Abstract
Myoglobinuric acute renal failure has three pathogenic mechanisms: tubular obstruction, renal vasoconstriction, and oxidative stress. The latter is generated through the iron released from the group hemo of the myoglobin. Iron induces the formation of high‐activity oxygen free radicals that increase oxidative stress and provoke lipid peroxidation and cellular death. This oxidative stress can be measured in several ways, both total or partially with the total antioxidant status or the intermediate enzymes. On the other hand, N‐acetylcysteine is a demonstrated substance with antioxidant properties. The aim of the present work was to assess the effect of N‐acetylcysteine on the oxidative stress in the glycerol‐induced acute renal failure in rats model. We observed that the animals treated with N‐acetylcysteine showed an improvement in the antioxidant activity given by an increase in the total antioxidant status and glutathione reductase levels in serum. This improvement was greater when treatment was administered before the induction of rhabdomyolysis. Nevertheless, the observed increase in antioxidant status was only statistically significant for glutathione reductase but not for total antioxidant status. Our results support an important role for N‐acetylcysteine in the treatment of this form of acute renal failure, although we think that oxidative stress is not the main pathogenic mechanism of the tubular necrosis induced by rhabdomyolysis, tubular obstruction and renal vasoconstriction being still more important.
Introduction
Acute renal failure (ARF) is the most important complication that can occur in the course of rhabdomyolysis.Citation[1] There are multiple causes of rhabdomyolysis, some of which suppose a social problem in addition to medical problems, for example, complications due to war, natural disasters, terrorism, drug abuse, and others.Citation[1] There is a classical developed experimental model for the study of the rhabdomyolysis associated renal insufficiency. In this model, the ARF is triggered after inducing rhabdomyolysis by means of the intramuscular injection of glycerol.Citation[2] The pathogenic mechanisms that are invoked, like mediators of the ARF, are several, between which is the oxidative stress produced by contained ferric proteins in released molecules of myoglobin to the bloodstream in rhabdomyolisis.Citation[3] This oxidative stress would participate in the tubular necrosis favoring lipid peroxidation of cellular membranes.Citation[4], Citation[5], Citation[6] Numerous studies have described the beneficial effect of diverse antioxidant agents on the function and renal morphology in this experimental model.Citation[4], Citation[5], Citation[7], Citation[8] The modification of oxidative status through indirect parameters has also been observed;Citation[4], Citation[9] nevertheless, there are no works that have studied this effect through the direct measurement of total antioxidant activity or of some of the enzymes that participate in this process. The objective of the present work was to study the variations produced on the serum total antioxidant state (TAS) and glutathione reductase activity (GR) in the model of myoglobinuric‐ARF when treating the animals with N‐acetylcysteine (NAC), a substance with recognized antioxidant properties.Citation[10]
Methods
Forty Sprague–Dawley rats weighing 150–300 g were included in the study. The animals were deprived of water but not food for the 24 h before induction of rhabdomyolysis. They were placed in metabolic cages where there was exact quantification of hydric balance. The rats were randomly assigned into four groups: Group A (n = 10) was administered NAC intraperitoneal, 10 mg/100 g body weight, 1 h before the induction of rhabdomyolysis; Group B (n = 10) received saline solution (0.9%) intraperitoneal in an equal dose of NAC in Group A, 1 h before the induction of rhabdomyolysis; Group C (n = 10) was administered NAC intraperitoneal, 10 mg/100 g body weight, immediately after rhabdomyolysis induction; Group D (n = 10) received saline solution (0.9%) intraperitoneal in an equal dose of NAC in Group C, immediately after rhabdomyolysis induction. Rhabdomyolysis was made, under light sedation with diazepam, by injecting glycerol (50% vol/vol in sterile saline), 10 mL/kg body weight, half in of the dose in each hind limb muscle. Then, the rats in all groups were allowed free access to water. Twenty‐four hours after glycerol injection, under anesthesia with ketamine and xylazine, blood samples were collected by cardiac puncture; immediately thereafter the animals were euthenized via intracardiac pentobarbital sodium administration. The TAS was determined by ABTS• +® commercialized kit (ABTS®, Randox® Laboratories, UK) a well‐established technique described by Miller and Rice‐Evans.Citation[11], Citation[12] The GR was determined by another commercialized kit by Randox® Laboratories and according to the reaction described by Spooner.Citation[13] Data are presented as mean values with their standard deviation. Significance of the results among animals was evaluated using the Student's t‐test. For comparisons involving more than two groups, analysis of variance (ANOVA) was applied. The differences were statistically significant considered when the p value was less than 0.05.
Results
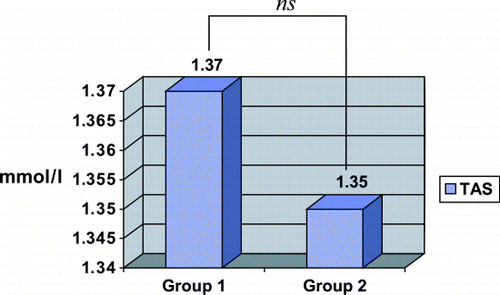
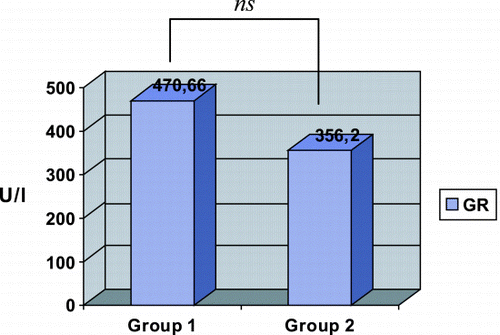
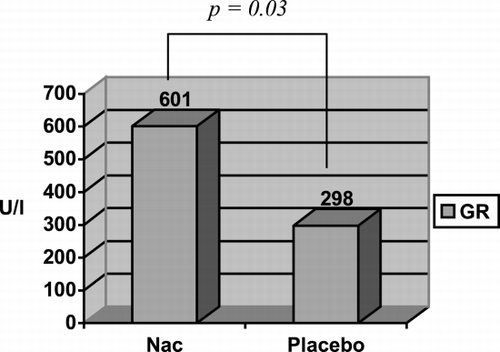
The results of the TAS and GR in the four groups that formed the study are shown in . In the groups in which was acted before inducing rhabdomyolysis (Groups A and B), the TAS level was greater in Group A, in which NAC was administered (TAS: 1.52 ± 0.21 mmol/L) than in Group B, which received a placebo (TAS: 1.38 ± 0.23 mmol/L), but without statistically significant difference (p > 0.05). However, with regard to GR we found differences (Group A: GR: 601 ± 148 U/L; Group B: GR: 298 ± 56 U/L) that reached statistical significance (p = 0.03) ( and ). When analyzing the groups in which was acted after inducing rhabdomyolysis (Groups C and D) we observed, on the contrary, that in the previous ones, the TAS levels (Group C: 1.23 ± 0.17 mmol/L; Group D: 1.32 ± 0.07 mmol/L) and the GR levels (Group C: 340 ± 130 U/L; group D: 443 ± 74 U/L) were lower in the group in which NAC was administered than they were in the placebo group. Nevertheless, these differences were not statistically significant for any of the two variables. With the aim of assessing whether the difference observed in the GR between Groups A and B (before rhabdomyolysis) that does not appear between Groups C and D (after rhabdomyolysis), was accidental or was derived from the administration of NAC before rhabdomyolysis, we reorganized the groups on the basis of the administration of NAC (Group 1) or placebo (Group 2), independently of which it was made before or after glycerol injection. The results of these groups are shown in . It is of note that the GR and TAS levels are greater in Group 1 (TAS: 1.37 ± 0.23 mmol/L; GR: 470 ± 189 U/L) than in Group 2 (TAS: 1.35 ± 0.14 mmol/L; GR: 356 ± 96 U/L) ( and ). These differences, however, were not statistically significant.
Table 1. Results of Glutathione Reductase and Total Antioxidant Status in the Four Groups of the Experiment
Table 2. Glutathione Reductase and Total Antioxidant Status Levels in the Rats Administered with N‐Acetylcysteine or Placebo
Figure 1. Comparison of total antioxidant status (TAS) level in serum with the administration of N‐acetylcysteine (Nac) or placebo before rhabdomyolysis (Groups A and B).
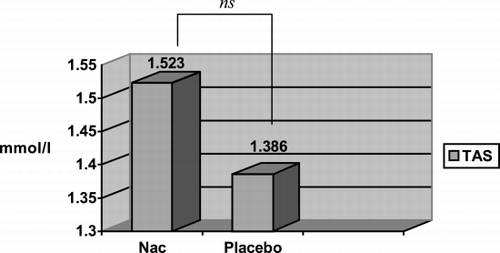
Figure 2. Comparison of glutathione reductase (GR) levels in serum with the administration of N‐acetylcysteine (Nac) or placebo before rhabdomyolysis (Groups A and B).
Discussion
In the glycerol‐induced ARF, three pathogenic mechanisms of tubular necrosis are invoked: tubular obstruction, renal vasoconstriction, and oxidative stress.Citation[14] Oxidative stress is produced by the generation of free oxygen radicals that cause lipid peroxidation of cellular membranes that leads to epithelial tubular cell necrosis.Citation[15], Citation[16], Citation[17] In this process, it has been demonstrated that the iron contained in the porphyritic ring of the myoglobin plays a critical role.Citation[14], Citation[15], Citation[16] The released iron from the group hemo of the myoglobin is introduced into the epithelial tubular cellCitation[9] where it is able to generate among others the hydroxyl radical,Citation[4], Citation[16] a free oxygen radical highly reactive and that participates in the cellular destruction. These data are based in studies in which the administration of iron chelator and free radical scavengers produce functional and histologic renal improvement.Citation[17], Citation[18], Citation[19], Citation[20] Most of the studies have assessed the oxidative stress and lipid peroxidation through indirect data as the production of malondialdehyde or lacticdehydrogenase.Citation[4], Citation[5], Citation[6], Citation[9] In our study, we have determined the total antioxidant activity of the serum that may give a more approximate idea of the role that this mechanism plays in the production of the acute tubular necrosis in this model. The data of our study support the existence of an oxidative stress in the renal tubule because of an increase in the levels of both TAS and GR, although it was only significant for the last group. The glutathione system is the most powerful natural antioxidant that exists.Citation[16] In this system, glutathione acts like a substrate of three enzymes: glutathione peroxidase, glutathione reductase, and glutathione S‐transferase.Citation[21] Glutathione reductase is a flavoenzyme that transforms oxidized glutathione again into reduced glutathioneCitation[22], Citation[23] to serve as a substrate of the other two enzymes, which reduces the hydrogen peroxide (H2O2) and other products of the peroxidation, turning them into less toxic substances and easily removable.Citation[22], Citation[23] N‐acetylcysteine has a direct antioxidant effect when reacting with several free oxygen radicals, and in addition plays a indirect role facilitating the synthesis of glutathione.Citation[24] In our study, the increase observed in the levels of GR and TAS in the animals that were treated with N‐acetylcysteine confirms the findings of Thielemann et al. that demonstrated an increase in renal intracellular glutathione levels after the administration of N‐acetylcysteine.Citation[25] The beneficial effect of N‐acetylcysteine only appears when it is administered before the acute renal insufficiency is induced, after it is administered a posteriora, although its positive effects not significant. This evidence is justified by the fact that when adding the effect of the N‐acetylcysteine administered before and after rhabdomyolysis, the increase observed in the levels of TAS and GR is not as great as when it is administered previously, losing even statistical significance for the GR. In a previous study, it was observed that in the first 24 h a drastic diminution of the TAS levels occurs, probably by glutathione consumption, with a spontaneous recovery of these levels in the following 72 h.Citation[26] Our results support the beneficial role of N‐acetylcysteine as an antioxidant since it achieves greater levels of TAS and GR in the treated groups than those in which a placebo was administered. The significant role of glutathione and N‐acetylcysteine in the decrease of oxidative stress also has been demonstrated in different in vitro studies.Citation[10], Citation[25], Citation[27] However, a clear correlation between the decrease of the oxidative parameters in vitro and the improvement of the renal function in vivo does not exist. In several works, it has been demonstrated that the antioxidant treatment improves renal function or even recovers it.Citation[7], Citation[8], Citation[28] In contrast, other authors have not found such beneficts.Citation[29], Citation[30], Citation[31] Therefore, although our results support the participation of oxidative stress in the pathogenesis of the myoglobinuric renal failure, we think, like other authors,Citation[24], Citation[31] that this mechanism does not exert a key role in the development of the glycerol‐induced renal failure, even though the antioxidant therapy could become one of the cornerstones of the classic treatment of this process, based on the volume replacement, urinary alkalinization, and diuretics.Citation[14]
References
- Fernández‐Fúnez A. La insufficientia renal aguda en la rhabdomyolysis. Rev. Clin. Esp. 1998; 198: 758–764
- Oken D. E., Arce M. L., Wilson D. R. Glycerol‐induced hemoglobinuric acute renal failure in the rat. I. Micropuncture study of the development of oliguria. J. Clin. Invest. 1966; 45: 724–735, [PUBMED], [INFOTRIEVE]
- Zager R. A. Heme protein‐ischemic interactions at the vascular, intraluminal and renal tubular cell levels: implications for therapy of myoglobin‐induced renal injury. Ren. Fail. 1992; 14: 341–344, [PUBMED], [INFOTRIEVE]
- Shah S. V., Walker P. D. Evidence suggesting a role for hydroxyl radical in glycerol‐induced acute renal failure. Am. J. Physiol. 1988; 255: F438–F443, [PUBMED], [INFOTRIEVE]
- Paller M. S. Hemoglobin‐ and myoglobin‐induced acute renal failure in rats: role of iron in nephrotoxicity. Am. J. Physiol. 1988; 255: F539–F544, [PUBMED], [INFOTRIEVE]
- Zager R. A., Burkhart K. M., Conrad D. S., Gmur D. J. Iron, heme oxygenase, and glutathione: effects on myohemoglobinuric proximal tubular injury. Kidney Int. 1995; 48: 1624–1634, [PUBMED], [INFOTRIEVE]
- Abul‐Ezz S. R., Walker P. D., Shah S. V. Role of glutathione in an animal model of myoglobinuric acute renal failure. Proc. Natl. Acad. Sci. U. S. A. 1991; 88: 9833–9837
- Stefanovic V., Savic V., Vlahovic P., Cvetkovic T., Najman S., Mitic‐Zlatkovic M. Reversal of experimental myoglobinuric acute renal failure with flavonoids from seeds of grape. Ren. Fail. 2000; 22: 255–266, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Zager R. A. Mitochondrial free radical production induces lipid peroxidation during myohemoglobinuria. Kidney Int. 1996; 49: 741–751, [PUBMED], [INFOTRIEVE]
- Aruoma O. I., Halliwell B., Hoey B. M., Putler J. The antioxidant action of N‐acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide and hypochlorous acid. Free Radic. Biol. Med. 1989; 6: 593–597, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Miller N. J., Rice‐Evans C., Davies M. J., Gopinathan V., Milner A. A novel method for measuring antioxidant capacity an its application to monitoring the antioxidant status in premature neonatus. Clin. Sci. (Lond.) 1993; 84: 407–412
- Rice‐Evans C., Miller N. J. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994; 234: 279–293
- Spooner R. J., Delides A., Goldberg D. M. Heat stability and kinetic properties of human serum glutathione reductase activity in various disease states. Biochem. Med. 1981; 26: 239–248, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Zager R. A. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996; 49: 314–326, [PUBMED], [INFOTRIEVE]
- Slater M. S., Mullins R. J. Rhabdomylysis and myoglobinuric renal failure in trauma and surgical patients: a review. J. Am. Coll. Surg. 1998; 186: 693–716, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Nath K. A., Norby S. M. Reactive oxygen species and acute renal failure. Am. J. Med. 2000; 109: 655–678, [CROSSREF]
- Shah S. V., Walker P. D. Reactive oxygen metabolites in toxic acute renal failure. Ren. Fail. 1992; 14: 363–370, [PUBMED], [INFOTRIEVE]
- Paller M. S., Hedlund B. E. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988; 34: 474–480, [PUBMED], [INFOTRIEVE]
- Zager R. A., Foerder C. A., Bredl C. The influence of mannitol on myoglobinuric acute renal failure: functional, biochemical, and morphologic assessments. J. Am. Soc. Nephrol. 1991; 2: 848–855, [PUBMED], [INFOTRIEVE]
- Parry W. L., Schaefer J. A., Meuller C. B. Experimental studies of acute renal failure. Protective effect of mannitol. J. Urol. 1963; 89: 1–6, [PUBMED], [INFOTRIEVE]
- Cruz R., Almaguer‐Melian W., Bergado‐Rosado J. A. El glutation en la función cognitiva y la neurodegeneración. Rev. Neurol. 2003; 36: 877–886, [PUBMED], [INFOTRIEVE]
- Gérard‐Monnier D., Acudiere J. Métabolisme et fonction antioxidante du glutation. Pathol. Biol. 1996; 44: 77–85
- Báez S., Seguraaguilar J., Widersten M., Johansson A. S., Mannervik B. Glutathione tranferases catalyze the detoxication of oxidized metabolites (o‐quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem. J. 1997; 324: 25–28
- DiMari J., Megyesi J., Udvarhelyi N., Price P., Davis R., Safirstein R. N‐acetyl cysteine ameliorates ischemic renal failure. Am. J. Physiol. Renal. Physiol. 1997; 272(41)F292–F298
- Thielemann L. E., Rosenblut E. W. Sulfur‐containing amino acids that increase renal glutathione protect the kidney against papillary necrosis induced by 2‐bromothylamine. Cell Biochem. Funct. 1990; 8: 19–24, [PUBMED], [INFOTRIEVE]
- Fernández‐Fúnez A., Polo F. J., Broseta L., Atienza M. P., Mora A., Sánchez Gascón F. Evolution of total antioxidant status in a model of acute renal insufficiency in rats. Ren. Fail. 2003; 25: 535–543, [CROSSREF]
- Paller M. S., Patten M. Protective effects of glutathione, glycine, or alanine in an in vitro model of renal anoxia. J. Am. Soc. Nephrol. 1992; 2: 1338–1344, [PUBMED], [INFOTRIEVE]
- Ishizuka S., Nagashima Y., Numata M., Yano T., Hagiwara K., Ozasa H., Sone M., Nihei H., Horikawa S. Regulation and immunohistochemical analysis of stress protein heme oxygenase‐1 in rat kidney with myoglobinuric acute renal failure. Biochem. Biophys. Res. Commun. 1997; 240: 93–98, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Fernández‐Fúnez A., Polo F. J., Broseta L., Valer J., Zafrilla L. Effects of N‐acetylcysteine on myoglobinuric‐acute renal failure in rats. Ren. Fail. 2002; 24: 725–733, [CROSSREF]
- Akpolat T., Akpolat I., Öztürk H., Sarikaya S., Cosar A. M., Bedir A., Kandemir B. Effect of vitamin E and pentoxifylline on glycerol‐induced acute renal failure. Nephron 2000; 84: 243–247, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Zurovsky Y., Grossman S. Evidence against oxidant injury and endotoxin underlying glycerol‐induced fatal rhabdomyolysis in rats. J. Basic Clin. Physiol. Pharmacol. 1992; 3: 239–251, [PUBMED], [INFOTRIEVE]