Abstract
Background: The role of apoptosis in the pathogenesis of renal diseases has not been clearly established. Proliferating cell nuclear antigen (PCNA) is also a proliferation marker. In this study, we investigated the relationship between clinical course and PCNA apoptosis on baseline renal biopsy in patients with Lupus nephritis (LN) and membranoproliferative glomerulonephritis (MPGN). Methods: Thirty-nine patients with proliferative glomerulonephritis [lupus nephritis (LN)[21] and MPGN[18]] were included in this study. PCNA and apoptosis on renal biopsies were detected by immunohistochemical and terminal deoxynucleotidyl transferase mediated dUTP nick end labelling TUNEL methods, respectively. We calculated the ratios of intraglomerular apoptotic cells and PCNA positive cells per glomeruli, and total numbers of apoptotic tubular cells and PCNA positive tubular cells among the 100 tubular cells, and PCNA positive cell and apoptotic cell on two different tubulointerstitial areas (40 × 10). Results: In LN: Apoptotic indexes of glomerulus and tubulus were 1.08 ± 0.49 and 3.71 ± 1.38, respectively. PCNA positivities were found at 16.76 ± 11.34%, 46.57 ± 22.54%, and 40.28 ± 23.14% on glomerulus, tubulus, and interstitium, respectively. The activity index was 11.23 ± 3.41, and the chronicity index was 3.81 ± 1.99. In MPGN: Apoptotic indexes were found at 0.83 ± 0.25 and 3.55 ± 1.75 on glomerulus and tubulus, respectively. PCNA positivities were found at 21.33 ± 18.42%, 35.5 ± 25.99%, and 34.66 ± 26.84% on glomerulus, tubulus, and interstitium, respectively. In controls, apoptosis was not found. In LN: PCNA positivity on tubulus and interstitium were correlated with the activity index (r = 0.768, p < 0.001, r = 0.721, and p < 0.001, respectively). Glomerular PCNA and apoptosis on interstitium and glomerulus were not correlated with the activity index. The activity index also was not correlated with creatinine clearance and daily proteinuria (p = 0.35 for both). At the end of the first year, patients with recovered or stabilized renal function had higher interstitial and tubular PCNA than others in G1 and G2. Conclusion: It can be said that expression of PCNA on renal biopsy was correlated with activity indexes in LN. PCNA may be a prognostic indicator in MPGN and LN. However, apoptosis does not have a predictive value for MPGN and LN.
Introduction
Apoptosis or programmed cell death was described by Kerr et al. in 1972. Apoptosis is different from cell necrosis. In healthy glomerulus, the cell cycle is very slow. It was calculated as 0.01% in mouse glomerulus and 0.03% in human glomerulus.Citation[1] Apoptosis increases during the recovery phase of acute renal failure and proliferative glomerulonephritis.Citation[2&3] Severe apoptosis can be the cause of parenchymal cell loss and tubular atrophy, and this process contributes to the progression of chronic renal disease.Citation[4] Fibrosis may increase due to inhibition of the fibroblast apoptosis. Increased apoptosis in neutrophils is found in postinfectious glomerulonephritis.Citation[5]Increased apoptosis in renal tissue (50–100 times) has also been reported in antineutrophil cytoplasmic antibodies (ANCA) positive vasculitis, IgA nephritis, and proliferative nephritis. Increased apoptosis and apoptosis-related gene expression have been reported in polycystic kidney disease.Citation[6]
PCNA is a nuclear protein occurring during normal cellular proliferation. PCNA is synthesized in every phase of the cell cycle except S1. PCNA can be used as a proliferation marker. It is an acidic, nonhistone polypeptide with 36 kD weight and a cofactor for DNA replication. When the PCNA is used to measure the proliferative capacity of normal renal cells, the highest PCNA ratio was found in glomerular and peritubular endothelial cells (38–43%),Citation[7-9] respectively.
We evaluated apoptosis and PCNA in renal biopsy examples of patients with primary MPGN and class IV lupus nephritis. The aim of this study is to investigate the correlation between clinical course and apoptotic indexes and PCNA ratio.
Subjects and Methods
In this study, there were 21 patients with lupus nephritis class IV (G1) and 18 patients with MPGN (G2). All of the patients had proteinuria. Patients also had microscopic hematuria, 33% had decreased renal function, 65% had hypertension, and 75% had edema. American Rheumatism Association (ARA) 1982 criteria were used to diagnose the lupus nephritis.Citation[10] Primary MPGN was diagnosed with appropriate renal histopathologic findings and with the exclusion of systemic disease, including collagen tissue disease and infectious causes. Physical examination, biochemical tests, including blood levels of blood urea nitrogen (BUN), creatinine, glucose, total protein, albumin, lipids, sodium, potassium, calcium, phosphorus, uric acid and creatinine clearance (Ccr), daily proteinuria, complete blood count (hematocrit, hemoglobin, white blood cell, platelets), and erythrocyte sedimentation rate were measured at the beginning, 3rd, 6th, and 12th months.
Staining Procedure
Renal biopsy specimens were fixed in Do Bosoq Brasil solution, and tissues were embedded in paraffin and cut in 4 mm sections. The sections were stained with hematoxylen eosin, periodic acid Schiff reagent, methanamine silver, and Masson trichrom. TUNEL method (Apoptag, peroxidase kits S7100) was used for determination of apoptosis. Dark black colored and pyknotic nuclei were accepted as apoptotic cells. All glomerular apoptotic cells were counted, and the apoptotic index was calculated as the ratio of apoptotic cells to the glomerulus number. Apoptotic cells and PCNA positive cells and total cells were also counted in interstitium in a 40 × 10(400) power field area that did not contain glomerulus. Apoptotic cells and PCNA positive cells were counted among the 100 tubular cells. A PCNA-positive cell was accepted as reddish-brown nuclear staining by monoclonal anti-PCNA antibody PC-10 (Biogenex).
Statistical Analyses
Data are expressed as mean ± standard deviation or percent. A logit binary model for repeated response was used for statistical evaluation.βhD and βipcna measured effects of PCNA and apoptosis with error groups in response (Ccr and proteinuria). β measured repeated effects, γi measured between PCNA and apoptosis and repeated measure correlation.Citation[11]
Results
Mean ages of patients were 29.28 ± 9.66 (R = 16–51), 33.88 ± 15.85 (R = 16–71), and 31.41 ± 12.92 (R = 16–71) in G1, G2, and G1 + G2, respectively. In G1 only one patient (4.8%) was male. Thirteen patients (72.2%) were men in G2. The ratio of males to females was significantly different in two groups (p < 0.0001).
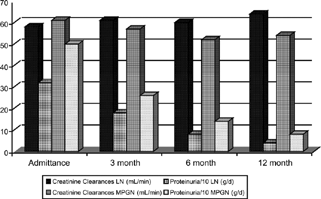
There were no differences between two groups for blood pressure, hematocrit, white blood cells, levels of blood urea nitrogen, Ccr, cholesterol, or triglyceride. In G1, daily proteinuria was lower than G2 on baseline, 3, 6, and 12 month measures (p = 0.0025, p = 0.025, p = 0.015, p = 0.015). There were significant differences between the values of baseline and 12 months on G1 and G2 for daily proteinuria (p < 0.001 for both). shows levels of proteinuria and Ccr of patients at 1, 3, 6, and 12 months, apoptotic indexes and PCNA ratios and activity and chronicity indexes in renal biopsies in G1. shows levels of proteinuria and Ccr of patients at 1, 3, 6, and 12 months, apoptotic indexes and PCNA ratios in G2. shows the mean value of proteinuria and creatinine clearance of patients at baseline and follow-up.
Table 1. In Patients with Lupus Nephritis: Apoptosis, PCNA, Activity and Chronicity Indexes, Creatinine Clearance, and Daily Proteinuria.
Table 2. In Patients with Membranoproliferative Glomerulonephritis: Apoptosis, PCNA, Creatinine Clearance, and Daily Proteinuria.
Figure 1. The mean value of proteinuria and creatinine clearance of patients at baseline and follow-up.
In G1 there were significant correlations between activity indexes and PCNA positivities of tubular andinterstitial (r = 0.789, p < 0.001, r = 0.721, p < 0.0001, respectively). There were no correlations between apoptosis (interstitial and glomerular) and activity indexes. Activity indexes and glomerular PCNA positivity were not correlated.
In G1 there was one patient with Ccr less than 10 mL/m. In this patient, renal function did not recover at the end of 1 year, the activity index was 8, and the chronicity index was 6. PCNA were found to be 9%, 8%, and 14% in glomerulus, tubuli, and interstitium, respectively. PCNA was 20% in normal glomerulus. The apoptotic index in this patient was found to be 0.4 and 1 in glomerulus and in interstitium, respectively.
There were two patients who had Ccr less than 10 mL/min in G2. The PCNA ratio was lower, but the tubular and interstitial apoptotic indexes were higher in these patients. In the follow-up period, clinical improvement did not develop.
In six patients in G1, Ccr was 10–50 mL/min. In four of these patients, renal dysfunction improved at the end of 1 year. This increment was not statistically significant. Activity indexes of these patients were 8–16, and chronicity indexes were lower. The glomerular PCNA ratio was between 5% and 8% except for one patient (glomerular PCNA ratio was 20%). However, PCNAs of tubular and interstitial were between 15–72% and 18–83%, respectively. These levels were high as compared to patients who have lower Ccr (< 10 mL/min). In this group, apoptotic indexes in glomerulus and interstitium were found to have increased. There was statistically significant correlation between the PCNA ratio in renal biopsy and daily proteinuria. There was also a significant correlation between Ccr and PCNA in tubuli and interstitium.
In G2 there were four patients with Ccr less than 10–50 mL/min. Two of these four patients developed end-stage renal failure at the end of 1 year. In one patient, renal function recovered. There were no correlations between PCNA ratio and apoptotic indexes and Ccr and daily proteinuria. PCNA ratios were 8–18% in glomerulus, 8–58% in tubuli, and 7–33% in interstitium. In patients who had Ccr 10–50 mL/min, the PCNA ratio was lower in MPGN than in ln.
In patients with ln who had Ccr > 50 mL/min, loss of renal function was not determined. In these patients, there was no correlation between apoptotic index and Ccr. Glomerular PCNA ratio and Ccr were not correlated. However, the PCNA ratios in tubuli and interstitium were increased. Chronicity indexes were lower, and activity indexes were markedly higher.
In 12 patients with MPGN, who have Ccr > 50 mL/min, renal function did not decrease at the end of 1 year. Although no correlation was found between apoptotic indexes and Ccr, there were statistically significant correlations in tubular and interstitial PCNA and Ccr. In patients with higher PCNA ratios, proteinuria also improved.
Positive correlations were found between activity indexes and tubular and interstitial PCNA ratios in patients with ln. In patients with higher activity indexes and PCNA ratio, proteinuria improved. Patients with a lower activity index, higher chronicity index, and lower PCNA ratio, did not improve.
There was significant correlation between activity indexes and PCNA in tubuli and interstitium in G1(r = 0.789, p < 0.001, r = 0.721, p < 0.001, respectively). However, activity indexes were not associatedwith apoptotic indexes (in tubuli and interstitium), PCNA ratios (in glomerulus), Ccr, and daily proteinuria (p > 0.05 for all).
For higher Ccr probability (>50 mL/min) tubular PCNA in G1 increased 1.1 times according to G2, and this value was not statistically significant. The same value for PCNA in interstitium was 1.006.
Ccr levels were not different when the patients were classified as having lower and higher PCNA ratios of tubular and interstitial tissue. The probability of higher Ccr for higher PCNA ratio in tubular was 0.73 according to lower tubular PCNA. The same value was 0.54 for interstitial PCNA. The ratio of higher Ccr (> 50 mL/m) in G1 was 1.05 times increased according to G2, but this proportion was not significant. The same parameter was found to be 0.96 for apoptosis in interstitium. Higher (> 1) and lower (< 1) apoptosis in glomerulus and interstitium did not cause differences for Ccr in G1 and G2 (p = 0.53, p = 0.56, respectively).
The ratios for higher proteinuria in G1 were 0.98 and 0.97 according to G2 for PCNA in tubular and interstitium, respectively, but were not statistically significant. Daily proteinuria was not found to be different in patients with lower or higher PCNA levels. The probability of lower proteinuria in 6 months and 12 months for a higher PCNA ratio in tubular and interstitial increased 1.9 times according to the lower PCNA ratio in tubular and interstitium. A higher PCNA ratio in glomeruli was not found in G1, so glomerular PCNA for proteinuria could not be evaluated. The probabilities of lower proteinuria in G1 were 1.19 and 1.18 (glomerular and interstitial) according to G2, but were not significant. Higher and lower apoptosis ratios did not differentiate for Ccr levels in G1 and G2.
Conclusion
We investigated the relationship between the course of renal function, daily proteinuria, and renal histopathologic findings (including activity and chronicity indexes, PCNA, apoptosis) in patients with class IV lupus nephritis and primary MPGN.
Two patients with MPGN had Ccr less than < 10 mL/min. PCNA ratios of these patients were lower. However, apoptosis was found to be increased both in glomerulus and interstitium. So, it can be said that when disease-related inflammation decreases, fibrosis and tubular damage increases. Treatment did not affect the course of renal function. In patients with ln who had Ccr lower than 10 mL/min, the PCNA ratio was also lower. However, the relationship between response to therapy and apoptosis cannot be evaluated because of the limited number of patients in this study. Irreversible loss of renal function can be suggested, as there was an increase of fibrosis and a regression of inflammation. In patients who have Ccr 10–50 mL/min (in G1 and G2), renal function improved or remained the same. There were positive correlations between tubular and interstitial PCNA and activity indexes in G1. The same findings were also found in patients with Ccr > 50 mL/min in G1. Activity indexes and tubular and interstitial PCNA ratios were higher in the case of improved renal function in G1. In addition to activity and chronicity indexes, tubular and interstitial PCNA can be markers of renal injury and response to treatment. No loss of renal function suggests that there was acute inflammation and no progression to fibrosis.Citation[3], Citation[12&13] Proteinuria was also improved at the end of 1 year in these patients. In case of lower Ccr or deteriorated renal function, the PCNA ratio was lower, whereas higher and lower Ccr were not different for apoptosis in glomerulus and interstitium.
In patients with MPGN, the PCNA ratio was lower than in patients with ln. Response to treatment in MPGN was not as good as with ln.
Positive correlations were found between activity indexes and tubular and interstitial PCNA ratios in patients with lupus nephritis. In patients with higher activity indexes and PCNA ratio, proteinuria improved. Patients with lower activity indexes, higher chronicity indexes, and lower PCNA ratios, did not improve.
We did not found correlations between apoptosis and renal function or proteinuria in G1 or G2. Was increased apoptosis a signal of fibrosis or inflammation? We cannot comment, because the number of patients was limited in our study. However, similar conclusions were found in some other studies.Citation[3&4], Citation[14&15]
In summary:
Tubular and interstitial PCNA correlated with activity and chronicity indexes of ln.
PCNA can be used as prognostic marker of MPGN, as can activity and chronicity indexes of ln.
Higher PCNA of tubular and interstitial in baseline renal biopsy may suggest good clinical course in both ln and primary MPGN.
Higher PCNA can be used as an indication of aggressive treatment in proliferative glomerulonephritis.
Increased apoptosis can be a marker of fibrosis (or inflammation). There was no correlation between apoptosis in renal biopsy and clinical course.
Acknowledgment
This study was supported by Çukurova University Research Foundation (TF.98.U.2).
References
- Baker A J.; Mooney A.; Hughes J.; Lombardi D.; Johnsville R J.; Savill J. Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J. Clin. Invest. 1994, 94, 2105–2116. [PUBMED], [INFOTRIEVE], [CSA]
- Ledda-Columbano G M.; Columbano A.; Coni P.; Faa G.; Pani P. Cell deletion by apoptosis during regression of renal hyperplasia. Am. J. Pathol. 1989, 135, 657–662. [PUBMED], [INFOTRIEVE]
- Shimizu A.; Kitamura H.; Masuda Y.; Ishizaki M.; Sugisaki Y.; Yamanaka N. Apoptosis in the repair process of experimental proliferative glomerulonephritis. Kidney Int. 1995, 47, 114–121. [PUBMED], [INFOTRIEVE]
- Shimizu A.; Masuda Y.; Kitamura H.; Ishizaki M.; Seigisaki Y.; Yamanaka N. Apoptosis in progressive cresentric glomerulonephritis. Lab. Invest. 1996, 74, 941–951. [PUBMED], [INFOTRIEVE], [CSA]
- Amore A.; Coppo R. Role of apoptosis in pathogenesis and progression of renal disease. Nephron 2000, 86, 99–104. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Woo D. Apoptosis and loss of renal tissue in polycystic kidney diseases. N. Engl. J. Med. 1995, 333, 18–25. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Nakopoulo L.; Stefanaki K.; Salpigidis K.; Boletis J.; Papadakis J.; Zeiss P M.; Vosnides G. The value of proliferating cell nuclear antigen (PCNA)/cyclin in the assessment of cell proliferation in glomerulonephritis. Histol. Histopathol. 1997, 12, 655–662. [CSA]
- Oda T.; Yoshizawa N.; Takeuchi A.; Nakabayashi I.; Nishiyama J.; Ishida A.; Tazawa K.; Murayama M.; Hotta O.; Taguma Y. Glomerular proliferating cell kinetics in acute post-streptococcal glomerulonephritis (APSGN). J. Pathol. 1997, 183, 359–368. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Thomas G L.; Yang B.; Wagner B E.; Savill J.; El Nahas A M. Cellular apoptosis and proliferation in experimental renal fibrosis. Nephrol. Dial. Transplant. 1998, 13, 2216. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Tan B M.; Cohen A S.; Fries J F.; Masi A T.; Mcshane D J.; Rothfield N F.; Schaller J G.; Talal N.; Winchester R J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [PUBMED], [INFOTRIEVE]
- Agresti A.Logit models for repeated binary responses. In Categorical Data Analysis; John Wiley: New York, 1990; 395–400.
- Savill J. Apoptosis: a mechanism for regulation of the cell complement of inflamed glomeruli. Kidney Int. 1992, 41, 607–612. [PUBMED], [INFOTRIEVE]
- Savill J. Regulation of glomerular cell number by apoptosis. Kidney Int. 1999, 56, 1216–1222. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Soto H.; Mosquera J.; Rodrigez-Iturbe B.; Henriquez La Roche C.; Pinto A. Apoptosis in proliferative glomerulonephritis: decreased apoptosis expression in lupus nephritis. Nephrol. Dial. Transplant. 1997, 12, 273–280. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Surgiyama H.; Kashihara N.; Makino H.; Yamasaki Y.; Ota A. Apoptosis in glomerular sclerosis. Kidney Int. 1996, 40, 103–111.