQ‐induced renal damage. This could be due to the formation of highly reactive radicals as a consequence of oxidative threat caused by CQ. The accumulated hydroperoxides can cause cytotoxicity, which is associated with the peroxidation of membrane phospholipids by lipid hydroperoxide.Citation[43], Citation[44] The preservation of renal cell membrane integrity by LA against CQ may depend on the favorable capacity of LA to pass through the membranes and can accommodate both hydrophilic and hydrophobic environments in which it neutralizes the oxidative reactions.Citation[45] In addition, due to its nucleophilic and antioxidant properties, LA reacts with endogenous electrophiles, including free radicals and reactive drug metabolites, thereby inhibiting the lipid peroxidation in kidney tissue.Citation[34] This may be the mechanism by which LA preserves the architecture of renal tissue from CQ.
In conclusion, these observations suggest that lipoate can prevent the CQ‐induced lipid peroxidation with overall enhancement of renal antioxidant defense system by its antioxidant and free radical scavenging activity. Therefore, lipoic acid has been suggested to be helpful in protection of CQ‐induced oxidative damage in renal tissue.
References
- Brucechwatt L. J. Chemotherapy of MalariaRevised 2nd Ed. Geneva WHO Monograph Series No. 27, WHO Publications Center USA distributor, Geneva, Albany, NY 1986; 119–165
- Savarino A., Boelaert J. R., Cassone A., Majori G., Cauda R. Effects of chloroquine in viral infections: an old drug against today's diseases?. Lancet Infect. Dis. 2003; 3: 722–727, [PUBMED], [INFOTRIEVE], [CSA]
- Taylor W. R.J., White N. T. Antimalarial drug toxicity. Drug Saf. 2004; 27: 25–61, [PUBMED], [INFOTRIEVE], [CSA]
- Bhattacharya B., Chatterjee T. P., Ghosh J. J. Effect of chloroquine on lysosomal enzymes. NADPH‐induced lipid peroxidation and antioxidant enzymes of rat retina. Biochem. Pharmacol. 1983; 42: 2965–2968, [CROSSREF]
- Magwere T., Naik Y. S., Hosler J. A. Effects of chloroquine treatment on antioxidant enzymes in rat liver and kidney. Free Radic. Biol. Med. 1997; 22: 23–25, [CROSSREF]
- Musabayane C. T., Copper R. G., Prasada Rao P. V.V., Balen R. J. Effects of ethanol on the changes in renal fluid and electrolyte handling and kidney morphology induced by long term chloroquine induced administration to rats. Alcohol 2000; 22: 129–138, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Packer L., Roy S., Sen C. K. Alpha‐lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Adv. Pharmacol. 1997; 38: 79–101, [PUBMED], [INFOTRIEVE]
- Bustamante J., Lodge J. K., Marcocci L., Tritschler H. J., Packer L., Rihn B. H. Alpha‐lipoic acid in liver metabolism and disease. Free Radic. Biol. Med. 1998; 24: 1023–1039, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sumathi R., Baskaran G., Varalakshmi P. Effect of DL‐α‐lipoic acid on tissue redox state in acute cadmium‐challenged tissues. J. Nutr. Biochem. 1996; 7: 85–92, [CROSSREF]
- Santhya P., Mohandoss S., Varalakshmi P. Role of DL‐α‐lipoic acid in gentamicin induced nephrotoxicity. Mol. Cell. Biochem. 1995; 145: 11–17, [CSA]
- Somani S. M., Hussian K., Whitworth C., Trammell G. L., Malafa M., Rybak L. P. Dose‐dependent protection by lipoic acid against cisplatin induced nephrotoxicity in rats: antioxidant defense system. Pharmacol. Toxicol. 2000; 86: 234–241, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Gurer H., Ozgunes H., Oztezcan S., Exal N. Antioxidant role of α‐lipoic acid in lead toxicity. Free Radic. Biol. Med. 1999; 27: 75–81, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Malarkodi K. P., Balachandar A. V., Sivaprasad R., Varalakshmi P. Prophylactic effect of lipoic acid against adriamycin induced peroxidative damage in rat kidney. Ren. Fail. 2003; 25: 367–377, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Dass E. E., Shah K. K. Paracetamol and conventional antimalarial drugs induced hepatotoxicity and its protection by methionine in rats. Indian J. Exp. Biol. 2000; 38: 1138–1142, [PUBMED], [INFOTRIEVE], [CSA]
- Pari L., Murugavel P. Protective effect of α‐lipoic acid against chloroquine induced hepatotoxicity in rats. J. Appl. Toxicol. 2004; 24: 21–26, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Spencer K. Analytical reviews in clinical biochemistry: the estimation of creatinine. Ann. Clin. Biochem. 1986; 23: 1–25, [PUBMED], [INFOTRIEVE]
- Natelson S. Microtechniques of Clinical Chemistry for the Routine Laboratory, C. C. Thomas. Springfield, Illinois 1957; 381
- Nichans W. G., Samuelson D. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968; 6: 126–130
- Jiang Z. Y., Hunt J. V., Wolff S. P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992; 202: 384–387, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Ellman G. C. Tissue sulphydryl groups. Arch. Biochem. Biophys. 1959; 82: 70–77, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Omaye S. T., Turbull T. P., Sauberclich H. C. Selected methods for determination of ascorbic acid in cells, tissues and fluids. Methods Enzymol. 1979; 6: 3–11
- Desai I. D. Vitamin E analysis method for animal tissues. Methods Enzymol. 1984; 105: 138–143, [PUBMED], [INFOTRIEVE]
- Kakkar P., Das B., Viswanathan P. N. A modified spectroscopic assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984; 21: 130–132, [PUBMED], [INFOTRIEVE], [CSA]
- Singha A. K. Colorimetric assay of catalase. Anal. Biochem. 1972; 47: 389–394, [CROSSREF]
- Rotruck J. T., Pope A. L., Ganther H. E. Selenium: biochemical role as a component of glutathione peroxidase purification assay. Science 1973; 179: 588–590, [PUBMED], [INFOTRIEVE]
- Habig W. R., Pbst M. J., Jakpoly W. B. Glutathione transferase. A first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974; 249: 7130–7139, [PUBMED], [INFOTRIEVE]
- Lowry O. H., Rosenbrough N. J., Farr A. I., Randall R. J. Protein measurement with the folin‐phenol reagent. J. Biol. Chem. 1951; 193: 265, [PUBMED], [INFOTRIEVE]
- Mohamed M., Abdel Latif M. D., Sahar A., Elglammal M. D. Sodium fluoride ion and renal function after prolonged sevoflurane or isoflurane anesthesia. Eng. J. Anesth. 2003; 19: 79–83
- Lyman J. L. Blood urea nitrogen and creatinine. Emerg. Med. Clin. North Am. 1986; 4: 223–233, [PUBMED], [INFOTRIEVE]
- Kagan V. E., Shvedova A., Serbinova E., Khan S., Swanson C., Powell R., Packer L. Dihydrolipoic acid—a universal antioxidant both in the membrane and in the aqueous phase, reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem. Pharmacol. 1992; 44: 1637–1649, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Arivazhagan P., Juliet P., Thilagavathy T., Paneerselvam C. DL‐α‐lipoic acid supplementation attenuates the age related changes in membrane bound ATPases in rats. J. Clin. Biochem. Nutr. 1999; 27: 111–120
- Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury. J. Gastroenterol. Hepatol. 2000; 15: 718–724, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Viani P., Cervato G., Fiorilli A., Cestaro B. Age related differences in synaptosomal peroxidative damage and membrane properties. J. Neurochem. 1991; 5: 253–258
- Biewenga G. P., Haenen G. R., Bast A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997; 29: 315–333, [PUBMED], [INFOTRIEVE], [CSA]
- Winkler B. S. Unequivocal evidence in support of non‐enzymatic redox coupling between glutathione/glutathione disulphide and ascorbic acid/dehydroascorbic acid. Biochim. Biophys. Acta 1992; 1117: 287–290, [PUBMED], [INFOTRIEVE]
- Flohe L., Packer L. Lipoic acid increases denovo synthesis of cellular glutathione by improving cysteine utilization. Biofactors 1997; 6: 321–338, [PUBMED], [INFOTRIEVE], [CSA]
- Wei Y. H. Oxidative stress and mitochondrial DNA mutations in human aging. Proc. Soc. Exp. Biol. Med. 1998; 217: 53–63, [PUBMED], [INFOTRIEVE], [CSA]
- Mansour M. A., Mostafa A. M., Nagi M. N., Khattab M. M., Al‐shahanah O. A. Protective effect of aminoguanidine against nephrotoxicity induced by cisplatin in normal rats. Comp. Biochem. Physiol., Part C 2002; 132: 123–128, [CSA], [CROSSREF]
- Vos R. M.E., Van Bladeren P. Glutathione‐S‐transferase in relation to their role in the biotransformation of xenobiotics. Chem. Biol. Interact. 1990; 75: 241–265, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Halliwell B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996; 16: 33–50, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- De Feo P., Volpi E., Lucidi P., Cruciani G., Santeusanio F., Bolli G. B., Brunetti P. Chloroquine reduces whole body proteolysis in humans. Am. J. Physiol. 1994; 267: E183–E186, [PUBMED], [INFOTRIEVE]
- Arivazhagan P., Thilakavathy T., Paneerselvam C. Antioxidant lipoate and tissue antioxidants in aged rats. J. Nutr. Biochem. 2000; 11: 122–127, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kaneko T., Baba N., Matsuo M. Suppression of lipid hydroperoxide‐induced oxidative damage to cellular DNA by esculetin. Biol. Pharm. Bull. 2003; 26: 840–849, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Pacifici E. H., McLeod L. L., Peterson H., Savanian A. Linoleic acid hydroperoxide‐induced peroxidation of endothelial cell phospholipids and cytotoxicity. Free Radic. Biol. Med. 1994; 17: 285–295, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Cao X., Phillis J. W. The free radical scavenger α‐lipoic acid protects against cerebral ischemia reperfusion injury in gerbils. Free Radic. Res. 1995; 23: 365–370, [PUBMED], [INFOTRIEVE], [CSA]