Abstract
Objective: The clinical relevance of altered lymphocyte function and the possible relation with uremic toxins, such as parathyroid hormone (PTH) is not well understood. We studied the changes in cellular immunity in patients in hemodialysis (HD) therapy and examined the relationship between T-lymphocyte function and plasma levels of PTH. Patients and Methods: Thirty-four patients (14 male) were enrolled in this study (mean age: 63.20 ± 10.01 years, M ± SD, 12 h/week HD). Our study population was divided into two groups: 18 patients with increased levels of PTH and 16 patients with normal levels of PTH. Lymphocyte subsets (CD2 +, CD3 +, CD3 +/4 +, CD3 +/8 +, CD19 +, CD3-/16 + 56 +, CD4/CD8 ratio) were quantitated in both groups using monoclonal antibodies (Immunotech, Coulter) and flow cytometric analysis. Following analysis of variance (ANOVA) testing was performed to test differences between groups (SPSS version 10). Results: A significant increase of CD2 was noticed in patients with increased levels of PTH (84.8 ± 5.5 vs. 79.8 ± 4, p < 0.05). The CD3 population was also increased in patients with elevated PTH (72 ± 8.6 vs. 68 ± 9.2, p = NS). This group of patients had also significantly increased levels of CD3/8 (44.8 ± 9.8 vs. 37.1 ± 5.8, p < 0.05). The CD4/CD8 ratio levels were higher in patients with elevated PTH compared with those who had normal PTH (2.2 ± 1.5 vs. 1.5 ± 0.8, p = NS). Conclusions: The elevated level of PTH seems to affect the lymphocyte function and is associated with changes in cellular immunity in the hemodialysis population. Our study is in progress in order to enlarge our study population and collect more data, which will lead us to more solid conclusions.
INTRODUCTION
Chronic renal failure is accompanied by various immunologic abnormalities of innate and acquired immunity. The clinical relevance of this altered function is related to the morbidity and mortality observed in uremic patients. The immunological deficiency due to uremia can not be corrected by any replacement therapy, hemodialysis (HD), or continuous ambulatory peritoneal dialysis (CAPD).Citation[1-3]
On the other hand, chronic renal failure is frequently associated with secondary hyperparathyroidism and immunological disorders. Recent studies support the hypothesis that high levels of parathyroid hormone (PTH) may contribute to the impairment of the cellular and humoral response.Citation[4] It has been postulated that PTH may affect the immune system of hemodialysis patients either by increasing cytosolic calciumCitation[5] or by direct stimulatory effect on T lymphocytes.Citation[6] On the other hand, other researchers have reported an immunosuppressive effect of PTH on T and B lymphocytes.Citation[7&8]
In general, several references support the hypothesis that high levels of PTH have an immumodulatory effect; however the data and the conclusions remain controversial.Citation[8&9] It is clear that the clinical relevance of altered lymphocyte function and the possible relation with uremic toxins, such as parathyroid hormone (PTH) is not well understood. For this reason, we studied the changes incellular immunity in patients in HD therapy and examined the relationship between T-lymphocyte function and plasma levels of PTH.
PATIENTS AND METHODS
The present study was carried out in 34 patients (14 male, 20 female) under HD (12 h/week HD) with a mean age of 63.20 ± 10.01 years, M ± SD. Our patients received HD for 35 ± 43 months. Our study population was divided in two groups: 18 patients with increased levels of PTH (Group I) and 16 patients with normal levels of PTH (Group II). All of the patients received regular hemodialysis treatment with polysulfone membranes and Kt/V equal to or higher than 1.2. The selection of the low and high PTH patient groups was based on at least three PTH determinations, but only the last one was considered, because this sample was collected at the same time as were the samples for the immunological assays. None of the patients showed evidence of immunological diseases.
Lymphocyte subsets (CD2 +, CD3 +, CD3 +/4 +, CD3 +/8 +, CD19 +, CD3 −/16 + 56 +, CD4/CD8 ratio) were determined using monoclonal antibodies (Immunotech, Coulter) and flow cytometric analysis (). Briefly, 20 µL of the appropriate monoclonal antibody was incubated with 100 µL of blood sample for 20 min in the dark. The samples were then lysed by the Immuno Prep reagent system (Beckman Coulter Company) and analyzed in the flow cytometer (Epics Elite ESP, Coulter).
Table 1 Monoclonal antibodies used for lymphocyte analysis
Intact PTH levels were measured in patient's serum. All samples were assayed by the solid-phase, two-site chemiluminescent enzyme–labeled immunometric method, in the Immulite 2000 analyte, using reagents of DPC Diagnostic Products.
Statistical evaluation was performed by analysis of variance (ANOVA) to test differences between groups (SPSS version 10).
RESULTS
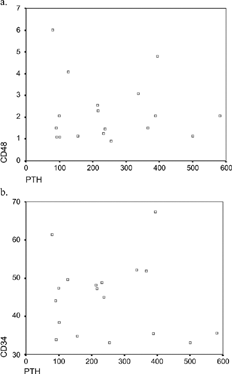
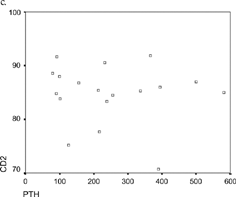
The results of lymphocyte subsets mean values in patients on HD in both groups are presented in . A significant increase of CD2 was noticed in patients with increased levels of PTH (84.8 ± 5.5 vs. 79.8 ± 4, p < 0.05). The CD3 population was also increased in patients with elevated PTH (72 ± 8.6 vs. 68 ± 9.2, p = NS). This group of patients also had significantly increased levels of CD3/8 (44.8 ± 9.8 vs. 37.1 ± 5.8, p < 0.05) and CD3/4 (44.5 ± 0.7 vs. 37.13 ± 5.78, p < 0.05). The CD4/CD8 ratio levels were slightly higher in patients with elevated PTH compared with those who had normal PTH (2.2 ± 1.5 vs. 1.5 ± 0.8, p = NS). No specific differences were noticed among those two groups of patients comparing CD19 and Natural Killer cells.
Table 2 Mean values of PTH levels and phenotypic analysis in patients on HD in two groups
There was a significant correlation between PTH levels and CD4/CD8, CD3/4, and CD2 in patients of Group II (, , ).
DISCUSSION
The data presented here suggested that uremic patients, irrespective of their PTH levels, present some immune dysfunction and corroborate with several studies.Citation[10&11] The putative causes suggested for these alterations were uremic toxins, dialysis treatment, and hyperparathyroidism. Our patients were under HD withbiocompatible membranes and very few of them had secondary hyperparathyroidism. Therefore, other components present in uremic serum may be responsible for the lymphocyte dysfunction.
On the other hand, our data comparing HD patients between two groups support the notion that PTH may play a role in the genesis of cell immunity abnormalities in uremic patients.
Over the last decades it was well established that chronic renal failure exhibits peripheral blood lymphopenia, which is accompanied by decreased lymphocyte proliferative response when stimulated by different antigens.Citation[12&13] This disorder has been related to decreased production of interleukin-2 and interferon-γ and it is more apparent in HD patients.Citation[12&13] Uremic toxins, LDL, prostaglandin E2, and parathyroidhormone seem to be incriminated for such insufficiency.Citation[13] Many theories have been established in order to explain immunological deficiencies of uremic patients. These include the direct damage of monocytes and the defect of soluble receptor of T lymphocytes in a uremic environment 13. No matter what the cause of this deficiency, immunological abnormalities increase the rate of infections, affect erythropoiesis, and affect the clinical outcome of end stage uremic patients.Citation[12&13]
Uremic patients with high levels of PTH showed an enhanced T cell–proliferative response compared with patients with normal levels of PTH. The mode of action of PTH on the cellular immune system is not yet understood fully. It has been showed that acute exposure to PTH in vitro increased T-cell proliferation in healthy controls.Citation[4] In contrast, exposure to PTH failed to stimulate proliferation of T cells from dialysis patients. Therefore, it is possible that the observed T-cell activation and proliferation in healthy subjects could be mediated by PTH-PTH-receptor interactions, which are known to affect the phospholipid turnover of cell membranes and to stimulate protein kinase C.Citation[14]
Another possible explanation for T-cell proliferation upon high PTH levels relies on the basal Ca+ 2 levels. It has been shown that high levels of PTH are associated with increased basal Ca+ 2 levels.Citation[15] An increase in the cell calcium content and in protein kinase C have an effect on T-cell activation. High intracellular calcium concentration is responsible for many cellular dysfunctions.Citation[6]
A B-cell dysfunction in the presence of PTH in vitro and in vivo has been reported by some authors.Citation[7], Citation[16] However, we found no significant differences between the two study groups when comparing B lymphocytes. Our results may be explained by the suggestion that B lymphocytes are pre-activated in hemodialysis patients. These B cells could be committed to nonspecificimmunoglobulin secretion, which is apparently preserved in patients with chronic renal insufficiency and hyporesponsive, to a certain degree, to proliferative stimuli.Citation[11]
In conclusion, our data suggest that elevated levels of PTH seem to affect the lymphocyte function and are associated with changes in cellular immunity in the hemodialysis population. It has been also proposed that other uremic factors in chronic renal failure may affect T-cell functions. Our study remains in progress in order to enlarge our study population and collect more data, which will lead us to more solid conclusions.
REFERENCES
- Sakellariou, G. Immunologic abnormalities in uremia. Hell. Nephrol. 1999; 11 (1), 232–238.
- Davies, S J.Suassuna, J.; Ogg, C S.; Cameron, J S. Activation of immunocompetent cells in the peritoneum of patients treated with CAPD. Kidney Int. 1988; 36, 661–668.
- Casciani, C V.; DeSimone, C.; Bonini, S. Immunological aspects of chronic uremia. Kidney Int. 1978; 13 (Suppl. 8), 49–54.
- Massry, S G.; Alexiewich, J M.; Gaciong, Z.; Klinger, M. Secondary hyperparathyroidism and the immune system in chronic renal failure. Semin. Nephrol. 1991; 11, 186–201. [PUBMED], [INFOTRIEVE]
- Alexiewich, J M.; Smogorzewski, M.; Akmal, M.; Klin, M.; Massry, S G. Nifedipine reverses the abnormalities in Ca and proliferation of B-cells from dialysis patients. Kidney Int. 1996; 50, 1249–1254.
- Klinger, M.; Alexiewich, J M.; Linker-Israeli, M.; Pitts, T O.; Gaciong, Z.; Fadda, G Z.; Massry, S G. Effect of parathyroid hormone on human T-cell activation. Kidney Int. 1990; 37, 1543–1551. [PUBMED], [INFOTRIEVE]
- Jiang, Y.; Yoshiba, A.; Ishioka, C.; Kinnota, H.; Mikawa, H. Parathyroid hormone inhibits immunoglobulin production without affecting cell growth in human B cells. Clin. Immunol. Immunopathol. 1992; 65, 286–293. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Shasha, S M.; Kristal, B.; Barzilai, M.; Makov, E U.; Shkolnik, T. In vitro: effect of PTH on normal T cell function. Nephron 1988; 50, 212–216. [PUBMED], [INFOTRIEVE]
- Lewin, E.; Ladefoged, J.; Brandi, L.; Olgaard, K. Parathyroid hormone-dependent T-cell proliferation in uremic rats. Kidney Int. 1993; 44, 379–384. [PUBMED], [INFOTRIEVE]
- Beaurain, G.; Naret, C.; Marcon, C.; Grateau, G.; Drueke, T.; Urena, P.; Nelson, D L.; Bach, J F.; Chatenoud, L. In vivo T cell pre-activation in chronic uremic hemodialyzed and non-hemodialyzed patients. Kidney Int. 1989; 36, 636–640. [PUBMED], [INFOTRIEVE]
- Descamps-Latscha, B.; Herbenin, A. Long term dialysis and cellular immunity: a critical survey. Kidney Int. 1993; 43, 135–142.
- Chatenoud, L.; Herbelin, A.; Beaurain, G.; Descamps-Latscha, B. Immune deficiency of the uremic patients. Adv. Nephrol. 1990; 19, 259–274.
- Coen, G.; Çaag-Weber, M.; Horl, W H. Immune dysfunction in uremia. Kidney Int. 1997; 52, 79–82.
- Farese, R B.; Bidot-Lopez, P.; Sabir, A.; Larson, R E. Parathyroid hormone acutely increases phosphoinositidies of the rabbit kidney cortex by a cycloheximide-sensitive process. J. Clin. Invest. 1980; 65, 1523–1526. [PUBMED], [INFOTRIEVE]
- Massry, S.; Smorgorzewski, M. Mechanisms through which parathyroid hormone mediates its deleterious effects on organ function in uremia. Semin. Nephrol. 1994; 14, 219–231. [PUBMED], [INFOTRIEVE], [CSA]
- Gaciong, Z.; Alexiewicz, J M.; Linker-Israeli, M.; Shulman, I A.; Pitts, T O.; Massry, S G. Inhibition of immunoglobulin production by parathyroid hormone. Implications in chronic renal failure. Kidney Int. 1991; 40, 96–106. [PUBMED], [INFOTRIEVE]