Abstract
Objective: The objective was primary to evaluate the safe use of a new calcium channel blocker, lercanidipine, in patients with chronic renal failure (CRF). The secondary objective was to study the protective effect of calcium channel blocker on renal function in CRF patients previously treated with ACE inhibitors or angiotensin receptor blockers. Design and Methods: The study recruited 203 CRF patients (creatinine > 1.4 mg/dL for males, creatinine > 1.2 mg/dL for females, or creatinine clearance < 70 mL/min). All patients were receiving ACE inhibitors (63.4%) or angiotensin II antagonist (36.6%) therapy, but they had higher blood pressure than recommended for CRF (130/85 mmHg). No patients were under diuretic treatment. Patients were clinically evaluated 1, 3, and 6 months after starting treatment with lercanidipine. Samples for urine and blood examination were taken during the examination. When needed, a third drug was added to the treatment, excluding diuretics. Creatinine clearance was measured using 24 h urine collection. Results: 175 patients rendered valuable for the study (age 63.9 ± 11.9 years, 52.9% males and 47.1% females). Blood pressure (BP) significantly decreased from 162 ± 17/93 ± 8.3 mmHg to 132 ± 12/78 ± 6 mmHg. 89.2% of patients showed a significant BP reduction, and 58.1% achieved optimal BP control (< 130/85 mmHg). Seven patients (3.4%) showed untoward effects. Not one case of edema was detected, and the prevalence of adverse effects related to vasodilatation was extremely low (three patients, 1.48%). Plasmatic creatinine did not change (1.9 ± 0.5 baseline versus 1.9 ± 0.6 mg/dL), but creatinine clearance increased at the end visit (41.8 ± 16.0 baseline versus 45.8 ± 18.0 mL/min, p = 0.019). Plasmatic cholesterol also decreased from 221 ± 46 to 211 ± 35 mg/dL (p = 0.001). Conclusions: Lercanidipine showed a high antihypertensive effect in CRF patients. It has a good tolerability profile and showed an interesting effect on plasmatic lipids. An improvement in renal function, measured through creatine clearance, was detected.
Introduction
Hypertension is a major determinant of progression of renal disease, irrespective of cause, and the relative risk of developing end-stage renal disease in hypertensive patients (compared with that of patients with “optimal” BP) increases threefold when diastolic BP increases to 90 mmHg.Citation[1] It is widely known that the cardiovascular system is affected profoundly by the presence of advanced renal failure.Citation[2] The Hypertension Detection and Follow-up Program (HDFP) studyCitation[3] showed that baseline serum creatinine had a significant prognostic value for 5-and 8-year all-cause mortality. The presence of proteinuria in hypertensive patients is also a powerful predictor of higher cardiovascular morbidity and mortality.Citation[4] Tighter BP control is the main mechanism for preventing the progression of chronic renal failure.Citation[5&6] Antihypertensive agents, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARB), seem to have an additional organ-protective role and are routinely used in renal disease.Citation[5]
In spite of its antihypertensive efficacy, dihydropyridine calcium antagonists are often reported to induce side effects responsible for treatment withdrawal or replacement with a drug of a different class. Lercanidipine is a new dihydropyridine calcium antagonist with high lipophilicity and high vascular selectivity, which confer it a gradual and prolonged antihypertensive effect and a good tolerability as compared with other dihydropyridine calcium channel blockers.Citation[7-9] However, the renoprotective effect of calcium antagonist is a controversial issue, in spite of a growing amount of information regarding its beneficial effect.Citation[10] In this way, it has been suggested that calcium antagonist could improve renal function in patients previously treated with angiotensin-converting enzyme inhibitors.Citation[11]
This article reports the results of the ZAndip en Función Renal Alterada (ZAFRA) Study with the aim to assess the safety and effectiveness of lercanidipine in patients with chronic renal failure and the protective effects of calcium channel blockers on renal function in patients with reduced baseline renal function.
Material and Methods
A total of 203 hypertensive renal disease patients from 16 Spanish centers was recruited. All patients werereceiving treatment with either ACE inhibitors or ARB and have chronic renal failure defined as increased plasma creatinine (≥ 1.4 for males or ≥ 1.2 for females) or decreased creatinine clearance (< 80 mL/min). To be included, the patients should have high blood pressure defined by the Clinical Guidelines of the WHO-IHS for renal disease patients (systolic BP ≥ 130 or diastolic BP ≥ 85 mmHg). No patients received diuretic treatment or other cardiovascular therapy simultaneous to angiotensin axis blocking agents.
Antihypertensive therapy with the long-acting calcium antagonist lercanidipine at a dose of 10 mg once a day was given to all patients. They were followed for 6 months, and four visits were scheduled (at inclusion, 1, 3, and 6 months after beginning). Additional therapy (alfa blocker or beta blockers) was prescribed to reach the randomized target BP when it was not achieved at 1 month: Patients who did not reach the target BP were scheduled for a facultative visit 30 days after adding a third antihypertensive agent (2 months). If BP was still higher than the target value, the patient could be excluded for follow-up by the investigator clinic criteria. Blood pressure, heart rate, adverse effects, symptom checklist, and compliance to treatment were assessed at each visit. BP was measured by a standard mercury sphygmomanometer approximately 24 h after the last drug intake. Two measurements, taken at 3 min intervals in the sitting position, were averaged and used as the clinical BP reference value. Heart rate was measured from the radial pulse for 30 sec.
According to the protocol, serum creatinine had to be measured, at each recruiting center, by standard laboratory techniques at every visit. Creatinine clearance was estimated using 24 h urine collection, which was also used to stimulate proteinuria or microalbuminuria. Blood samples were also analyzed for cholesterol, triglycerides, glucose, urate, and ionogram. A complete hemogram was also performed at every visit.
Statistical analyses were performed by a computer program. Data are reported as mean with one standard deviation. Differences between continuous variables were compared by the use of student's t test for paired samples. Differences in proportion were challenged using the McMemar test due to the existence of paired values. A P value of < 0.05 was considered statistically significant; all P values are two-tailed.
Results
Twenty-eight patients were excluded from evaluation due to protocol violations during the inclusion. The study ended with 175 patients: 92 men and 82 women, mean age 63.9 ± 11.0 years. There were 95 (54.3%) overweight patients (BMI ≥ 25 and < 30 Kg/m2), and 45 (25.7%) were obese (IMC ≥ 30 kg/m2). Twenty-nine patients were smokers.
Causes of renal failure are shown in . Forty-two patients were diabetics (39 type 2 and three type 1). Forty-six patients have proteinuria ≥ 500 mg/day. Fifty (28.6%) patients have mild chronic renal failure (creatinine clearance < 80 mL/min and > 50 mL/min); 103 patients (58.9%) have moderate chronic renal failure (creatinine clearance < 50 mL/min and ≥ 25 mL/min), and 22 (12.6%) showed advanced renal failure (creatinine clearance < 25 mL/min).
Table 1. Causes of Renal Failure.
A total of 43 patients discontinued the study because of adverse events (n = 1), poor compliance to treatment(n = 1), high blood pressure in spite of treatment (n = 26), poor compliance with study procedures (n = 10), end-stage renal failure (n = 1) and interruption of follow-up (n = 4). Four patients showed untoward effects during the follow-up (erectile disfunction, n = 1; urine incontinence, n = 1; mouth dryness; eosinophilia, n = 1). No patients complained about lower limb swelling or heaviness, and edema was not detected at inspection. Three more subjects from the group of patients excluded from evaluation presented adverse reactions (flush, n = 2; unspecific complains, n = 1). These three latter patients have been taken into account to calculate incidence of adverse reactions.
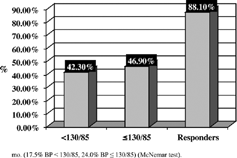
After 1 month of treatment, SBP and DBP were significantly (p < 0.001) reduced by lercanidipine (from 162.0 ± 16.6/93.2 ± 8.3 baseline to 142.8 ± 15.5/83.1 ± 8.1 mmHg). Similar results were observed for values entering in the analysis at 3 and 6 months (see values in ). At 6 months mean BP reduction from baseline was − 29.8/ − 14.5 mmHg (relative reduction 18.0/15.1%). Heart rate () was similar before and during treatment. After 6 months of treatment, the percentage of normalized patients was 42.3%, and the proportion of patients who needed to add a third antihypertensive agent was 41.5% ().
Table 2. Changes in Blood Pressure.
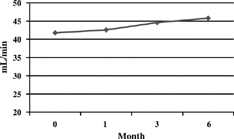
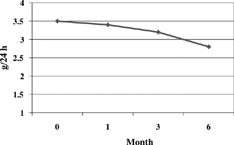
Treatment with lercanipine was accompanied by either no change or by small and no significant variations in the various hematology values considered in the study. Evolution of biochemical values has been reported in . Cholesterol (p = 0.001) and triglycerides (p = 0.018) significantly decreased at 6 months. No increase in the number of patients with abnormal or hematologic findings was seen during treatment as compared to pretreatment values. Creatinine clearance significantly improved at 6 months. (P = 0.019) (). No changes in plasmatic or urea creatinine were detected. Proteinuria diminished significantly at the end of thefollow-up (2.8 ± 2.8 versus baseline, 3.5 ± 3.2 g/day, p = 0.0155) ().
Table 3. Biochemical Changes.
Discussion
The present study in kidney disease patients demonstrates that lercanidipine, a calcium antagonist, is a safe and effective antihypertensive drug in this kind of patient, with a clean side effect profile. In addition, it showed that a calcium channel blocker slightly improves the renal function at medium term and lowers proteinuria when it was associated with an angiotensin axis-blocking drug.
Calcium antagonist is a widely used antihypertensive agent, and its use has increased dramatically since the 1980s. Its wide appeal can be attributed to several features, including its well-documented antihypertensive efficacy and metabolic neutrality.Citation[12] Calcium antagonists have been tested against placebo and against other active therapies in several studies. A careful prospective meta-analysis of all studies in December 1999 showed equal morbidity and mortality reduction of calcium channel blockers when compared with diuretics, beta-blockers, and ACE inhibitors.Citation[13] Another recent meta-analysis has shown that calcium antagonists seem to be more efficacious in preventing strokes than conventional drugs.Citation[14] More specifically, in diabetic nephropathy patients, ARB failed to show significant differences in morbidity and mortality compared with calcium antagonists.Citation[15]
In spite of their antihypertensive efficacy, dihydropyridine calcium antagonists are often reported to induce side effects responsible for treatment withdrawal or replacement with drugs of a different class. Lercanidipine is a new compound with high lipophilicity and high vascular selectivity, which ensures a gradual and prolonged antihypertensive effect.Citation[7-9], Citation[16-18] In all these studies, tolerability of lercanidipine was very good, and, when compared with other dihydropyridine calcium antagonists, it appeared to be better. The most common adverse effect of dihydropyridine is pedal edema. It seems to be related to arterial dilation, which increases intracapillary pressure and squeezes fluid from the intravascular space into the interstitium.Citation[19] Compared with amlodipine and lacidipine, lercanidipine showed a lower intercadence of pedal edema in essential hypertensives.Citation[8] In our study, the incidence of edema and vasodilatory related side effects was really low in spite of the proteinuria of a high number of patients, a well-known edema-prone status. Because vasodilatory edema associated with dihydropyridine calcium antagonists responds well to agents that dilate the postcapillary vessel, such as ACE inhibitors or ARB,Citation[20&21] the simultaneous treatment with those drug classes might have influenced our results.
It is well established that BP reduction, regardless of the antihypertensive agent used, slows the progression of renal disease.Citation[22-27] Moreover, ACE inhibitors and, more recently, ARB, seem to provide an added degree of protection to the kidney damage, independent of their arterial pressure–reducing effects.Citation[15], Citation[25-30] So, it was considered ethically necessary for all patients in this study to be previously treated with one of those drugs.
The results from this study should be viewed with caution, as this study has some potential limitations. They include the small number of studied subjects, the use of creatinine clearance rather than a more precise marker of glomerular filtration rate, and last, the use of an open label rather than double-blind, double-dummy design. The use of creatinine clearance as a marker is a potential limitation of this study. Although we are aware that it is imprecise as a measure of glomerular filtration rate, recent studies found it to be a good predictor of renal function decline.Citation[31&32] Therefore, even with the limitations of this marker, we feel our data are reliable and meaningful.
There are scant reports on the protective effect of calcium antagonists. Data from the SYST-EUR study demonstrate a protective effect of calcium channel blockers compared with placebo in hypertensive patients, even those with diabetes and those with proteinuria.Citation[33] Verapamil treatment decreased proteinuria and renal failure progression in diabetic nephropathy in African Americans.Citation[34] The results of the INSIGHT study suggest that antihypertensive treatment with nifedipine GITS also offers a renoprotective effect higher than do thiazides.Citation[35] Our findings, combined with the evidence from other intervention trials, raise the possibility that long-term antihypertensive therapy with long-acting dihydropyridine may produce specific reno protection beyond their effect on blood pressure. On the other hand, several studies using amlodipine as calcium antagonist agent failed to show any renal protective effect in diabetic or nondiabetic renal disease.Citation[15], Citation[30], Citation[36]
It has been suggested that calcium antagonists may improve renal function when it has been impaired by the previous use of ACE inhibitors, and it must be taken into account that all recruited patients were treated with angiotensin axis blocking drugs previously to initiate lercanidipine treatment.
Theoretically, the renal microcirculatory effects of calcium channel blockers cannot explain this renoprotective effect, because they preferentially dilate the afferent glomerular arteriole. This does not favor a decrease of glomerular hypertension, one of the postulated mechanisms to explain the protective effect of ACE inhibitors and ARB.Citation[37] But, it has been reported that lercanidipine could dilate efferent arteriole in spontaneous hypertensive rats.Citation[38] However, there are possible mechanisms otherthan the reduction of intraglomerular capillary pressure, whereby calcium channel blockers could prevent renal dysfunction such as the attenuation of the mitogenic effects of growth factors,Citation[39] the modulation of macromolecular traffic across and entrapment within the mesangium, the inhibition of the renal effects of endothelin, and the decrease in free radical formation.Citation[37]
Other possible reasons for differences in renal preservation between drug groups are differences in antiproteinuric effects of the antihypertensive agents. Reduction in proteinuria in individuals with insulin-dependent or noninsulin-dependent diabetes mellitus is known to correlate with the preservation of renal function.Citation[25-27] A report by Hebert et al.Citation[40] demonstrated that only those individuals who manifested reductions in proteinuria had a slowed progression of diabetic nephropathy. Two different meta-analyses, however, demonstrate that reductions in proteinuria observed with ACE inhibitors are out of proportion to the level of BP reduction.Citation[25&26] Thus, these and other studies suggest that ACE inhibitors and nondihydropyridine calcium channel blockers reduce proteinuria to a degree greater than predicted by simple BP reduction.Citation[25-27], Citation[41] Combination therapy of ACE inhibitors with either nondihydropyridine or dihydropyridine might have a deeper effect on urine protein reduction.Citation[42&43] Our data support this latter contention, because there was a greater reduction in proteinuria when lercanidipine was added to ACE inhibitors or ARB therapy.
Lercanidipine shows good efficacy as an antihypertensive agent in renal disease. The incidence of untoward effect was very low. Furthermore, this study supports the concept that the dihydropyridine calcium channel blocker lercanidipine decreases proteinuria and improves the progression of established renal disease when it is associated with an ACE inhibitor or ARB. It should be remembered that all subjects had chronic renal failure before entry into the study, and most of them have lost at least 50% of their renal function. Further large-scale clinical trials are needed to confirm the observations made in this study.
Acknowledgment
Supported by grants from Recordati-Spain (Madrid, Spain).
References
- Klag, M J.; Whelton, P K.; Randall, B L.; Neaton, J D.; Brancati, F L.; Ford, C E.; Shulman, N B.; Stamler, J. Blood pressure and end-stage renal disease in men. N. Engl. J. Med. 1996, 334, 13–18. [PUBMED], [INFOTRIEVE], [CSA]
- Rostand, S G.; Brunzell, J D.; Cannon, R O.; Victor, R G. Cardiovascular complications in renal failure. J. Am. Soc. Nephrol. 1991, 2, 1053–1058. [PUBMED], [INFOTRIEVE]
- Shulman, N B.; Ford, C E.; Hall, W D.; Blaufox, M D.; Simon, D.; Langford, H G.; Schneider, K A. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The hypertension detection and follow-up program cooperative group. Hypertension 1989, 13 (Suppl 5), I180–I193.
- Samuelsson, O.; Wilhelmsen, L.; Elmfeldt, D.; Pennert, K.; Wedel, H.; Wikstrand, J.; Pennert, K.; Wedel, H.; Wikstrand, J.; Berglund, G. Predictors of cardiovascular morbidity in treated hypertension: results from the primary preventive trial in Göteborg, Sweden. J. Hypertens. 1985, 3, 167–176. [PUBMED], [INFOTRIEVE]
- Ruilope, L M.; Campo, C.; Rodriguez-Artalejo, F.; Lahera, V.; Garcia-Robles, R.; Rodicio, J L. Blood pressure and renal function: therapeutic implications. J. Hypertens. 1996, 14, 1259–1263. [PUBMED], [INFOTRIEVE]
- Mandhavan, S.; Stockwell, D.; Cohen, H.; Alderman, M H. Renal function during antihypertensive treatment. Lancet 1995, 345, 749–751. [CROSSREF]
- Barrios, V.; Navarro, A.; Esteras, A.; Luque, M.; Romero, J.; Tamargo, J.; Prieto, L.; Carrasco, J L.; Herranz, I.; Navarro-Cid, J.; Ruilope, L M. Antihypertensive efficacy and tolerability of lercanidipine in daily clinical practice. The ELYPSE study. Blood Pressure 2002, 11, 95–100. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Leonetti, G.; Magnani, B.; Pessina, A C.; Rappelli, A.; Trimarco, B.; Zanchetti, A.; COHORT Study Group. Tolerability of long-term treatment with lercanidipine versus amlodipine and lacidipine in elderly hypertensives. Am. J. Hypertens. 2002, 15, 932–940. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- DiGiorgio, L A.; Orlandini, F.; Malasoma, P.; Zappa, A. A double-blind cross-over study of lercanidipine versus amlodipine in the treatment of mild-to-moderate essential hypertension. Curr. Ther. Res. 1999, 60, 511–520. [CROSSREF]
- Tobbe, S.; Epstein, M. The use of calcium antagonists in the treatment of hypertensive persons with kidney disease. Curr. Hypertens. Rep. 2002, 4, 191–194. [CSA]
- Bitar, R.; Flores, O.; Reverte, M.; Lopez-Novoa, J M.; Macias, J F. Beneficial effect of verapamil added to chronic ACE inhibitor treatment on renal function in hypertensive elderly patients. Int. Urol. Nephrol. 2000, 32 (2), 165–169., [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Messerli, F H. Calcium antagonists in hypertension: from hemodynamics to outcomes. Am. J. Hypertens. 2002, 15 (Pt2), 94S–97S. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Neal, B.; MacMahon, S.; Chapman, N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomized trials. Blood pressure lowering treatment trialists' collaboration. Lancet 2000, 356, 1955–1964. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Opie, L H.; Schall, R. Evidence-based evaluation of calcium channel blocker for hypertension: equality of mortality and cardiovascular risk relative to conventional therapy. J. Am. Coll. Cardiol. 2002, 39, 315–322. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lewis, E.; Huncksicker, L G.; Clarke, W R.; Berl, T.; Pons, M A.; Lewis, J B.; Ritz, E.; Atkins, R C.; Rohde, R. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Rengo, F.; Romis, L. Activity of lercanidipine in double-blind comparison with nitrendipine in combination treatment of patients with resistant essential hypertension. J. Cardiovasc. Pharmacol. 1997, 29 (Suppl. 2), S54–S58.
- Morisco, C.; Trimarco, B. Efficacy and tolerability of lercanidipine in comparison to and in combination with atenolol in patients with mild to moderate essential hypertension in a double blind controlled study. J. Cardiovasc. Pharmacol. 1997, 29 (Suppl. 2), S54–S58.
- Barbagallo, M.; Barbagallo, G. Sangiorgi, Efficacy and tolerability of lercanidipine in monotherapy in elderly patients with isolated systolic hypertension. Aging Clin. Exp. Res. 2000, 12, 375–379. [CSA]
- Messerli, F H. Vasodilatory edema: a common side effect of antihypertensive therapy. Am. J. Hypertens. 2001, 14, 978–979. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hanson, L.; Lindholm, L H.; Ekbom, T.; Dahlöf, B.; Lanke, J.; Schersten, B.; Wester, P.-O.; Hedner, T.; d Faire, U.; STOP-Hypertension-2 study group, Randomized trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity. The Swedish trial in old patients with hypertension-2 study. Lancet 1999, 354, 1751–1756. [CROSSREF]
- Messerli, F H.; Oparil, S.; Feng, Z. Comparison of efficacy and side effects of combination therapy of angiotensin-converting enzyme inhibitor (benazepril) with calcium antagonists (either nifedipine or amlodipine) versus high-dose calcium antagonists monotherapy for systemic hypertension. Am. J. Cardiol. 2000, 86, 1182–1187. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Parving, H H. The impact of hypertension and antihypertensive treatment on the course and prognosis of diabetic nephropathy. J. Hypertens. 1990, 8, S187–S191.
- Peterson, J C.; Adler, S.; Burkart, J M.; Greene, T.; Hebert, L A.; Hunsicker, L G.; King, A.; Klahr, S.; Massry, S G.; Seifter, J L. Blood pressure control, proteinuria and the progression of renal disease: the modification of diet in renal disease study. Ann. Intern. Med. 1995, 123, 754–762. [PUBMED], [INFOTRIEVE], [CSA]
- Toto, R D.; Mitchell, N C.; Smith, R D. “Strict” blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney Int. 1995, 48, 851–859. [PUBMED], [INFOTRIEVE]
- Gansevoort, R T.; Sluiter, W J.; Hemmelder, M H.; de Zeeuw, D.; de Jong, P E. Antiproteinuric effect of blood pressure lowering agents: a meta-analysis of comparative trials. Nephrol. Dial. Transplant. 1995, 10, 1963–1974. [PUBMED], [INFOTRIEVE], [CSA]
- Maki, D D.; Ma, J Z.; Louis, T A.; Kasiske, B L. Long-term effects of antihypertensive agents on proteinuria and renal function. Arch. Intern. Med. 1995, 155, 1073–1080. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Weidmann, P.; Schneider, M.; Bohlen, L. Therapeutic efficacy of different anti-hypertensive drugs in human diabetic nephropathy: an updated meta-analysis. Nephrol. Dial. Transplant. 1995, 10 (Suppl. 9), 39–45. [PUBMED], [INFOTRIEVE], [CSA]
- National High Blood Pressure Education Program Working Group on Hypertension and Renal Disease, 1995 Update of the working group reports on chronic renal failure and renovascular hypertension. Arch. Intern. Med. 1996, 156, 1938–1947. [CROSSREF], [CSA]
- Brenner, B M.; Cooper, M E.; de Zeeuw, D.; Keane, W F.; Mitch, W E.; Parving, H H.; Remuzzi, G.; Snappin, S M.; Zhang, Z.; Shahinfar, S. Effect of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Parving, H H.; Lehnert, H.; Bröchner-Mortensen, J.; Gomis, R.; Andersen, S.; Arner, P. For the study group Irbesartan in patients with type 2 diabetes and microalbuminuria. N. Engl. J. Med. 2001, 345, 870–878. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kesteloot, H.; Joossens, J V. On the determinants of the creatinine clearance: a population study. J. Hum. Hypertens. 1996, 10, 245–249. [PUBMED], [INFOTRIEVE], [CSA]
- Lemann, J.; Bidani, A.; Bain, R P.; Lewis, E.; Rohde, R D.; Collaborative Study Group, Use of serum creatinine to estimate glomerular filtration rate in health and early diabetic nephropathy. Am. J. Kidney Dis. 1990, 16, 236–243., [PUBMED], [INFOTRIEVE]
- Voyaki, S M.; Staessen, J A.; Thijs, L.; Wang, J G.; Efstratopoulos, A D.; Birkenhäger, W H.; de Leeuw, P W.; Leonetti, G.; Nachev, C.; Rodicio, J L.; Tuomilehto, J.; Fagard, R.; Systolic Hypertension in Europe (Syst-Eur) Trial Investigators, Follow-up of renal function in treated and untreated older patients with isolated systolic hypertension. J. Hypertens. 2001, 19, 511–519. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Bakris, G L.; Mangrum, A.; Copley, J B.; Vicknair, N.; Sadler, R. Effect of calcium channel or ß-blockade on the progression of diabetic nephropathy in African Americans. Hypertension 1997, 29, 744–750. [PUBMED], [INFOTRIEVE], [CSA]
- Brown, M J.; Palmer, C R.; Castaigne, A.; de Leeuw, P W.; Mancia, G.; Rosenthal, T.; Ruilope, L M. Morbidity and mortality in patients randomized to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the international nifedipine GITS study: intervention as a goal in hypertension treatment (INSIGHT). Lancet 2000, 356, 366–372. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Agodoa, L Y.; Appel, L.; Bakris, G L.; Beck, G.; Bourgoignie, J.; Brigs, J P.; Charlestown, J.; Cheek, D.; Cleaveland, W.; Douglas, J G.; Douglas, M.; Dowie, D.; Faulkner, M.; Gabriel, A.; Gassman, J.; Greene, T.; Hall, Y.; Hebert, L.; Hiremath, L.; Jamerson, K.; Johnson, C J.; Kopple, J.; Kusek, J.; Lash, J.; Lea, J.; Lewis, J B.; Lip Kowitz, M.; Massry, S.; Middleton, J.; Miller, E R., III.; Norris, K.; O'Conner, D.; Ojo, A.; Phillips, R A.; Pogue, V.; Rahman, M.; Randall, O S.; Rostand, S.; Schulmar, G.; Smith, W.; Thornley-Brown, D.; Tisher, C C.; Toto, R D.; Wright, J T., Jr.; Xu, S. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001, 285, 2719–2728. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Epstein, M. Calcium antagonists and renal protection: emerging perspectives. J. Hypertens. 1998, 18 (Suppl. 4), S17–S25.
- Sabbatini, M.; Leonardi, A.; Testa, R.; Vitaioli, L.; Amenta, F. Effect of calcium antagonists on glomerular arterioles in spontaneously hypertensive rats. Hypertension 2000, 35, 775–779. [PUBMED], [INFOTRIEVE]
- Schultz, P.; Raij, L. Inhibition of human mesangial cell proliferation by calcium channel blockers. Hypertension 1990, 15 (Suppl. 1), 76–80.
- Hebert, L A.; Bain, R P.; Verme, D.; Cattran, D.; Whittier, F C.; Tolchin, N.; Rohde, R D.; Lewis, E J.; Collaborative Study Group, Remission of nephrotic range proteinuria in type I diabetes. Kidney Int. 1994, 46, 1688–1693. [PUBMED], [INFOTRIEVE]
- Bakris, G L.; Williams, B. ACE inhibitors and calcium antagonists alone or combined: is there a difference on progression of diabetic renal disease?. J. Hypertens. 1995, 13 (suppl 2), S95–S101. [CSA]
- Bakris, G L.; Weir, M R.; DeQuattro, V.; McMahon, F G. Effects of an ACE inhibitor/calcium antagonists combination on proteinuria in diabetic nephropathy. Kidney Int. 1998, 54, 1283–1289. [PUBMED], [INFOTRIEVE], [CROSSREF]
- The effects of an ACE inhibitor and a calcium antagonists on the progression of renal disease: the nephross study. Nephrol. Dial. Transplant. 2001, 16, 2158–2166. [CROSSREF], [CSA]