Abstract
Aim. Aside from lowering lipid levels; statins improve endothelial function, decrease platelet aggregation, reduce procoagulant blood factors, and decrease vascular tone. This study was conducted to investigate the possible effect of atorvastatin on blood pressure (BP) in a group of hypertensive and dyslipidemic patients. Methods. Thirty-six hypertensive and dyslipidemic patients with inadequately controlled lipid levels by diet were treated with atorvastatin 20 mg/day for 8 weeks and compared with 24 patient matched controls treated with diet alone. The type and dosage of antihypertensive medications were not altered during statin therapy. Blood lipid profile including total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglyceride (TG) levels were noted at inclusion and after 8 weeks. Ambulatory BP monitoring (ABPM) was carried out at study entry and at the end of week eight. Results. A total of 49 patients (32 patients in the atorvastatin group and 17 patients in the control group) completed the 3-month follow-up period of observation. The ABPM studies indicated significant reductions in total average systolic BP, total average diastolic BP, total average mean BP, day average systolic BP, day average diastolic BP, night average systolic BP, night average diastolic BP, and night average mean BP levels in the atorvastatin group, whereas these reductions were not observed in the control group. Conclusion. Our results indicate that atorvastatin therapy significantly improves BP control in hyperlipidemic hypertensive patients. However, the effects of other statins on BP, as well as, the different dosages need to be further investigated.
INTRODUCTION
Dyslipidemia and hypertension are both independent risk factors and if they are together, they have a synergistic effect on the risk of experiencing coronary artery disease.Citation[1] Dyslipidemia is commonly seen in hypertensive patients,Citation[2&3] where it could cause significant increase in the risk for cardiovascular events. A Multiple Risk Factor Intervention Trial (MRFIT) showed that hypertensive patients with dyslipidemia have higher cardiovascular mortality than do other groups.Citation[4]
According to a series of large randomized endpoint trialsCitation[5-7] statin treatment of subjects with dyslipidemia produced important reductions in major fatal and nonfatal cardiovascular events, both in people with established coronary disease and in those with no overt evidence of atherosclerotic disease.Citation[8&9] The relationship between dyslipidemia and blood pressure is still not clearly understood. Some clinical trials have found a link between high cholesterol levels and impaired endothelium-dependent vascular relaxation.Citation[10&11] In animal studies, they demonstrated that hypercholesterolemia induces the overexpression of the gene coding for angiotensin II receptors.Citation[12] In the light of these data, treatment of dyslipidemia could be directly involved in the control of vascular tone and reactivity and could contribute to the development of systemic hypertension.
Hydroxymethylglutaryl coenzyme A reductase inhibitors, commonly known as statins, have multiple actions above and beyond their lipid lowering capabilities. In the last decade, statins brought a major revolution in the field of preventive cardiology. The cholesterol and recurrent events trials revealed that patients treated with statins had a lower risk of cardiovascular events when compared with age-matched placebo-treated patients.Citation[13-15] Meta-analysis of cholesterol-lowering trials have demonstrated that for a give degree of cholesterol reduction, the risk of cardiovascular events and the incidence of stroke in patients with coronary artery disease are less in patients treated with statins compared to those treated with other cholesterol-lowering treatments.Citation[16]
It is well known that other effects of statins are unrelated to lowering the serum cholesterol level. The pleiotropic actions include direct effects on vascular tissue, kidney, bone, and glucose metabolisms. Resultant effects consist of increased nitric oxide levels and improved endothelial function,Citation[17] stabilization of plaque, reduction of the progression of nephropathy,Citation[18] development of diabetes,Citation[18] inflammation, thrombogenic responses,Citation[19] and fracture rates.Citation[20] Moreover, recent animal data indicate that statins improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species.Citation[21] The aim of the present study was to investigate the effect of statin therapy on blood pressure in patients with hypertension and dyslipidemia.
PATIENTS AND METHODS
Hypertensive and dyslipidemic patients attending the outpatient cardiology and nephrology clinics of our hospital on regular basis were screened and those who met the inclusion criteria were enrolled. Inclusion criteria are: 1) Arterial hypertension, defined as either antihypertensive use or systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg; 2) No history of lipid lowering medication; 3) No laboratory evidence of liver, renal, or thyroid dysfunction on routine screening; 4) Documented fasting serum total cholesterol (TC) ≥ 200 mg/dL even with dietary fat restriction comprising fat less than 30% of calories for 2 months; 5) No history of corticosteroid or hormone replacement therapy. Patients with chronic alcoholism, smoking, uncontrolled hypertension, diabetes mellitus (> 110 mg/dL fasting glucose level), and severe dyslipidemia (TC ≥ 300 mg/dL) were excluded.
Eligible patients were enrolled into a 4-week run-in phase to stabilize blood pressure and plasma cholesterol levels. During this period, all patients maintained their usual antihypertensive treatment. The patients were instructed to maintain the same diet throughout the study. At the end of the run-in phase, which represents the baseline of the trial, 36 patients with high plasma cholesterol levels were treated with 20 mg atorvastatin for 8 weeks. A dietary treatment alone was maintained in the 24 hypercholesterolemic patients. During the 3-month period of observation, the antihypertensive treatment was not modified.
Atorvastatin was started regardless of patients' ages and weight at a dose of 20 mg/day in all patients whose serum levels, TC, and low-density-lipoprotein (LDL)were above 200 mg/dL and 130 mg/dL respectively. During the follow-up the antihypertensive therapy, as well as the concurrent medications, were kept constant, and also the patients were instructed to maintain a similar diet throughout the study. At baseline and 8 weeks after starting atorvastatin therapy, venous blood specimens after overnight fasting were drawn between 8 and 10 a.m. Serum TC, high density lipoprotein (HDL), LDL, and triglyceride (TG) were measured by the same laboratory with standard methods. The LDL cholesterol levels were calculated by using the Friedwald formula.
Ambulatory blood pressure monitoring (ABPM) was performed with Reynolds Medical Tracker NIBP2 oscillometric monitor. Each patient used an arm cuff of similar size to the one used for routine office blood pressure measurement in the nondominant arm. The ABPM was obtained over 24 hour periods at baseline and after 8 weeks from all patients. The device was programmed to measure blood pressure every 10 minutes between 06:00 a.m. and 10:00 p.m., and every 20 minutes between 10:00 p.m. and 06:00 a.m.
Statistical analysis was carried out using an SPSS statistical package (version 10.0). The results are presented as mean ± SD (standard deviation). Wilcoxon signed ranks test was used to compare the blood pressure results before and after 8 weeks to determine the effect of atorvastatin on blood pressure. The P value of < 0.05 was considered significant.
RESULTS
A total of 49 patients (32 patients in the atorvastatin group and 17 patients in the control group) completed the 3-month follow-up period of observation. Four patients in the statin group and seven in the diet group dropped out of the study because of poor compliance (5 patients), and poor blood pressure control (6 patients). Blood pressure and cholesterol measurements were obtained in all patients. The baseline demographic characteristics of the study population along with those of the 2:1 matched controls are shown in . No significant differences have been were observed between both groups of patients in term of demographic variables (p > 0.05). As expected, after 8 weeks of atorvastatin therapy, TC, LDL, HDL, and TG levels were improved (p < 0.05), whereas in thecontrol group no significant changes were found (p > 0.05). Atorvastatin was well tolerated by all patients, and no clinical side effects, such as myopathy, diarrhea, or abdominal discomfort, were noted during the follow-up. There were no episodes of elevation of transaminases and creatinine phosphokinase.
Table 1. Baseline clinical characteristics of the study population.
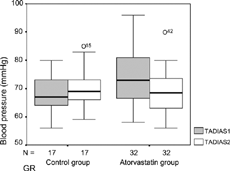
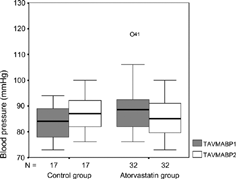
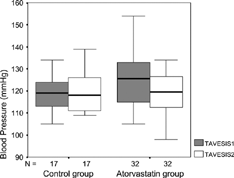
The effects on systolic and diastolic blood pressure of atorvastatin were compared with the control group. Patients treated with atorvastatin had a significantly greater decrease in systolic and diastolic blood pressure than did patients treated with diet therapy alone. Ambulatory blood pressure monitoring (ABPM) studies indicated significant reductions in total average systolic blood pressure (TAVESIS) (), total average diastolic blood pressure (TADIAS) (), total average mean blood pressure (TAVMABP) (), day average systolic blood pressure (DASBP), day average diastolic blood pressure (DADBP), night average systolic blood pressure (NASBP), night average diastolic blood pressure (NADBP) and night average mean blood pressure (NAMBP) levels compared to baseline where as these reductions were not observed in control group ().
Table 2. ABPM results baseline and after 8 weeks in atorvastatin and control groups.
Figure 1. Total average means blood pressure (TAVMABP) findings before and after 8 weeks in the atorvastatin and control group.
DISCUSSION
Dyslipidemia and hypertension are both a major risk factor for coronary artery disease. Previous studies found that coexistent risk factors, such as high blood pressure and high cholesterol levels, generally exert a cumulative effect on the risk of cardiovascular events.Citation[1] Observational data demonstrated that hypertension-prone patients whose incidence of stable hypertension over time was reported to be significantly increased in the presence of a serum cholesterol level higher than 200 mg/dL.Citation[2-4], Citation[22], Citation[23] In addition, a retrospective analysis of this study revealed that blood pressure decreased, especially in patients treated with statins.Citation[24] In our present study, we demonstrate that atorvastatin not only improved the lipid profile, but it also significantly reduced the day- and nighttime systolic and diastolic blood pressure as well.
Previous studies found that combination of statins and antihypertensive drugs may control the blood pressure much better than does either drug alone,Citation[25-27] this may be due to a possible additive effect between these drugs. In the Brigsella Heart Study,Citation[28] they found that reduction of blood pressure observed with the combination of statins and antihypertensive drugs could not be achieved in patients treated with fibrates and antihypertensive drugs. These newer evidences suggest that statins may possess effects extending beyond their lipid-lowering effect. In the light of these studies, statins, which are organ protective and improve vascular endothelial function, could contribute to better blood pressure apart from cholesterol reduction. Sporito et al.Citation[25] investigated the possible effects of pravastatin and lovastatin on blood pressure in patients taking enalapril and lisinopril. They demonstrated a significant improvement in mean arterial blood pressure after 4 weeks of therapy. In another study, an average decrease in systolic and diastolic blood pressure of 6–3 mmHg respectively was noted in 17 hypercholesterolemic but normotensive individuals after 20 weeks of treatment with atorvastatin.Citation[29] Conversely, in some previous studies, they found no blood-pressure lowering effect in normotensive and well-controlled hypertensive patients.Citation[19], Citation[27], Citation[30]
The positive clinical effects of statins could not be explained only by improving the serum lipid profile. Endothelial nitric oxide (NO) synthase upregulation is associated with protection of organ function by NO such as in cardiovascular disease.Citation[31-34] Moreover, inhibition of basal NO synthesis in vivo can induce atherosclerosis. In previous studies, they demonstrate that withdrawal of statins down regulates NO synthase,Citation[35] inhibit the generation of oxygen-derived free radicals such as superoxide anion (O2−) and hydroxy (OH−) and promote endothelial dysfunction in mice.Citation[35&36]
Additional mechanisms could involve decreasing vascular reactivity to norepinephrine and angiotensin II,Citation[37] and down-regulating angiotensin I receptors, decreasing aldosterone-endothelium levels.Citation[19] Patients with high cholesterol levels have impairment in peripheral compliance.Citation[35] Cholesterol reduction with statin improves endothelium-dependent arterial compliance; thereby increase the vasodilator capacity of the large arteries.Citation[38] This may explain the sensitivity of the vascular wall to the blood pressure–lowering effect in patients taking the combination antihypertensive drugs and statins.
The limitations of this study must be mentioned. We have demonstrated that statin has a beneficial effect on blood pressure control in hypertensive-hyperlipidemic patients; insight into possible mechanisms behind this association requires pharmacologic evaluation. First, the duration of our trial was short. We have no idea about the long-term benefits of statin in patients with hypertension and hyperlipidemia. We did not investigate whether the effect of statins on blood pressure was dose related or not. A small sample size is another limitation. Meanwhile, although baseline BP values of the atorvastatin group appeared to be greater than did those of the control group, we found a more significant decrease in blood pressure levels in the atorvastatin group than that in control group. In addition, 60% of our patients treated with atorvastatin had already received ACE inhibitors, angiotensin II receptor blockers, or calcium channel blockers. Therefore, we cannot exclude the contribution of the synergistic or additive effects of these drugs to possible blood pressure–lowering effects of statins. This relationship needs to be confirmed in further prospective studies.
In conclusion, our study demonstrates that atorvastatin may improve the blood pressure in patients with hyperlipidemia and hypertension. Statins are always surprising us, not all of their profits can be explained only by cholesterol lowering.
| ABBREVIATIONS | ||
| HL: | = | Hyperlipidemia |
| TC: | = | Total cholesterol |
| HDL: | = | High density lipoprotein |
| LDL: | = | Low density lipoprotein |
| TG: | = | Triglyceride |
| TAVESIS: | = | Total average systolic blood pressure |
| TADIAS: | = | Total average diastolic blood pressure |
| TAVMABP: | = | Total average mean blood pressure |
| DASBP: | = | Day average systolic blood pressure |
| DADBP: | = | Day average diastolic blood pressure |
| NASBP: | = | Night average systolic blood pressure |
| NADBP: | = | Night average diastolic blood pressure |
| NAMBP: | = | Night average mean blood pressure |
REFERENCES
- Kannel, W B. Importance of hypertension as a major risk factor in cardiovascular disease. In Hypertension: Physiopathology and Treatment; Bosch, J., Grozsman, R J., Eds.; McGraw Hill: New York, 1999; 888–910.
- Kannel, W B. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA 1996, 275, 1571–1576. [PUBMED], [INFOTRIEVE]
- Njolstad, I.; Arnesen, E.; Lund-Larsen, P G. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction: a 12-year follow-up of the Finnmark Study. Circulation 1996, 93, 450–456. [PUBMED], [INFOTRIEVE], [CSA]
- Neaton, J D.; Blackburn, H.; Jacobs, D.; Kuller, L.; Lee, D J.; Sherwin, R.; Shih, J.; Stamler, J.; Wentworth, D. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial: Multiple Risk Factor Intervention Trial Research Group. Arch. Intern. Med. 1992, 152, 1490–1500. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial. Lancet 2002, 360, 7–22. [CROSSREF]
- Prosper Study Group. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomized controlled trial. Lancet 2002, 360, 1623–1630. [CROSSREF]
- Athyros, V G.; Papageorgiou, A A.; Mercouris, B R. The Greek atorvastatin and coronary heart disease evaluation (GREACE) study. Curr. Med. Res. Opin. 2002, 18, 220–228. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Randomized trial of cholesterol lowering in 4444patients with coronary heart disease: Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [CROSSREF]
- Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a range of initial cholesterol levels: the Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) Study Group. N. Engl. J. Med. 1998, 339, 1349–1357. [CROSSREF], [CSA]
- Lewis, T V.; Cooer, B A.; Dart, A M.; Chin-Dusting, J P. Responses to endothelium-dependent agonists in subcutaneous arteries excised from hypercholesterolemic men. Br. J. Pharmacol. 1998, 124, 222–228. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Wever, R.; Stroes, E.; Rabelink, T J. Nitric oxide and hypercholesterolemia: a matter of oxidation and reduction? Atherosclerosis 1998, 137 (suppl), 51–60. [CROSSREF]
- Nickening, G.; Bohm, M. Regulation of the angiotensin AT receptor expression by hypercholesterolemia. Eur. J. Med. Res. 1997, 2, 285–289. [CSA]
- Shepherd, J.; Cobbe, S M.; Ford, I.; Isles, C G.; Lorimer, A R.; MacFalane, P W.; McKillop, J H.; Packard, C J. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia: West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 1995, 333, 1301–1307. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Sacks, F M.; Pfeffer, M A.; Moye, L A.; Rouleau, J L.; Rutherford, J D.; Cole, T G.; Brown, L.; Warnica, J W.; Arnold, J M.; Wun, C C.; Davis, B R.; Braunwald, E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels: Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996, 335, 1001–1009. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Pekkanen, J.; Linn, S.; Heiss, G.; Suchindran, C M.; Leon, A.; Rifkind, B M.; Tyroler, H A. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N. Engl. J. Med. 1990, 322, 1700–1707. [PUBMED], [INFOTRIEVE]
- Brown, B G.; Zhao, X Q.; Sacco, D E.; Albers, J J. Lipid lowering and plaque regression: new insights into prevention of plaque disruption and clinical events in coronary disease. Circulation 1993, 87, 1781–1791. [PUBMED], [INFOTRIEVE]
- Asberg, A.; Hartmann, A.; Fiedelsa, E.; Holdaas, H. Atorvastatin improves endothelial function in renal transplant recipients. Nephrol. Dial. Transplant. 2001, 16, 1920–1924. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Tonolo, G.; Melis, M G.; Formato, M.; Angius, M F.; Carboni, A.; Brizzi, P.; Ciccareese, M.; Cherchi, G M.; Maioli, M. Additive effects of simvastatin beyond its effects on LDL cholesterol in hypertensive type 2 diabetic patients. Eur. J. Clin. Investig. 2000, 30, 980–987. [CROSSREF], [CSA]
- Glorioso, N.; Troffa, C.; Filigheddu, F.; Dettori, F.; Soro, A.; Pinna, P.; Collatina, S.; Pahor, M. Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension 1999, 34, 1281–1286. [PUBMED], [INFOTRIEVE]
- Meier, C R.; Schlinger, R G.; Kraenzlin, M E.; Schlegel, B.; Jick, H. HMG-CoA reductase inhibitors and the risk of fractures. JAMA 2000, 283, 3205–3210. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Wassmann, S.; Laufs, U.; Baumer, A T.; Muller, K.; Ahlbory, K.; Linz, W.; Itter, G.; Rosen, R.; Bohm, M.; Nickenig, G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension 2001, 37, 1450–1457. [PUBMED], [INFOTRIEVE]
- Borghi, C.; Bacchelli, S.; Degli Esposti, D.; Ambrosioni, E.; Boricelli, L. Pressor and metabolic correlates to long-term development of stable hypertension in borderline hypertensive patients. J. Hypertens. 1997, 15, 111.
- Julius, S.; Jamerson, K.; Mejia, A.; Krause, L.; Schork, N.; Jones, K. The association of borderline hypertension with target organ changes and higher coronary risk: Tecumseh Blood Pressure study. JAMA 1990, 264, 354–358. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Goode, G K.; Miller, J P.; Heagerty, A M. Hyperlipidemia, hypertension, and coronary heart disease. Lancet 1995, 345, 362–364. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Sposito, A C.; Mansur, A P.; Coelho, O R.; Nicolau, J C.; Ramires, J A. Additional reduction in blood pressure after cholesterol-lowering treatment by statins (lovastatin or pravastatin) in hypercholesterolemic patients using angiotensin-converting enzyme inhibitors (enalapril or lisinopril). Am. J. Cardiol. 1999, 83, 1497–1499. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Bachelli, S.; Borghi, C.; Prandin, M G.; Immordino, V.; Ambrosioni, E. Improved blood pressure control in hypertensive and hypercholesterolemic patients treated with HMG-CoA reductase inhibitors. Am. J. Hypertens. 1998, 11, 25A. [CROSSREF]
- Borghi, C.; Prandin, M G.; Costa, F V.; Bachelli, S.; Esposti, D.; Ambrosioni Use of statins and blood pressure control in treated hypertensive patients with hypercholesterolemia. J. Cardiovasc. Pharmacol. 2000, 35, 549–555. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Dormi, A.; Gaddi, A.; Borghi, C. Lipid lowering drugs and blood pressure control in patients at high CV risk: evidences from a population surveyin the Brighella Heart Study. J. Hypertens. 1999, 17, 60.
- Leibovitz, E.; Hazanov, N.; Shargorodsky, M.; Gavish, D. Treatment with atorvastatin improves small artery compliance in patients with severe hypercholesterolemia. Am. J. Hypertens. 2001, 14, 1096–1098. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Fogari, R.; Zopi, A.; Lusardi, P.; Preti, P.; Poleti, L.; Salvetti, A. Effects of pravastatin given every other day in dyslipidemic hypertensive patients treated with different antihypertensive drugs. High Blood Press. 1996, 5, 219–225. [CSA]
- Ross, R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993, 362, 802–809. [CROSSREF]
- Ross, R. Cell biology of atherosclerosis. Annu. Rev. Physiol. 1995, 57, 791–804. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Abetel, G.; Poget, P N.; Bonnabry, J P. Hypotensive effect of an inhibitor of cholesterol synthesis: a pilot study. Schweiz. Med. Wochenschr. 1998, 128, 272–277. [PUBMED], [INFOTRIEVE]
- Muramatsu, J.; Kobayashi, A.; Haseegawa, N.; Yokouchi, S. Hemodynamic changes associated with reduction in total cholesterol by treatment with HMG-CoA reductase inhibitor pravastatin. Atherosclerosis 1997, 130, 179–182. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Kool, M.; Lustermans, F.; Kragten, H.; Strijker Boudier, H.; Hoeks, A.; Reneman, R.; Rila, H.; Hoogendam, I.; Van Bortel, L. Does lowering of cholesterol levels influence functional properties of large arteries? Eur. J. Clin. Pharmacol. 1995, 48, 217–223. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kaesemeyer, W H.; Caldwell, R B.; Huang, J.; Caldwell, R W. Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J. Am. Coll. Cardiol. 1999, 33, 234–241. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Straznicky, N E.; Howes, L G.; Lam, W.; Louis, W J. Effects of pravastatin on cardiovascular reactivity to norepinephrine and angiotensin II in patients with hypercholesterolemia and systemic hypertension. Am. J. Cardiol. 1995, 75, 582–586. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Knopp, R H. Drug treatment of lipid disorders. N. Engl. J. Med. 1999, 341, 498–510. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]