Abstract
Parenteral iron has been recommended for the treatment of iron deficiency in the majority of maintenance hemodialyzed (HD) patients. However, iron supplementation and consequent over saturation of transferrin and high iron levels, may aggravate oxidative stress already present in these patients. This study aimed to further clarify the role of repeated intravenous iron therapy as a supplementary cause of oxidative stress in HD patients. Markers of free radical activities (carbonyl reactive derivatives, CRD, thiol groups, SH, malondialdehyde, MDA) and antioxidant enzyme activities (superoxide dismutase, SOD and glutathione peroxidase, GPX) were determined in plasma and red blood cells (RBC) of 19 hemodialysis patients given a total iron dose of 625 mg (ferrogluconat, Ferrlecit, 62.5 mg). Blood samples were taken before the first and after the last dose of iron. Twenty apparently normal subjects served as healthy controls. Before iron treatment, HD patients exhibited increased concentrations of MDA and CRD in plasma and red blood cells, accompanied with impaired antioxidant capacity. All patients responded to iron therapy with a significant increase in their serum ferritin, serum iron, hemoglobin, and red blood cells levels. However, iron treatment resulted in enhanced oxidative stress in plasma of HD patients, since significant increase in plasma MDA and CRD concentrations, together with a decrease in nonprotein SH groups levels were detected. Supplementation with iron did not significantly influence plasma SOD and GPX activities, nor did any of the red blood cell parameters tested. Our data show that, despite improvement in hematological parameters, an increase in iron stores due to supplementation could also contribute to increased free radical production in HD patients.
INTRODUCTION
Anemia is most often very serious complication in chronic renal disease patients on hemodialysis, and it has a multifactorial origin. There are three key components for the proper erythropoiesis and hemoglobin synthesis in maintenance hemodialysis patients. Erythropoietin (EPO) is a stimulant factor in the acceleration of hematopoietic activity. Adequate body iron storage is a prerequisite for iron supply and effective iron mobilization and transport is the most important factor for iron utilization.Citation[1-3] Therefore, iron may be a rate-limiting factor in erythropoiesis.
Sometimes EPO can accelerate hematopoiesis to such an extent that iron demand exceeds the iron mobilization and transport rate; the occurrence of iron deficiency underEPO treatment is not a rare phenomenon.Citation[4&5] Because of that, end-stage renal disease patients require adequate iron therapy to benefit from treatment with EPO. Thus, parenteral iron has been recommended for the treatment of iron deficiency in the majority of maintenance hemodialysis patients receiving or not receiving erythropoietin, and most hemodialysis patients receive the supplemental iron intravenously.Citation[6] However, concerns have been raised about parenteral iron supplementation leading to an ”over saturation” of transferrin and excessively high iron levels, which may aggravate oxidative stress already present in these patients.Citation[7] Namely, it is established that an imbalance between pro-oxidant reactions and antioxidant defense begins in the early phase of chronic renal failure and gradually becomes worse during the progress of renal insufficiency.Citation[8-10] Thus, the resulting oxidative stress is most pronounced in patients on dialysis.Citation[8-12] In these patients oxidative stress increases as antioxidant defenses are weakened by pro-oxidant hemodialysis factorsCitation[12&13] and even further by an increase in renal anemia.Citation[14] The likeliest sources of oxidative stress in anemic states are, in addition to other mechanisms, an inadequate tissue oxygen supply, which leads to increased free radical production,Citation[14] a very low level of circulating red cells and mobile free radical scavengers which provide antioxidant protection to tissues and organs.Citation[15] Recent clinical studies suggest that complete correction of renal anemia using recombinant human erythropoietin (rhEPO) could reduce free radical production and improve endogenous red blood cell and plasma antioxidant capacity in hemodialysis patients.Citation[16&17]
Concern over the possibility of accelerated oxidative deterioration in iron-treated hemodialysis patients arose mainly from the studies performed after i.v. application of a single dose of iron.Citation[18&19] On the other hand, the effects of repeated iron supplementation were less studied. In order to establish a firm relationship between iron therapy, excess iron, and worsening of oxidative stress it is necessary to evaluate the effects of repeated i.v. iron supplementation on oxidative stress parameters in hemodialysis patients. Thus, the present investigation was undertaken to clarify whether partial correction of renal anemia with iron treatment influences free radical generation and endogenous antioxidant capacity in patients on maintenance hemodialysis. Therefore, markers of free radical activities (carbonyl reactive derivatives, CRD, thiol groups, SH, malondialdehyde, MDA) and antioxidant enzyme activities (superoxide dismutase, SOD, and glutathione peroxidase, GPX) were determined in plasma and red blood cells (RBC) of hemodialysis patients before and after repeated i.v. iron therapy.
MATERIALS AND METHODS
Patients
The study included 19 stable patients undergoing regular hemodialysis treatment for 6 ± 1.2 years. The average age of the patients (nine male and 10 female) was 46.6 ± 5.8 (mean ± SD) years. Hemodialyzed patients were given a total iron dose of 625 mg (ferrogluconat, Ferrlecit, 62.5 mg) i.v. during 10 consecutive dialyses. Patients did not receive epoetin or darbepoetin therapy. Blood samples were taken before the first and last dose of iron. Twenty apparently normal subjects served as healthy controls. Their average age was 42 ± 6 years old and included 11 males and nine females. All patients gave informed consent to participate in the study.
Collection and Preparation of Blood Samples
Venous blood samples were taken from each patient and control subject over trace-element free heparin, and used immediately for the analysis of hematological and biochemical parameters. In hemodialyzed patients, blood samples were taken immediately before hemodialysis. Hematological and biochemical parameters were checked prior to and after 10 supplemental iron doses.
Isolation of Red Blood Cells
Whole blood was immediately centrifuged at 3000 × g at 4°C for 10 min and plasma discharged. Packed red blood cells were washed in cold saline until supernatant was clear. The cells were lysed with distilled water and three freeze-thaw cycles. The supernatant solution, obtained by centrifugation of cell lysate for 20 min at 10 000 × g, was used for biochemical assays.
Enzyme Assays
Copper (Cu) and Zinc (Zinc) superoxide dismutase (SOD) activity in the plasma and red blood cells was measured by the method of Misra and Fridovich,Citation[20] based on the ability of SOD to inhibit autooxidation of epinephrine at alkaline pH (pH 10.2). One unit of SOD activity was defined as the amount of enzyme, that inhibits the oxidation of epinephrine by 50%.
Glutathione peroxidase (GPX) activity was determined by the coupled assay procedure of Gunzler et al.,Citation[21] and one unit of enzyme activity is reported as µmol nicotinamide adenosine dinucleotide phosphate (NADPH)oxidized/min, assuming 6.22 × 103 to be the molar absorbency of NADPH at 340 nm.
Measurement of Malondialdehyde, Thiol Groups, and Carbonyl Reactive Derivatives
Plasma and red blood cell extent of lipid peroxidation was measured as malondialdehyde (MDA) spectrophotometrically with 2-thiobarbituric acid. The calibration curve was prepared for each run using 1,1,3,3-tetraetoxipropane as a standard. The MDA concentration is expressed as µmol/L.Citation[22]
Plasma thiol (SH) groups' concentration was determined according to the method of Jocelyn et al.Citation[23] Red blood cell nonprotein thiol groups (NP-SH) were measured by the spectrophotometric assay of Ellman.Citation[24] The concentration of thiol groups is expressed as mmol/L.
Plasma extent of oxidative modifications of proteins was measured as carbonyl reactive derivatives (CRD) by the spectrophotometric method of LevineCitation[25] using 2,4-dinitrophenylhydrazine (DPNH) as a classic carbonyl reagent. The amount of CRD is expressed as µmol/g proteins.
Laboratory Evaluation
Hematological parameters including hemoglobin, hematocrit, ferritin, iron, and erythrocyte indexes were measured on an automated counter. The transferrin saturation index (TSAT index) was calculated according the following formula: saturation (%) = serum iron/total iron binding capacity.
Statistical Analysis
Data are presented as mean ± SD. Paired Student's t-test was used to determine if the calculated means of the obtained values have significantly changed during the trial. The limit of significance was set up at p < 0.05.
RESULTS
The hematological and biochemical data of the hemodialyzed patients and healthy controls included in the study are presented in . Regarding hematological parameters, we observed significantly decreased levels of serum iron, hemoglobin, and red blood cell levels in hemodialyzed patients as compared to normal values (). Total iron binding capacity (TIBC) in hemodialyzed patients was at the lower limit of reference range values for healthy subjects (). The evolution of hematological parameters in our patients during i.v. iron therapy is similar to that described in the already extensive literature about correction of nephrogenic anemia with iron.Citation[5&6], Citation[18] All the patients responded to iron therapy with a statistically significant increase in their serum ferritin, serum iron, hemoglobin, and red blood cell levels (). The ferritin concentrations, which showed a great dispersion at baseline (mean 127 ± 105 µg/L), showed significant changes with treatment (292 ± 133, ). Transferrin saturation index (TSAT), calculated from serum iron and total iron binding capacity, also increased in response to the i.v. iron therapy. There were no differences in mean corpus mean corpuscular volume (MCV), albumin, and uric acid levels between pre- and post-iron supplementation ().
Table 1. Comparison of Various Hematological and Biochemical Parameters in Hemodialysis Patients Before and After Iron Supplementation.
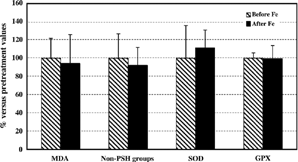
In plasma of hemodialyzed patients before iron treatment, MDA level, carbonyl groups, and SOD activity were increased, while low level of thiol groups and diminished GPX activity were observed (). Collectively, the hemodialyzed patients prior to iron therapy exhibited increased red blood cell (RBC)malondialdehyde and non-protein thiol groups levels, as well as baseline superoxide dismutase hypoactivity, compared to control subjects ().
Table 2. Markers of Oxidative Damage and Antioxidative Enzyme Activities in Plasma of Controls and Hemodialyzed Patients Supplemented with Iron.
Table 3. Markers of Oxidative Damage and Antioxidative Enzyme Activities in Red Blood Cells of Controls and Hemodialyzed Patients Supplemented with Iron.
Iron treatment resulted in enhanced oxidative stress in plasma of hemodialyzed patients (, ). Namely, after 10 consecutive i.v. administrations of iron to hemodialysis patients a significant increase in plasma MDA and CRD concentrations, together with a decrease in protein thiol groups levels (, ) was detected. Supplementation with iron did not significantly influence plasma superoxide dismutase and glutathione peroxidase activities (, ). In contrast to markers of oxidative stress in plasma, no significant differences in either of the red blood cell parameters tested in hemodialyzed patients who received i.v. iron therapy was identified (, ).
Figure 1. Plasma markers of oxidative damage and antioxidative enzyme activities in hemodialyzed patients supplemented with iron. All values after iron supplementation are expressed as a percentage (mean ± SD) of values before iron treatment. Statistically significant differences were evaluated by Student's t-test (*p < 0.05). MDA, malondialdehyde; RCD, reactive carbonyl derivates; PSH-groups, protein thiol groups; SOD, superoxide dismutase; GPX, glutathione peroxidase.
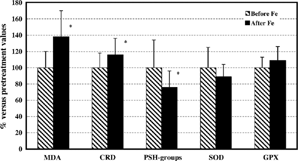
Figure 2. Red blood cell markers of oxidative damage and antioxidative enzyme activities in hemodialyzed patients supplemented with iron. All values after iron supplementation are expressed as a percentage (mean ± SD) of values before iron treatment. MDA, malondialdehyde; Non-PSH groups, non-protein thiol groups; SOD, superoxide dismutase; GPX, glutathione peroxidase.
DISCUSSION
The occurrence of oxidative stress in chronic renal failure (CRF) patients, whether on dialysis or not, has been suggested in studies showing an imbalance between oxidant and antioxidant systems and their cofactors in plasma and blood cells.Citation[8-13]
Apart from the kidney disease itself and the uremia, numerous other factors appear to contribute to oxidative stress including renal anemia which, per se, leads overall to marked decrease in antioxidant and mobile free radical scavenger capacity.Citation[14] The treatment of anemia in CRF patients is frequently hindered by the presence of suboptimal iron store. Iron deficiency should be corrected before treatment with recombinant human erythropoietin (rhEPO) or novel erythropoiesis stimulating protein (NESP) is initiated. Thus, regular, intravenous iron supplements represent a standard adjuvant therapy for the treatment of anemia in chronic kidney disease.Citation[6]
In this study we observed that intravenous administration of iron improves the anemia in hemodialyzed patients. All patients responded to i.v. iron therapy with a progressive improvement in their iron status and other hematological parameters tested. The evolution of the hematological parameters in our patients is similar to that described in the already extensive literature about correction of nephrogenic anemia with parenteral iron therapy.Citation[5&6], Citation[16], Citation[18] However, iron supplementation is not without side effects, particularly if high dose intravenous iron is administered.Citation[18&19], Citation[26] There is some controversy as to whether correction of anemia with Epo or/and with iron supplementation improves or worsens oxidative stress in end-stage renal disease patients.Citation[16-19], Citation[26] Some studies have suggested that single intravenous injection of iron, may at least transiently enhance the oxidative stress.Citation[18&19] However, potential deleterious effects of repeated intravenous iron supplementation on the plasma and red blood cell antioxidant status are less studied. Thus, this study aimed to further clarify the role of repeated intravenous iron therapy as a supplementary cause of oxidative stress in hemodialysis patients.
Given that free radicals have very short half-lives (seconds), the assessment of oxidative stress in this study is based on the measurement of different stable oxidized compounds (such as lipid peroxidation products, MDA, and DPNH-carbonyl reactive derivatives, CRD). At the same time, both enzymatic antioxidants (superoxide dismutase, glutathione peroxidase) and nonenzymatic antioxidants (glutathione, protein, and nonprotein thiol groups) are also evaluated. Our results confirmed increased concentrations of MDA and CRD in plasmaand red blood cells of hemodialysis patients before iron therapy, suggesting increased lipid and protein oxidation.Citation[17], Citation[27&28] Similarly, these patients had an impaired antioxidant capacity as measured by superoxide dismutase and glutathione peroxidase activities.
However, the oxidative damage present before treatment, does not appear to regress after iron supplementation. Namely, iron administration did not affect any red blood cell parameters tested. Moreover, oxidative stress in plasma is strongly enhanced after repeated i.v. iron administration as shown by the increased concentrations of MDA, CRD, and low levels of thiol groups. It is generally believed that blood plasma is exposed to more severe oxidative stress than intracellular fluids. The level of plasma antioxidant enzymes is much lower than in intracellular space.Citation[15] Sulphydryl groups of serum proteins, including serum albumin, have been suggested to be a “sacrificial” antioxidant in plasma and extravascular spaces.Citation[15] Besides, inflammation plasma proteins such as ferritin, transferrin, and even albumin exert a nonenzymatic antioxidant effect by sequestrating transition metal ions.Citation[15] Intravenous iron administered to anemic HD patients to correct anemia, seems to lead to an “over saturation” of transferrinCitation[29&30] and other iron-sequestrating plasma proteins that can release free iron, which have been suggested to act as catalytic agents in oxygen radical formation.Citation[29] Namely, with the dosage and timing of intravenous iron used in this study, we found that the transferrin saturation (TSAT) is over 50% in many patients. The high TSAT values found in this study upon repeated iron infusions suggest that the capacity of transferrin to bind and transport the available iron has been exceeded over the entire observation period. As a transition metal, iron is a main source of hydroxyl radical formation by reaction involving hydrogen peroxide and superoxide anion. Red blood cells, granulocytes, and macrophages, as well other metabolically active cells generate hydrogen peroxide and superoxide anions. These oxygen species can diffuse into the extracellular environment and potentially interact with redox-active iron present in plasma. As a result, non-transferrin-bound, redox-active iron might induce lipid and protein oxidation.
In this study, a significant increase in plasma DNPH-reactive carbonyl derivatives (CRD) and malondialdehyde levels was found. The DNPH-activity of proteins is postulated to indicate the presence of CRD by free radical-initiated reactions of side chains of amino acids residues.Citation[25], Citation[31] These data, together with our findings on reduced level of protein thiol groups, support the hypothesis that during periods of repeated i.v. iron therapy, the excessive amounts of extracellular iron promotes iron-mediated formation of free radical species, which could result in unscheduled lipid peroxidation and oxidation of plasma proteins. This idea is further supported by a recent study of Drüecke et al.Citation[28] who concluded that advanced oxidation protein products were elevated in end-stage renal disease patients, and its level correlated with annual intravenous iron dose administered.
In summary, our data clearly show that, despite marked improvement in hematological parameters related to a partial correction of renal anemia, a relative increase in iron stores due to parenteral iron supplementation could also contribute to increased free radical production in hemodialysis patients. The elevation of plasma carbonyl reactive derivatives and malondialdehyde levels, observed after repeated i.v. iron therapy, could only partially be explained by the increase of serum transferrin saturation and iron load,Citation[30] in keeping with the view that uremic patients have numerous defects of antioxidant defense unrelated to iron. Thus, iron load could amplify the consequences of these defects and increase the plasma pro-oxidant activity. We believe that in patients with lower iron stores the possibility of inducing free-radical toxicity will be significantly reduced by usage of low-dose intravenous iron therapy, together with regular monitoring of iron status, especially the transferrin saturation index.
REFERENCES
- Fisher, J W. Mechanism of the anemia of chronic renal failure. Nephron 1980, 25, 106–111. [PUBMED], [INFOTRIEVE]
- Eschenbach, J W.; Adamson, J W. Anemia of end stage renal disease (ESRD). Kidney Int. 1985, 28, 1–5.
- Eckardt, K U. Pathophysiology of renal anemia. Clin. Nephrol. 2000, 53 (S1), S2–S8. [PUBMED], [INFOTRIEVE], [CSA]
- Fishbane, S.; Frei, G L.; Maesuka, J. Reduction of human erythropoietin dose by the use of chronic intravenous iron supplementation. Am. J. Kidney Dis. 1995, 7, 204–207.
- Tarng, D C.; Chen, T W.; Huang, T P. Iron metabolism indices for early prediction of the response and resistance to erythropoietin therapy in maintenance hemodialysis patients. Am. J Nephrol. 1995, 15, 230–237. [PUBMED], [INFOTRIEVE], [CSA]
- National Kidney Foundation-Dialysis Outcomes Quality Initiative. Clinical guide lines for the treatment of anemia of chronic renal failure. Eknoyanv G., Ed.; III Iron support. Am. J. Kidney Dis. 1997, 30 (4 Suppl 3), S192–S240. [CSA]
- Deicher, R.; Horl, W H. Intravenous iron: theiatrogenic kick to lose control over oxygen? Kidney Blood Press. Res. 2002, 25 (5), 284–288. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Mimic-Oka, J.; Simic, T.; Djukanovic, Lj.; Stefanovski; Ramic, Z. Glutathione and its associated enzymes in peripheral blood cells in different stages of chronic renal insufficiency. Amino Acids 1992, 2, 215–224.
- Mimic-Oka, J.; Simic, T.; Ekmescic, V.; Dragicevic, P. Erythrocyte glutathione peroxidase and superoxide dismutase activities in different stages of chronic renal failure. Clin. Nephrol. 1995, 44 (1), 44–48. [PUBMED], [INFOTRIEVE], [CSA]
- Mimic-Oka, J.; Simic, T.; Djukanovic, Lj.; Reljic, Z.; Davicevic, Z. Alteration in plasma antioxidant capacity in various degrees of chronic renal failure. Clin. Nephrol. 1999, 4, 223–241.
- Jackson, P.; Loughrey, C M.; Lightbody, J H.; Mc Namel, P T.; Young, I S. Effects of hemodialysis on total antioxidant capacity and serum antioxidants in patients with chronic renal failure. Clin. Chem. 1995, 41, 1135–1138. [PUBMED], [INFOTRIEVE], [CSA]
- Tetta, C.; Biasoli, S.; Schiavon, R.; Inguaggiato, P.; David, S.; Panichi, V.; Wratten, M L. An overview of hemodialysis and oxidant stress. Blood Purif. 1999, 17, 118–126. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Luciak, M.; Trznadel, K. Free oxygen species metabolism during hemodialysis with different membranes. Nephrol. Dial. Transplant. 1991, S3, 66–70.
- Grune, T.; Sommerburg, O.; Siems, W G. Oxidative stress in anemia. Clin. Nephrol. 2000, 53 (S1), S18–S23. [PUBMED], [INFOTRIEVE], [CSA]
- Halliwell, B.; Gutteridge, J M.C. Oxidative stress and antioxidant protection: some special cases. In Free Radicals in Biology and Medicine. Halliwell, B., Gutteridge, J M.C., Eds.; Oxford University Press: New York, 2000; 485–499.
- Ludat, K.; Sommerburg, O.; Grune, T.; Siems, W G.; Riedel; Hampl, H. Oxidation parameters in complete correction of renal anemia. Clin. Nephrol. 2000, 53 (S1), 530–535.
- Mimic-Oka, J.; Simic, T.; Djukanovic, Lj. Epoetin treatment improves red blood cell and plasma antioxidant capacity in hemodialysis patients. Ren. Fail. 2002, 24 (1), 77–87. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Lim, P S.; Wei, Y H.; Yu, Y L.; Kho, B. Enhanced oxidative stress in hemodialysis patients receiving intravenous iron therapy. Nephrol. Dial. Transplant. 1999, 14, 2680–2687. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Rooyakkers, T M.; Stroes, E S.; Kooistra, M P.; van Faassen, E E.; Hider, R C.; Rabelink, T J.; Marx, J J. Ferric saccharate induces oxygen radical stress and endothelial dysfunction in vivo. Eur. J. Clin. Investig. 2002, S1, 9–16. [CROSSREF]
- Misra, H P.; Fridovich, I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [PUBMED], [INFOTRIEVE]
- Gunzler, W A.; Kremers, H.; Flohe, L. An improved coupled test procedure for glutathione peroxidase in blood. Z. Klin. Chem. Klin. Biochem. 1974, 12, 444–448. [PUBMED], [INFOTRIEVE]
- Dousset, J C.; Trouilh, M.; Foglietti, M J. Plasma malonaldehyde levels during myocardial infarction. Clin. Chim. Acta 1983, 129, 319–322. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Jocelyn, P C. Spectrophotometric assay of thiols. Methods Enzymol. 1987, 143, 44–67. [PUBMED], [INFOTRIEVE]
- Elman, G L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 74, 443–450. [CROSSREF]
- Levine, R L.; Williams, J A.; Stadtman, E R.; Shacter, E. Carbonyl assay determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [PUBMED], [INFOTRIEVE]
- Cavdar, C.; Temiz, A.; Yenicerioglu; Caliskan, S.; Celik, A.; Sifil, A.; Onvural, B.; Camsari, T. The effects of intravenous iron treatment on oxidant stress and erythrocyte deformability in hemodialysis patients. Scand. J. Urol. Nephrol. 2003, 37 (1), 77–82. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Tovbin, D.; Mazor, D.; Vorobiov, M.; Chaimovitz, C.; Meyerstein, N. Induction of protein oxidation by intravenous iron in hemodialysis patients: role of inflammation. Am. J. Kidney Dis. 2002, 40 (5), 1005–1012. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Drüeke, T.; Witko-Sarsat, V.; Massy, Z.; Descamps-Latscha, B.; Guerrin, A P.; Marchais, S J.; Gausson, V.; London, G M. Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation 2002, 106, 2212–2217. [CROSSREF]
- Roob, J M.; Koschsorur, G.; Tiran, A.; Horina, J H.; Holzer, H.; Winklhofer-Roob, B M. Vitamin E attenuates oxidative stress induced by intravenous iron in patients on hemodialysis. J. Am. Soc. Nephrol. 2000, 11, 539–549. [PUBMED], [INFOTRIEVE], [CSA]
- Esposito, B P.; Breuer, W.; Slotki, I.; Cabantchik, Z I. Labile iron in parenteral iron formulations and its potential for generating plasma nontransferrin-bound iron in dialysis patients. Eur. J. Clin. Investig. 2002, 32 (S1), 42–49. [CROSSREF]
- Davies, K J.A. Protein damage and degradation by oxygen free radicals. II Modification of amino acids. J. Biol. Chem. 1987, 262, 9902–9907. [PUBMED], [INFOTRIEVE]