Abstract
Background: Oxidative stress (OxS) induced by diabetes plays an important role in the development of diabetic nephropathy. Studies have shown that antioxidants are beneficial in its reduction. Vitamin E has been documented as providing the most improvement in the antioxidative status. Recently, pentoxifylline (PTX) has been proposed to have antioxidant properties. Aim: The aim of the study was to assess the ability of two antioxidants to reduce lipid peroxidation and renal hypertrophy in vivo. Methods: Diabetes was induced by streptozotocin (STZ) in rats. Treatment groups 7were divided as follows: healthy (H), diabetic without treatment (STZ), PTX treated group (STZ + PTX), and vitamin E supplemented (STZ + E) group. At 8 weeks, kidneys were removed; one was homogenized to quantify lipoperoxide levels (LPOS), and the other was used to study the morphological changes by electron microscopy (EM). Additionally, plasma total antioxidant activity (TAA) was quantified. Results: A reduction in LPOS was observed in both groups: PTX and vitamin E with regard to STZ group. PTX increased TAA compared to STZ + E, which restored it to its normal values. However, both treatments reduced the LPO/TAA ratio to lower basal levels; hence, similar results were obtained in terms of correcting functional parameters. Structural changes in STZ rats included a glomerular membrane thickening, podocyte flattening, as well as loss of fenestration in the endothelial layer. All these changes were less aggressive for treated rats. Conclusions: Vitamin E and PTX have potential therapeutic properties that may help to retard the rate of deterioration of diabetic kidneys.
Introduction
Glucose is the major energy source for most mammalian cells and provides the major part of the mass of macromolecular synthesis. Excessive glucose, however, can be transported intracellularly and metabolized to change the redox potential, to increase sorbitol production via aldose reductase.Citation[1&2] This leads to an increase in reactive oxygen species (ROS) production as well as to the decrease of free radical scavenging molecules.Citation[3] It can alter signal transduction pathways, such as the activation of diacylglycerol (DAG) and protein kinase C (PKC) levels.Citation[4] Therefore, maintaining control over blood glucose level plays an important role in the oxidant/antioxidant balance. Nevertheless, restoring blood glucose to near-normal values is not sufficient to provide complete protection from the development of complications including diabetic nephropathy.
Approaches aimed at modifying the influence of additional factors may prove to be useful in providing supplemental organ protection. Therapeutic strategies involved in the management and prevention of diabetic nephropathy include currently available treatments, such as intensified glycemic control and antihypertensive agents, particularly those that interrupt the renin–angiotensin system, i.e., angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB). Although data from the Diabetes Control and Complications Trial establish a central role for hyperglycemia in diabetic complications,Citation[5] strict control ofglucose in diabetic patients can be difficult and sometimes dangerous.
It has been suggestedCitation[6] that angiotensin II (AII) has direct effects on glomerular cells so that it may induce sclerosis independent of its hemodynamic actions, in view of the fact that agents that interfere with AII action may decrease glomerular injury without altering glomerular pressures. Studies that evaluate the effects of these agents on the development of microalbuminuria report a possible antioxidative mechanism of the nephroprotective action that modifies the oxidant/antioxidant status, supporting a central role of OxS in the pathogenesis of the kidney.Citation[7&8]
Treatment with antioxidants may be a promising intervention to prevent progression of kidney disease. Several studies have reported its benefits in models of glomerular diseaseCitation[9] as well as in diabetic glomerulosclerosis.Citation[10] Not only studies utilizing cultured rat mesangial cells in high glucose media show the effectiveness of vitamin E in preventing lipid peroxidationCitation[11] but also models of oxidative damage show its effectiveness, therefore revealing its nephroprotective properties.Citation[12&13]
It is well documented that vitamin E ameliorates glycemic control,Citation[14] probably by reducing auto-oxidation reactions, limiting advanced glycosylation end-product formation in vivo.
PTX has gained considerable interest as an oxygen free radical scavenger. Several in vitro studies have indicated its antioxidant effects.Citation[15-18] Likewise, we recently reported that PTX protects the diabetic kidney by reversing lipid peroxidation and stimulating the antioxidant status of diabetic rats.Citation[19] In addition to these properties, it is also a modulator of immune function, reducing microalbuminuria in patients with type 2 diabetes who were advancing toward nephropathy.Citation[20-22]
Findings support that both ACEI and PTX are capable of preventing glomerular damage in the early stages of diabetic nephropathy, and, when used together, additional protection has been reported.Citation[23] Therefore, not only ACEI but also other drugs (like PTX or vitamin E) must be considered when extra protection is required. In this study, we examined the ability of two antioxidants to reduce lipid peroxidation and renal hypertrophy in vivo from an early stage of diabetes induced with STZ.
Materials and Methods
Animals
Studies were performed using male Sprague-Dawley rats weighing 230–290 g. We observed the Institution's guide for the care and use of laboratory animals. Rats were fed with standard diet and were given water ad libitum.
Diabetes Induction
Diabetes was induced by a single intraperitoneal injection of streptozotocin [65 mg/kg body weight (b.w.)] freshly dissolved in sterile saline solution. Healthy control rats (H) received equivalent amounts of saline intraperitoneally (i.p.). Two days later, samples of blood were drawn, and the diabetic state was confirmed by measuring the level of blood glucose, using a reflectance meter (One Touch Basic, LifeScan J & J, Milpitas, CA, USA). Rats with blood glucose levels > 250 mg/dL were included in the study. Four groups (n = 9 each) were established by a randomized method as follows: control group (H); diabetic group without treatment (STZ); pentoxifylline-treated diabetic group (STZ + PTX), which was injected daily with 80 mg PTX/kg b.w. i.p.; and finally, the vitamin E supplemented diabetic group (STZ + E). The latter group received a dietary supplementation containing 500 IU of α-tocopherol/kg of chow (Labdiet). Prior to sacrifice, rats were weighed, and 24 h urine samples were collected to determine the albumin excretion rate (UalbV).
Tissue Preparation
Rats were anesthetized with ether 8 weeks after the onset of diabetes. Samples of blood were obtained by cardiac puncture and collected in EDTA tubes in order to analyze the biochemical parameters of interest [total antioxidant activity (TAA), creatinine, glucose, total cholesterol, and triglycerides]. One kidney was removed, cleaned of gross adventitial tissue, blotted dry, weighed, and analyzed for lipoperoxides (LPOS = malondialdehyde and 4-hydroxy-2-nonenal) as an index of kidney lipid peroxidation. The other kidney was perfused with buffered glutaraldehyde, cut, and fixed in the same solution to be studied by EM using standard techniques. All kidneys were coded and blindly evaluated.
The remnant kidney used to quantify LPOS was minced and then homogenized in 20 mM phosphate buffer (pH 7.4) containing butylated hydroxytoluene (BTH) at a final concentration of 5 mM at 4°C for 30 sec (2 × 15 sec with a 15 sec cooling interval) using a polytron homogenizer (Teckman Model TR-10, Cincinnati, OH, USA). The homogenate obtained was centrifuged at 3,000 × g for a period of 10 min at 4°Cin a refrigerated centrifuge (Sorvall RC-5B, Dupont Instruments, Wilmington, DE, USA). The resultant supernatant was used to measure LPOS by the colorimetric assay BIOXYTECH, LPO 586 (Oxis International, Inc.).
Biochemical Assays
Plasma concentrations of total cholesterol, triglycerides, and creatinine were measured enzymatically in a clinical chemistry analyzer (Hitachi 911, Roche). TAA was measured by a colorimetric technique as described by Miller et al.Citation[24] using a commercial kit (Randox Laboratories LTD, UK). Twenty-four-hour urine samples were obtained from animals kept in metabolic cages with access only to drinking water. Creatinine was measured in these samples as well as albumin concentration, which was determined by radioimmunoassay (Euro/Diagnostic Products Corporation, LA, USA).
Serum vitamin E levels were analyzed by high-pressure liquid chromatography.Citation[25&26] The assay was modified by including the antioxidant butylated hydroxytoluene during the extraction with heptane. All samples were analyzed in one run of the assay to avoid interassay variation.
Statistical Data Analysis
Results were expressed as mean ± standard error of the mean (SEM). Differences among the four groups were evaluated by one way ANOVA, followed by the Bonferroni method where appropriate. Statistical significance was ≤ 5%.
Results
All rats that received STZ became diabetic (blood glucose level 250 mg/dL, P < 0.001 versus H group); additionally, hypercholesterolemia and hypertriglyceridemia were observed (). PTX or vitamin E treatments had no effect on blood glucose concentration (P > 0.05). At the end of the study, PTX had not changed the levels of lipid parameters, although vitamin E treatment tended to normalize cholesterol and triglyceride levels and did not reach statistical significance after 8 weeks of treatment (P > 0.05). shows the ratio of vitamin E to the amount of triglycerides (vitamin E/TG) in three groups. At the start of the study, the ratio was significantly reduced in the STZ group (3.65 ± 0.082 and 3.07 ± 0.013 versus 11.87 ± 0.036 µg/mg, P < 0.05). This occurred as a result of a high level of TG, diminishingmore at 8 weeks (1.30 ± 0.085 µg/mg, P < 0.05). This was in contrast to the H group that showed no modification of TG level. However, vitamin E treatment returned this ratio toward normal levels (12.19 ± 0.09 versus 11.87 ± 0.036 µg/mg, P > 0.05). shows the renal function parameters with uncontrolled diabetes that resulted in glomerular hyperfiltration (Ccr = 1.63 ± 0.07 mL/min/100 g b.w., P < 0.05 at 8 weeks versus H group). Such hyperfiltration was reversed by antioxidant therapies (PTX: 0.82 ± 0.078 mL/min/100 g b.w. and 0.88 ± 0.05 mL/min/100 g b.w., vitamin E, P > 0.05).
Table 1. Effects of PTX and Vitamin E on Metabolic Parameters in STZ-Induced Diabetes.
Table 2. Vitamin E/TG Ratio (µg/mg).
Table 3. Renal Function Parameters.
There was a significant increase in UalbV 2 days after STZ (0.40 ± 0.07 versus the values previous to diabetogenic 0.0020 ± 0.0001 mg/day, P < 0.0001). In uncontrolled diabetes, this showed a clear advance toward proteinuria (0.70 ± 0.24 mg/day, at 8 weeks), while in the STZ + PTX group, a significant decrease was shown (0.27 ± 0.15 mg/day, P < 0.05). Vitamin E treatment reduced UalbV (0.41 ± 0.07 mg/day, P < 0.05), confirming the nephroprotective effects of these compounds. Both treatments significantly reduced UalbV, although the final values for proteinuria were not statistically different in these two groups.
LPOS levels were significantly elevated in all STZ groups compared to those in H group 2 days after STZ (P < 0.05) (). Levels increased throughout the experiment and were higher in STZ rats than in H group rats (9.9 ± 1.71 versus 2.1 ± 0.41 µM LPO/g, P < 0.001). LPOS levels in the STZ + PTX group were not different from those of H group (3.5 ± 1.1 versus 2.1 ± 0.41 µM LPO/g, P > 0.5). Vitamin E treatment reduced more LPO levels (0.74 ± 0.16 µM LPO/g, P > 0.5).
Table 4. Effect of PTX and Vitamin E on STZ-Induced Diabetes.
At the beginning of the experiment (2 days), TAA in plasma was found to be significantly increased in the STZ group in relation to the group of healthy rats (2.40 ± 0.46, 2.90 ± 0.54, 2.3 ± 0.40 mmol/L versus 1.0 ± 0.26 mmol/L, P < 0.001). When we examined the progression of TAA in STZ rats, this activity decreased significantly during a period of 8 weeks (1.6 ± 0.15 mmol/L versus 2.4 ± 0.46 mmol/L, P < 0.05). The effect of PTX at the end of the experiment was increasing the TAA with respect to the STZ group (3.82 ± 0.23 versus 1.6 ± 0.15 mmol/L,P < 0.001), contrary to the vitamin E group that had normalized the values (1.1 ± 0.06 versus 0.9 ± 0.17 mmol/L, P < 0.05).
The LPO/TAA ratio increased with time, threefold in the STZ group at 8 weeks (6.2 × 10− 3 versus 2.3 × 10− 3, P < 0.001). Contrary to oxidant/antioxidant balance with the antioxidant treatments, they were consistently lower compared to H group, confirming its effectiveness.
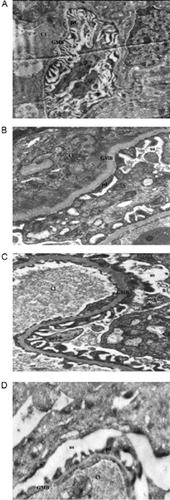
Histopathologic findings by EM at 8 weeks revealed that the structures in the H group () were preserved. There is evidence of glomerular basement membrane (GMB) thickening and podocyte flattening in STZ rats (), as well as loss of fenestrations in the endothelial layer of capillaries, classic changes of diabetic glomerulopathy. In the PTX-treated STZ group (), mild changes were evident in the thickness of the basement membrane, the podocytes were preserved, and fenestrations in endothelial layers occurred, contributing, therefore, to the improvement of renal function. These attenuated changes are indicative of a suppressive effect, at this point in time, by PTX. Vitamin E treatment caused only subtle changes in the glomerular structures ().
Discussion
Findings in the present study support the hypothesis that lipid peroxidation plays a vital role in the development of diabetic nephropathy. We found that by the end of 8 weeks, LPOS levels increased significantly in streptozotocin-induced diabetic rats, which was associated with a significant decrease in TAA. In the same way, previous studies showed that OxS occurs in the glomeruli from the early stage of diabetes, mainly in the renal cortex of rats during experimental diabetes, as a consequence of disturbances of redox balance, leading to renal excretory dysfunction.Citation[27&28]
In response to OxS induced by STZ, antioxidant enzymes are produced to protect cellular and tissue injury in the initial stage.Citation[29] In the same way, our results show a significantly increased TAA at 2 days after STZ. Nevertheless, by the end of the study, antioxidant levels were reduced in untreated STZ rats and were favorable to a significantly increased LPOS/TAA ratio. Thus, the differences in antioxidant levels during the experiment seem to depend on the duration of hyperglycemia.
Results of antioxidant treatments in diabetic animals have been mostly positive, namely, vitamin E supplementation exhibited a beneficial reduction in OxS in animal models of diabetes.Citation[30&31] In addition to their well-established effects, we observed an important reduction in LPOS levels in the kidney, as well as a normalized TAA.Also, when compared to the H group, the LPOS/TAA ratio appeared to be reduced. Our findings are consistent with previous studies reporting that vitamin E reduces urinary lipophilic aldehydes and renal enlargement in streptozotocin-induced diabetic rats.Citation[32] Wagner's studies also demonstrated that vitamin E slows the rate of free-radical-mediated lipid peroxidation in cells.Citation[33] In the same way, antioxidant enzyme activity in the kidney also demonstrated vitamin E protection.Citation[34]
Effects of this lipidic antioxidant tended toward normal lipid levels. Statistical significance was not reached after 8 weeks of treatment, even though its contribution could be observed in the normalization of vitamin E/TG ratio to control levels. The final result was a significantly reduced level of TG and a subtle increase in vitamin E levels. We took this ratio into account as a measure of the concentration of the liposoluble vitamin E in its natural environment. Thus, we observed that the highest values of this ratio appeared in the H group, as opposed to the STZ group, where it was gradually reduced during the experiment as a consequence of hypertriglyceridemia.
The effect of PTX on LPO was found to be less effective than the effect of vitamin E. In the same manner as vitamin E, however, it reduced LPOS levels, as PTX did. TAA had a more significant increase, taking into account its antioxidant strength effects. It also showed improvement in the LPO/TAA ratio, to the same extent as vitamin E. Both compounds were capable of buffering ROS; hence, similar results were obtained in terms of correcting functional renal parameters as well as conserving glomerular structures.
Recent studies have suggested that a range of pathways is implicated in the increase of ROS in diabetes.Citation[35] Different kinds of ROS are generated, so it is evident that vitamin E and PTX influence different specific oxidative pathways, i.e., vitamin E mainly stabilizes lipid radicals, as opposed to PTX, which is mainly a hydroxyl radical scavenger.Citation[22]
In this context, it is well documented that lipophilic antioxidants have more potent scavenging properties than hydrophilic antioxidants, because they react preferentially with ROS before more vital structures may be attacked, thus being preventive antioxidants. This property could explain our results of lower basal levels in LPO at the end of the study in the STZ + E group.
In this study, we employed lipid peroxidation as an index of oxidative damage, because it is perhaps the most extensively studied consequence of free radical attack, although other important molecules are also modified.
Our data demonstrate that α-tocopherol supplementation at 500 IU/kg chow is efficacious in controlling proteinuria as well as in ameliorating the OxS. Nevertheless, it has been demonstrated by Chan et al.Citation[9] that with a 2.5-to 5-fold reduction (100 and 250 IU/kg chow) in the same period, this vitamin E dose is still efficacious.
It is well documented that vitamin E supplementation can improve insulin efficiency and glycemic equilibrium, as has been shown by the decrease of glycemia, glycated hemoglobin, and fructosamine values.Citation[14] We found that PTX and vitamin E treatments had no suppressive effects on hyperglycemia in diabetic rats. The probable reason was that β cells had already been destroyed by STZ, and this damage was irreversible.
From the early stage of diabetes, OxS increased without reflecting a common consequence of diabetes-induced glomerular damage but supported the primary role of OxS in the pathogenesis of diabetic nephropathy.
The utility of antioxidant treatments during development of complications is justifiable, taking into account that the increased production of ROS by independent biochemical pathways is perhaps the common factor between hyperglycemia and the diabetic pathogenesis, thus evidenced by Nishikawa et al.Citation[36]
In summary, our results suggest the following: increased LPOS may be an important reason for morphological and functional disruption of glomeruli; the differences in antioxidant levels during the progression to proteinuria in the STZ group may be the consequence of a progressively debilitated antioxidative mechanism through the process of glycation; and vitamin E and PTX have potential therapeutic properties that may help to slow the rate of deterioration in kidney function.
Acknowledgments
MED-E received the fellowship 92290 from CONACyT-Mx during her doctoral training at UASLP. This work has been supported by grants 19980202034 from SIHGO-CONACyT and 25887-M from the National Council of Sciences (CONACyT-Mx) to FM.
References
- Nakamura N.; Obayashi H.; Fujii M.; Fukui M.; Yoshimori K.; Ogata M. Induction of aldose reductase in cultured human microvascular endothelial cells by advanced glycation end products. Free Radic. Biol. Med. 2000, 29 (1), 17–25. [PUBMED], [INFOTRIEVE], [CSA]
- Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Inter., Suppl. 2000, 77, S3–S12. [CROSSREF], [CSA]
- Opara E C.; Abdel-Rahman E.; Soliman S.; Kamel W A.; Souka S.; Lowe J E. Depletion oftotal antioxidant capacity in type 2 diabetes. Metabolism 1999, 48, 1414–1417. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Koya D.; King G L. Protein kinase C activation and the development of diabetic complications. Diabetes 1998, 47, 859–866. [PUBMED], [INFOTRIEVE]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977. [CROSSREF]
- Remuzzi A.; Fassi A.; Sangali F.; Malanchini B.; Mohamed E I.; Bertani T.; Remuzzi G. Prevention of renal injury in diabetic MWF rats by angiotensin II antagonism. Exp. Nephrol. 1998, 6, 28–38. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Ha H.; Kim K H. The amelioration of diabetic microalbuminuria and lipid peroxidation by captopril. Yonsei Med. J. 1992, 33, 217–223. [PUBMED], [INFOTRIEVE]
- de Cavanagh E M.; Inserra F.; Toblli J.; Stella I.; Fraga C G.; Ferder L. Enalapril attenuates oxidative stress in diabetic rats. Hypertension 2001, 38, 1130–1136. [PUBMED], [INFOTRIEVE]
- Chan W.; Krieg R J., Jr.; Norkus E P.; Chan J C.M. α-Tocopherol reduces proteinuria, oxidative stress, and expression of Transforming Growth Factor β in IgA nephropathy in the rat. Mol. Genet. Metab. 1998, 63, 224–229. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Koya D.; Lee I K.; Ishii H.; Kanoh H.; King G L. Prevention of glomerular dysfunction in diabetic rats by treatment with d-α-tocopherol. J. Am. Soc. Nephrol. 1997, 8, 426–435. [PUBMED], [INFOTRIEVE], [CSA]
- Trachtman H. Vitamin E prevents glucose-induced lipid peroxidation and increased collagen production in cultured rat mesangial cells. Microvasc. Res. 1994, 47, 232–239. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Mekinova D.; Chorvathova V.; Volkouova K.; Staruchova M.; Grancicova E.; Kivanova J.; Ondreicka R. Effect of intake of exogenous vitamins C, E and beta-carotene on antioxidative status in kidneys of rats with streptozotocin-induced diabetes. Nahrung 1995, 39, 257–261. [PUBMED], [INFOTRIEVE], [CSA]
- Fryer M J. Vitamin E as a protective antioxidant in progressive renal failure. Nephrology 2000, 5, 1–7. [CROSSREF]
- Ihara Y.; Yamada Y.; Toyokuni S.; Miujawaki K.; Ban N.; Adachi T. Antioxidant α-tocopherol ameliorates glycemic control of GK rats, a model of type 2 diabetes. FEBS Lett. 2000, 473, 24–26. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Horvath B.; Marton Z.; Halmosi R.; Alexy T.; Szapary L.; Vekasi J. In vitro antioxidant properties of pentoxifylline, piracetam, and vinpocetine. Clin. Neuropharmacol. 2002, 25 (1), 37–42. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Bhat V B.; Madyastha K M. Antioxidant and radical scavenging properties of 8-oxo derivatives of xanthine drugs pentoxifylline and lisofylline. Biochem. Biophys. Res. Commun. 2001, 288 (5), 1212–1217. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Freitas J P.; Filipe P.; Guerra Rodrigo F. Potential antioxidative effects of pentoxifylline. C.R. Seances. Soc. Biol. Fil. 1995, 189 (3), 401–405. [PUBMED], [INFOTRIEVE], [CSA]
- Freitas J P.; Filipe P M. Pentoxifylline: a hydroxyl radical scavenger. Biol. Trace Elem. Res. 1995, 47 (1–3), 307–311. [PUBMED], [INFOTRIEVE], [CSA]
- Dávila-Esqueda M E.; Martínez F. Pentoxifylline protects the streptozotocin induced oxidative damage to renal tissue in rats. Experimental Diabetes Research 2004, 5, 1–17. [CSA]
- Navarro J F.; Mora C. Antiproteinuric effect of pentoxifylline in patients with diabetic nephropathy. Diabetes Care 1999, 22, 1006–1008. [PUBMED], [INFOTRIEVE], [CSA]
- Guerrero-Romero F.; Rodríguez-Moran M.; Paniagua-Sierra J R.; Garcia-Bulnes G.; Salas-Ramírez M.; Amato D. Pentoxifylline reduces proteinuria in insulin-dependent and non-insulin-dependent diabetic patients. Clin. Nephrol. 1995, 43, 116–121. [PUBMED], [INFOTRIEVE], [CSA]
- Tripathi K.; Prakash J.; Appaiha D.; Srivastava P K. Pentoxifylline in management of proteinuria in diabetic nephropathy. Nephron 1993, 64 (4), 641–642. [PUBMED], [INFOTRIEVE]
- Harmankaga O.; Seber S.; Yuksel A.; Yilmaz M.; Unsal A. Combination of pentoxifylline with angiotensin converting enzyme inhibitors produces an additional reduction in microalbuminuria in hypertensive type 2 diabetic patients. Ren. Fail. 2003, 25 (3), 465–470. [CROSSREF]
- Miller N J.; Rice-Evans C.; Davies M J.; Gopinathan U.; Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [PUBMED], [INFOTRIEVE]
- Cooper J D.H.; Thadwal R.; Cooper M J. Determination of vitamin E in human plasma by high-performance liquid chromatography. J. Chromatogr., B 1997, 690, 355–358.
- Julianto T.; Yuen K H.; Noor A M. Simple high-performance liquid chromatography. J. Chromatogr., B. 1999, 732, 227–231.
- Koya D.; Hayashi K.; Kitada M.; Kashiwagi A.; Kikkawa R.; Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J. Am. Soc. Nephrol. 2003, 14, S250–S253. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Jachec W.; Tomasik A.; Tarnawski R.; Chwalinska R. Evidence of oxidative stress in the renal cortex of diabetic rats: favorable effect of vitamin E. Scand. J. Clin. Lab. Invest. 2002, 62, 81–88. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Torres M D.; Canal J R.; Pérez C. Oxidative stressin normal and diabetic rats. Physiol. Res. 1999, 48, 203–208. [PUBMED], [INFOTRIEVE], [CSA]
- Fryer M J. Vitamin E as a protective antioxidant in progressive renal failure. Nephrology 2000, 5, 1–7. [CROSSREF]
- Görgün F M.; Gümüstas M K. Vitamin E supplementation in streptozotocin-treated rats alters cerebelar and plasma nitric oxide metabolism. J. Toxicol. Environ. Health, Part A 2002, 65, 631–637. [CROSSREF], [CSA]
- Kim S S.; Gallaher D D.; Csallany A S. Vitamin E and Probucol reduce urinary lipophilic aldehydes and renal enlargement in streptozotocin-induced diabetic rats. Lipids 2000, 35 (11), 1225–1237. [PUBMED], [INFOTRIEVE], [CSA]
- Wagner B A.; Buettner G R.; Burns C P. Vitamin E slows the rate of free radical-mediated lipid peroxidation in cells. Arch. Biochem. Biophys. 1996, 334 (2), 261–267. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Van den Braden C.; Deman A.; Ceyssens B.; Pauwels M.; Empsen C.; Verbeelen D. Vitamin E protects renal antioxidant enzymes and attenuates glomeruloesclerosis in Adriamycin-treated rats. Nephron 2002, 91 (1), 129–133. [CROSSREF], [CSA]
- Lee H B.; Yu M R.; Yang Y.; Jiang Z.; Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14 (8), S241–S245. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Nishikawa T.; Edelstein D.; Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int.periodicpubfield>2000, 58, S26–S30. [CROSSREF]