Abstract
Secondary hyperparathyroidism (SHP) is a frequent complication of long-term dialysis patients, and surgical parathyroidectomy remains necessary in patients resistant to medical therapy. The present paper reports single center results in subtotal parathyroidectomy, presenting diagnostic procedure, indications for parathyroidectomy, and postoperative course of metabolic and endocrine disorders. Forty-seven patients (25 males and 22 females), aged 25–60 years, regularly hemodialyzed between 3–23 years, have undergone parathyroidectomy at our Clinical Center during the last 10 years. The patients had plasma iPTH levels 8–45 times higher than the top normal limit, high values of alkaline phosphatase, calcemia on the upper normal level, and hyperphosphatemia. Radiographic changes characteristic for SHP were seen in all patients before parathyroidectomy, and the most common were subperiosteal resorptions (100%), bone cysts and periosteal neostosis (66%), and extraskeletal calcifications (98%). Enlarged parathyroid glands were seen by ultrasound in 62% of patients. All patients manifested pruritus and bone pain, 89% of them had myopathy, while other symptoms and signs were present in lower proportions. After parathyroidectomy, pruritus and myopathy reduced significantly, while pain in bones and joints remained. One patient had brown tumor at the maxillary bone that regressed gradually after parathyroidectomy. Significant decreases of phosphate and calcium levels were recorded in all but two patients on the very first postoperative day. Regular peroral and parenteral supplementations of calcium and vitamin D metabolites were used, but calcemia was not normalized until the end of the third week of the postoperative period. Serum alkaline phosphatase showed an increase after the surgery, thereupon a sudden and then slower decrease up to 1 year from the surgery. Plasma iPTH levels, checked on the 21st postoperative day, were close to the lower normal limit in all but two (4.3%) patients with persistent SHP, who required reoperation. In conclusion, subtotal parathyroidectomy was proved as a successful and safe treatment for patients with SHP resistant to medical therapy, and treatment was followed by improvement of clinical symptoms and metabolic disorders.
Introduction
Secondary hyperparathyroidism (SHP) remains a frequent complication of chronic renal failure (CRF) despite the early start and regular use of calcium supplements, phosphate binders, and vitamin D metabolites.Citation[1-4] In patients demonstrating severe SHP, administration of vitamin D metabolites may be difficult and inconsistently effective because of hypercalcemia or uncontrolled hyperphosphatemia. When medical treatment fails, surgical reduction of the parathyroid glands mass becomes necessary.Citation[5&6]
The present paper reports single center results in subtotal parathyroidectomy and describes diagnosis, indications for parathyroidectomy, and changes in metabolic and endocrine disorders after parathyroidectomy.
Subjects and Methods
Forty-seven patients who have undergone subtotal parathyroidectomy at the Clinical Center of Serbia in Belgrade during the last 10 years were analyzed retrospectively. Patients from different hemodialysiscenters were directed to our clinic for subtotal parathyroidectomy after previous examination at our Outpatients Department when parathyroidectomy had been indicated. There were 25 males and 22 females ages between 25 and 60 (40.78 ± 10.41) years maintained with hemodialysis between 3 and 23 (8.97 ± 3.59) years before parathyroidectomy. All patients were hemodialyzed three times a week for 4 h. Medical histories and documentations showed that the patients used phosphate binders and vitamin D metabolites according to well-known indications, but the majority of them had to stop vitamin D usage due to hypercalcemia.
Preoperative examination was comprised of registration of clinical signs and symptoms characteristic for SHP and of the carrying out of the following diagnostic procedures: biochemical analysis—serum calcium, phosphate, alkaline phosphatase, creatinine, urea, and intact parathyroid hormone (iPTH); x-ray of hands (AP), shoulders (lateral), skull (lateral), and pelvis (AP); and ultrasonography of parathyroid glands.
Indications for parathyroidectomy were extremely high plasma iPTH (at least eight fold higher than the upper normal value), increased serum alkaline phosphatase, presence of radiographic changes as well as symptoms and signs characteristic for SHP, and resistance to medical treatment. The finding of enlarged parathyroid glands by ultrasound as well as hypercalcemia and hyperphosphatemia made it easier to set the indication.
Subtotal parathyroidectomy was carried out under general anesthesia by highly skilled endocrine surgeons. Once all the four parathyroid glands were identified, the gland, which appeared the least hyperplastic but had an adequate blood supply, was resected, and the others were removed.
Postoperative follow-up of clinical signs and symptoms and biochemical results was carried out according to the following schedule: serum calcium (twice a day during the first 7 days, once a day for the next 7 days, every other day until the 21st day), phosphate (once a day the first 7 days, then once a week), alkaline phosphatase (once a week until the 21st day, once a month until the 3rd month, after 1 year), creatinine, and urea (once a week). After the parathyroidectomy, most patients were supplemented with oral or parenteral calcium and vitamin D metabolites, and the dosages were adjusted according to serum calcium levels. Only in two patients did the serum calcium level remained unchanged, so supplementation was not necessary, and in these patients, persistent SHP was diagnosed.
Plasma calcium concentration was analyzed in the principle of O-cresolphthalein complex; serum phosphate level (like anorganic, orthophosphate) photometrically, with Urlich–Rabee method; and serum alkaline phosphatase by measuring P-nitrophenol on an absorption filter. Serum creatinine was measured by means of Jaffe reaction and urea with enzyme method (ureasa-gluthamat-dehidrogenasa). Intact PTH in plasma was measured by using the RIA method (Nicholas Institute Diagnostic).
Results are expressed as mean values and standard deviations, percentages, and frequency distributions. Statistical analyses include differences between two percentages, t-test, and Wilocxon Signed Ranks Test.
Results
All patients complained of the symptoms and signs characteristic for SHP. Before parathyroidectomy, almost all patients complained of pruritus, bone pain, and symptoms of myopathy, while 19–34% of patients had joint pain, skeletal deformities, and fractures, but only one patient had each periarteritis and tetiva rupture ().
Table 1. Symptoms and Signs of SHP Before and After Subtotal Parathyroidectomy.
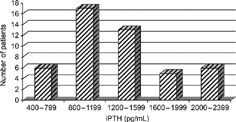
Plasma levels of iPTH in analyzed patients are shown in . All patients had high plasma iPTH levels that were 8 to 45 times higher than the upper normal value. Results of routine biochemical analyses are presented in . Before parathyroidectomy, the mean serum calcium level of the studied patients was near the upper normal level, while mean values of phosphate and alkaline phosphatase were higher than normal. Serum creatinine and urea levels were in the range expected for patients on this mode of treatment.
Table 2. Results of Biochemical Analyses Before and 21 Days After Subtotal Parathyroidectomy.
Figure 1 Distribution of patients according to plasma IPTH levels before subtotal parathyroidectomy.
The results of bone x-rays are presented in . The most frequent findings were subperiosteal resorptions, bone cysts, and periosteal neostosis. Extraskeletal calcifications were found in the majority of patients: periarticular or tumoral calcifications in 98%, vascular in 74%, and visceral in three patients (6%). One patient had brown tumor at the maxillary bone that regressed gradually after successful parathyroidectomy.
Table 3. Radiographic Findings in Patients Before Subtotal Parathyroidectomy.
In our center, the routine operative procedure for SHP is subtotal parathyroidectomy. When all four glands were found on operation (39 patients), three were extirpated, and the forth one was resected only partially. Total excision was done when less than four glands were found (eight patients). Except for intraoperative injury of unilateral recurrent laryngeal nerve in two patients, that completely recovered, there were no other surgical complications.
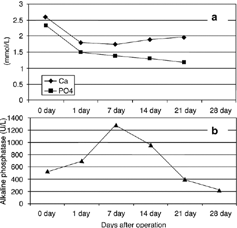
Symptoms and signs diminished after parathyroidectomy (). Significant decreases of calcium and phosphate levels were recorded on the first postoperative day in the majority of patients. Between the first and seventh days, serum calcium levels varied between 1.01–2.44 mmol/L despite the regular peroral and parenteral supplementation of calcium and the use of vitamin D metabolites. Calcemia was restored during the 3-week postoperative period (). The normal serum level of phosphate reached in the first days after operation was maintained during the first month without the use of phosphate binders. Hypophosphatemia appeared in a few patients only occasionally. Mild hyperkalemia was registered in several patients, but no additional therapy was necessary. Alkaline phosphatase concentration showed an increase after the surgery, there and after a sudden and then slower decrease (, ). Alkaline phosphatase reached normal values no earlier than 1 year after the surgery (data not presented). Plasma iPTH level, checked on the 21st postoperative day, was close to the lower normal level in all but two patients, whose iPTH, hypercalcemia,hyperphosphatemia, and increased alkaline phosphatase were maintained. Persistent SHP was diagnosed in these patients, and reoperation was indicated.
Discussion
Besides therapeutical advantage in the treatment of secondary hyperparathyroidism, the need for surgical treatment still exists. Since 1960, when the first description of subtotal parathyroidectomy in two patients with chronic renal failure was published,Citation[7] numerous authors have reported the use of parathyroidectomy in the treatment of secondary hyperparathyroidism. Over the last three decades, subtotal parathyroidectomy or total parathyroidectomy with autotransplantation of parathyroid gland tissue has been a widely used surgical approach, although some authors favored the use of total parathyroidectomy.Citation[8-12] In our Clinical Center, subtotal parathyroidectomy is used as the only method for surgical treatment of secondary hyperparathyroidism.
The indications for parathyroidectomy in patients with CRF are not clearly defined and are still a matter of debate.Citation[10], Citation[13] Hypercalcemia, if associated with mental symptoms or severe hypertension, is quickly reversed after parathyroidectomy. However, hypercalcemia alone may not reflect the presence of parathyroid hyperplasia, but could if combined with aluminium-induced osteomalacia, treatment with vitamin D, use of oral calcium supplements, or high dialysate calcium. Therefore, evidence for secondary hyperparathyroidism, that is, bone erosions, markedly elevated levels of PTH, and bone biopsy demonstrating osteitis fibrosa, should be obtained before surgery is undertaken. The appearance of ischemic skin ulcerations associated with marked vascular calcifications, that is, calciphylaxis, is another indication for parathyroidectomy. On rare occasions, pruritus may be so severe that surgery should be considered, although it is unlikely to produce benefits in the absence of other manifestations of secondary hyperparathyroidism. Persistently elevated serum phosphorus unresponsive to oral phosphate binders should raise the question of significant overt hyperparathyroidism. It has been shown that in this situation, a fraction of the increment in serum phosphate is due to release of phosphate from bone as a consequence of the increased bone resorption.Citation[9] Thus, severe hyperphosphatemia together with histological and radiographic evidence of osteitis fibrosa may be an indication for parathyroidectomy.
Because PTH has been considered to be a uremic toxin, other manifestations of hyperparathyroidism adversely affecting bone marrow (anemia), myocardial function (congestive heart failure), and neuromuscular tissue (peripheral neuropathy), may be considered potential indications for parathyroidectomy.Citation[10], Citation[14-16]
All our patients had a high plasma level of iPTH that was 8 to 45 times higher than normal (). Radiographic changes characteristic for SHP were also seen in all of them, most frequently, subperiosteal resorptions, bone cysts, and periosteal neostosis (). Twenty-one out of 47 patients (44.7%) had calcium levels higher than normal before parathyroidectomy; hyperphosphatemia was present in 42 patients (89.4%), while alkaline phosphatase was significantly higher than normal in all patients. Extraskeletal calcifications were present in 46 of 47 patients: periarticular or tumoral calcifications in 98%, vascular in 74%, while visceral calcification was found in 6% of patients (). As bone histology analysis was not available in our institution, the presence of several criteria numbered in the “Subjects and Methods” section had to be considered sufficient to indicate parathyroidectomy. High iPTH level and radiographic signs of SHP were considered the most important among them.
The clinical course after parathyroidectomy is predictable in dialysis patients. There is a significant and precipitous decline in serum calcium level, which may be accompanied by numbness, paresthesias, and tetany cramps that may appear by the second day. Postoperatively, the decline in serum calcium level correlated with the severity of the hyperparathyroidism as judged by bone histomorphometry.Citation[17] In general, thenadir of this hypocalcemia is usually attained within the period from the second to the fourth day after the operation.Citation[10] In our patients, a significant decrease in calcium level (for 31%) appeared by the first postoperative day and was associated with moderate manifestation of hypocalcemia. Serum calcium level increased gradually to the 21st postoperative day.Citation[13], Citation[18]
An abrupt decrease in serum phosphorus level is usually observed immediately after parathyroidectomy, as was the case with our patients. In general, patients with higher plasma alkaline phosphatase, indicating active bone resorption and formation, have more profound hypophosphatemia. Such data suggest that the severity of the osteitis fibrosa may be an important determinant of postoperative hypophosphatemia. It is of interest that the hypophosphatemia may be prolonged as long as 1 year after the parathyroidectomy.Citation[13], Citation[19] Although serum phosphorus levels decreased significantly in our patients, hypophosphatemia was rare and short-term, and supplementation with phosphate buffer was not necessary.
Although serum alkaline phosphatase, which is usually elevated prior to surgery, declines in the long term, a marked increase in the immediate postoperative period has been described recently.Citation[20&21] This observation was evaluated by Urena et al., who examined 37 hemodialysis patients undergoing parathyroidectomy.Citation[21] They observed a significant increase in serum alkaline phosphatase from day 4 after parathyroidectomy, with the highest value observed from days 7 to 14. This increase, in the absence of changes in liver function, was mainly due to the bone-specific iso-alkaline phosphatase. The author interpreted this increment as a flare-up of osteoblastic activity. By the third week post-parathyroidectomy, the serum alkaline phosphatase started to decrease steadily, reaching normal values after 6 months.Citation[10] In our patients, similar changes were registered (), but serum levels of alkaline phosphatase did not normalize in all patients until 1 year after parathyroidectomy.
The main problem with subtotal or total parathyroidectomy with autotransplantation is the gradual development of recurrent hyperparathyroidism. The incidence of recurrence is similar in both. This is confirmed by Kaye, who analyzed this problem in an excellent review of parathyroid surgery in renal failure.Citation[22] Many authors analyzing their own results or those of others found 6–14% of recurrence.Citation[23-26] The most common causes of reoperation were failure of the initial parathyroidectomy, leaving hypersecreting parathyroid tissue in place, or incomplete operation because of anatomical anomalies involving ectopic or supernumerary glands.Citation[27] Besides, SHP can recur several years later. So, Kessler and colleagues, in their own series, found 11.43% of reoperations, out of which 8.57% were due to signs of hyperparathyroidism persisting after the initial operation,Citation[27] while Berczi and colleagues found 10.42% patients with persistent SHP because of failure of the initial operation.Citation[28] In our short-term study, only three postoperative weeks were analyzed, and persistence of SHP was found in two (4.25%) out of 47 patients that made reoperation unavoidable, which is similar to the results of Tominaga and colleagues (4.2%).Citation[29]
Conclusion
Subtotal parathyroidectomy was proven as a successful and safe treatment of medicament resistant SHP that was followed by the improvement of metabolic disorders and clinical symptoms. Persistent hyperparathyroidism occurred when inadequate parathyroidectomy was performed.
References
- Reichel H.; Drueke T.; Ritz E. Bony complications in chronic renal failure. In Oxford Textbook of Clinical Nephrology; Cameron S.; Davison A M.; Grundfild J P.; Keer D.; Ritz E., Eds; Oxford University Press: Oxford, 1992; 1365–1389.
- Felsenfeld A J.; Llach F. Parathyroid gland function in chronic renal failure. Kidney Int. 1993, 43 (4), 771–789. [PUBMED], [INFOTRIEVE]
- Feinfeld D A.; Sherwood L M. Parathyroid hormone and 1,25(OH)2D3 in chronic renal failure. Kidney Int. 1988, 33 (6), 1049–1058. [PUBMED], [INFOTRIEVE]
- Torres A.; Lorenzo V.; Hernandez D.; Rodriguez J C.; Concepcion M T.; Rodriguez A P.; Hernandez A.; de Bonis E.; Darias E.; Gonzales-Posada J M.; Losada M.; Rufino M.; Felsenfeld A J.; Rodriguez M. Bone disease in predialysis, hemodialysis, and CAPD patients: evidence of better bone response to PTH. Kidney Int. 1995, 47 (5), 1434–1442. [PUBMED], [INFOTRIEVE]
- Malluche H H. Renal bone disease—an ongoing challenge to the nephrologist. Clin. Nephrol. 1995, 44 (Suppl. 1), S38–S41. [PUBMED], [INFOTRIEVE]
- Charhon S A.; Berland Y F.; Olmer M J.; Delawari E.; Traeger J.; Meunier P J. Effects of parathyroidectomy on bone formation and mineralization in hemodialyzed patients. Kidney Int. 1985, 27 (2), 426–435. [PUBMED], [INFOTRIEVE]
- Stanbury S W.; Lumb G A.; Nicholson W F. Elective subtotal parathyroidectomy for renal hyperparathyroidism. Lancet 1960, 1, 793–799., [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kaye M.; D'Amour P.; Henderson J. Elective total parathyroidectomy without transplantation in end-stage renal disease. Kidney Int. 1989, 35 (6), 1390–1399. [PUBMED], [INFOTRIEVE]
- Kaye M.; Rosenthall L.; Hill O.; Tabah R J. Long-term outcome following total parathyroidectomy in patients with end-stage renal disease. Clin. Nephrol. 1993, 39 (4), 192–197. [PUBMED], [INFOTRIEVE]
- Llach F. Parathyroidectomy in chronic renal failure: indications, surgical approach and the use of calcitriol. Kidney Int. 1990, 38 (Suppl. 29), S62–S68.
- De Francisco A L.; Fresnedo G F.; Rodrigo E.; Pinera C.; Amado J A.; Arias M. Parathyroidectomy in dialysis patients. Kidney Int. 2002, 80 (Suppl. May), 161–166. [CROSSREF]
- Tominaga Y.; Uchida K.; Haba T.; Katayama A.; Sato T.; Hibi Y.; Numano M.; Tanaka Y.; Inagaki H.; Watanabe I.; Hachisuka T.; Takagi H. More than 1,000 cases of total parathyroidectomy with forearm autograft for renal hyperparathyroidism. Am. J. Kidney Dis. 2001, 38 (4 Suppl. 1), S168–S171. [PUBMED], [INFOTRIEVE]
- Shpitz B.; Korzets Z.; Dinbar A.; Jedeikin R.; Hoffman S.; Bernheim J. Immediate postoperative management of parathyroidectomized hemodialyzed patients. Dial. Transplant. 1986, 15, 507–513.
- Brancaccio D.; Gallileni M.; Cozzolino M. Treatment of hyperparathyroidism—why is it crucial to control serum phosphate?. Nephrol. Dial. Transplant. 1996, 11 (3), 420–423. [PUBMED], [INFOTRIEVE], [CSA]
- Massry S G. Parathyroid hormone and uremic manifestations. Contrib. Nephrol. 1980, 20, 84–91. [PUBMED], [INFOTRIEVE]
- Statopolsky E.; Martin K.; Hurska K. Parathyroid hormone metabolism and ts potential as a uremic toxin. Am. J. Physiol. 1980, 239, F1–F12.
- Massry S G. Is parathyroid hormone a uremic toxin?. Nephron 1977, 19 (3), 125–130. [PUBMED], [INFOTRIEVE]
- Mozes M F.; Soper W D.; Jonasson O.; Lang G R. Total parathyroidectomy and autotransplantation in secondary hyperparathyroidism. Arch. Surg. 1980, 115 (4), 378–383. [PUBMED], [INFOTRIEVE]
- Massry S G.; Goldstein D A. Role of parathyroid hormone in uremic toxicity. Kidney Int. 1978, 8 (Suppl.), S39–S42.
- Klahr S.; Statopolsky E. Toxicity of parathyroid hormone in uremia. Am. Rev. Med. 1986, 37, 71–78. [CROSSREF]
- Urena P.; Basile C.; Brateau G.; Lalour B.; Vassault A.; Bourdeau A.; Bourdon R.; Dubost C.; Zingraff J.; Drueke T. Short-term effects of parathyroidectomy on plasma biochemistry in chronic uremia. Kidney Int. 1989, 36 (1), 120–126. [PUBMED], [INFOTRIEVE]
- Kaye M. Hyperparathyroidism and parathyroidectomy in renal failure. Int. J. Srtif. Organs 1990, 13 (12), 787–789.
- Niederle B.; Roka R.; Brennan M F. The transplantation of parathyroid tissue in man: development, indications, technique, and results. Endocrin. Rev. 1982, 3, 245–279. [CSA]
- Saxe A. Parathyroid transplantation: a review. Surgery 1984, 95, 507–526. [PUBMED], [INFOTRIEVE], [CSA]
- Rothmund M.; Wagner P K. Reoperations for persistent and recurrent secondary hyperparathyroidism in chronic renal failure. Ann. Surg. 1984, 200, 18–23.
- Johnson W J.; McCarthy J T.; Van Heerden J A.; Sterioff S.; Grant C S.; Kao P C. Results on subtotal parathyroidectomy in hemodialysis patients. Am. J. Med. 1988, 84, 23–32. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kessler M.; Avila J M.; Renoult E.; Mathieu P. Reoperation for secondary hyperparathyroidism in chronic renal failure. Nephrol. Dial. Transplant. 1991, 6 (3), 176–179. [PUBMED], [INFOTRIEVE]
- Berzi C.; Nagy A.; Matyus J.; Balazs G.; Kakuk G.; Lukacs G. Results and complications of parathyroidectomy in secondary hyperparathyroidism. Magy. Seb. 2001, 54 (6), 356–360.
- Tominaga Y.; Katayama A.; Sato T.; Matsuoka S.; Goto N.; Haba T.; Hibi Y.; Numano M.; Ichimori T.; Uvhida K. Re-operation is frequently required when parathyroid glands remain after initial parathyroidectomy for advanced secondary hyperparathyroidism in uremic parients. Nephrol. Dial. Transplant. 2003, 18 (Suppl. 3), iii65–iii70. [PUBMED], [INFOTRIEVE]