Abstract
In this study, we aimed to compare Cystatin C (Cys C) with other traditional glomerular filtration rate (GFR) markers and to evaluate its superiority over them in detecting early renal involvement in patients with primary hypertension. Fifty-one primary hypertensive patients and 29 healthy control subjects, who were similar in terms of age and gender, were included in the study. In all subjects serum levels of Cys C, beta-2 microglobulin, serum creatinine (SCr), uric acid, BUN, albumin; 24 h urinary levels of protein (Upro), albumin (Ualb) and creatinine were measured. The GFR was calculated according to Creatinine Clearance (CrCl), Cockcroft-Gault (CG) and Modification of Diet in Renal Disease (MDRD) formulas. The MDRD was used as the reference method. A GFR < 80 mL/min/1.73 m2 was considered as the lower cut-off limit. Mean levels of the serum parameters were found to be significantly higher in the patient group than they were in the control group (p < 0.05). Mean CrCl, CG, and MDRD levels were lower in patients than they were in controls but the difference was statistically significant for CG and MDRD. The serum parameter having the best correlation with MDRD was SCr (r = − 0.760) in patients and Cys C (r = − 0.622) in controls. However, in ROC analysis; the area under curve (AUC) for Cys C was found to be superior (AUC = 0.900) to the other markers. The CrCl was the parameter having the worst diagnostic efficiency (AUC = 0.598). As a conclusion, compared to other traditional markers, measurement of Cys C may be a better parameter to estimate GFR, especially to detect mild reductions of GFR in primary hypertensive patients.
INTRODUCTION
Arterial hypertension-related renal damage is an increasingly common problem,Citation[1] because “hypertensive nephrosclerosis” is moving up the charts to number 2 in terms of diagnostic frequency cited as causing end-stage renal disease in chronic dialysis patients.Citation[2] So, checking the renal function in hypertensive patients is important for both detecting early renal involvement and reducing the mortality and morbidity.
Glomerular filtration rate (GFR) is considered as the best overall index of renal function in health and disease. The most widely used and accepted endogenous filtration markers for clinical GFR estimation are the measurement of serum creatinine (SCr), alone, or inconjunction with a 24-hour urine collection for the determination of creatinine clearance (CrCl). Alterations in renal handling and metabolism of creatinine and methodological interferences in its measurement may influence the concentration of SCr.Citation[3] CrCl determinations involving timed urine collections may provide greater accuracy but they are difficult for patients to perform and there may be uncertainities in urine collections. Precise GFR can be measured using isotope clearance techniques but all of these techniques are relatively time-consuming, expensive, and involve radiation exposure.
Cystatin C (Cys C) is a basic, nonglycosylated, LMW protein (Mr = 13,359), consisting of 120 amino acids that is proposed to be a better marker of GFR than SCr since it is produced by all investigated nucleated cells at a constant rate, freely filtered in the glomeruli, and almost completely reabsorbed and catabolized by the proximal tubular cells.Citation[4-7] The serum Cys C concentration correlates inversely with GFR, and assays for Cys C are commercially available.Citation[8-10] Multiple studies have validated the use of Cys C as a renal marker in different patient groups such as those with diabetes, renal transplantation, liver cirrhosis, and rheumatoid arthritis.Citation[11-16]
In this study, we evaluated the diagnostic efficiency of Cys C in detecting early renal impairment in patients with primary hypertension (PH) as compared with SCr, CrCl, blood urea nitrogen (BUN), uric acid (UA), beta 2 microglobulin (BMG) and GFR estimated by the formula of Cockcroft and Gault (CG-GFR) using the MDRD (modification of diet in renal disease) formulaCitation[17] as a reference method for GFR.
METHODS
Study Groups
Fifty-one patients (24 male and 27 female, aged 35–56, mean 48.47 ± 0.77) with a diagnosis of PH and 29 healthy subjects (11 male and 18 female, aged 36–57, mean 45.79 ± 1.31) who were similar in terms of age and gender with the patient group participated in this study. A detailed medical history was obtained from all subjects. The patients with a known hypertension duration of more than 6 months (range: 6 months–25 years, median: 6 years) were included in the study. All patients were nondiabetic without any other chronic diseases except hypertension and they continued their antihypertensive treatment during the study. In all the patients, hypertension was under control (a mean of three measurements of blood pressure < 140/90 mmHg) and none of them exhibited renal hyperfiltration. The control subjects were considered to be healthy on the basis of a general medical examination and biochemical test results; no continuous drug using was in question for them. Clinical characteristics of the study subjects are shown in .
Table 1. Clinical Characteristics of the Study Groups.
Methods
Blood samples were drawn from the antecubital vein from fasting subjects early in the morning. Serum was isolated without delay by centrifugation at 4000 rpm for 5 minutes and serum samples were analyzed on the same day for SCr, BUN, UA, albumin, and BMG measurements. Some part of the serum samples was stored at − 80°C for Cys C measurement and analyzed within one month. For urinary protein and albumin measurements and CrCl calculation; a 24-h urine sample was obtained for each subject on the same day as blood sampling. Serum concentrations of creatinine, BUN, UA, albumin, Cys C, BMG and urinary concentrations of creatinine, albumin, and protein were measured with the following commercially available methods: serum creatinine, albumin, BUN, UA; urinary protein, albumin, and creatinine with an autoanalyzer (Roche/Hitachi Modular) using kits of Roche Diagnostics; Serum Cys C and BMG by particle-enhanced immunonephelometry (N-Latex Cystatin C, Code No OQNM and N-Latex ß2 Microglobulin, Code No OQWU; Dade Behring) on Dade Behring Nephelometer Systems BN II. To calculate CrCl (mL/min), we used the classical formula: CrCl = U × V/P × T where U is urinary creatinine concentration (mg/dL), V is the urinary volume (mL), P is the plasma creatinine concentration (mg/dL), and T is time period for urine collection (minutes). To estimate GFR, we used the MDRD formula as a reference method: MDRD-GFR (mL/min) = 170 × [SCr in mg/dL]− 0.999× [age]− 0.176× [0.762; if female] × [1.180; if black] × [BUN in mg/dL]− 0.170× [Alb in g/dL]+ 0.318.Citation[17] We also estimated GFR according to the Cockcroft-Gault formula: CG-GFR (mL/min) = (140 − Age) × 2,12 × weight (kg) × K/SCr where K = 0.85 in women and 1.00 in men.Citation[18] All clearances were expressed as mL/min/1.73 m2 after correction for body surface area (BSA) according to the Du Bois-Du Bois formula:Citation[19] BSA (m2) = 0,007184 × [height (cm)]0.725× [weight (kg)]0.425.
Statistical Analysis
Statistical analysis was performed using SPSS for Windows (Ver. 10,0,1) program. All data are expressed as mean values ± SE. Differences between the groups were tested with the Independent Samples T Test and the Mann-Whitney U Test. Correlation analysis was performed by calculation of Pearson’s correlation coefficient (nonparametric). A p value less than 0.05 was considered statistically significant. The sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively), and the diagnostic validities of Cys C, SCr, BUN, UA, BMG, CG-GFR, and CrCl to detect reduced GFR in comparison to MDRD-GFR were evaluated by receiver operating characteristic (ROC) analysis.Citation[20] Med-Calc for Windows (Ver. 7.0) was used for calculations and comparisons of the areas under curves.
RESULTS
Comparison of the Study Groups
Although serum BUN, SCr, and UA levels were between reference ranges both in the control and the patient group; mean levels of these parameters were found to be significantly higher in the patient group than they were in the control subjects (p values 0.004, 0.002, and 0.008; respectively). The difference between the study groups was more evident for mean serum Cys C and BMG concentrations (p = 0.000 for both). For all subjects in the control group; serum Cys C and BMG levels were in normal ranges. On the other hand; 15 patients had serum BMG levels higher than the upper reference limit whereas 21 patients had increased serum Cys C levels.
Mean levels of Upro and Ualb were significantly higher in patients than in the controls (p values 0.021 and 0.011; respectively). 11 patients had proteinuria (Upro > 150 mg/d) and 1 patient had albuminuria (Ualb > 30 mg/d).
All the subjects in the control group had GFR levels higher than 80 mL/min/1.73 m2, which was considered asthe lower cut-off limit of GFR. In the patient group, 6 subjects had CrCl levels lower than 80 mL/min/1.73 m2, whereas 3 had CG-GFR, and 12 had MDRD-GFR levels lower than the cut-off limit. Mean levels for CG-GFR and MDRD-GFR were significantly lower in the patient group than they were in the control subjects (p values 0.007 and 0.000; respectively), but there was no statistically significant difference between the mean levels of CrCl of the study groups (p = 0.813).
The comparison of the study groups for these parameters are presented in .
Table 2. The Comparison of the Study Groups.
Correlations of the Parameters
In order to evaluate the correlation of different markers with GFR, comparison with the MDRD-GFR was carried out. Reciprocals of the serum concentrations of BUN, SCr, UA, Cys C, and BMG were all found to increase with increasing GFR. The correlation coefficients (r) of all parameters for both of the study groups are shown in . The 1/SCr correlated best with MDRD-GFR (r: 0.760, p: 0.000) in the patient group and 1/Cys C (r: 0.622, p: 0.000) in the control group.
Table 3. Correlations of the Parameters with MDRD-GFR in Study Groups.
When the correlations of the GFR parameters were analyzed; it was found that there is a strong correlation between CG-GFR and MDRD-GFR in both patient and control groups (r: 0.650, p: 0.000 and r: 0.707, p: 0.000; respectively). The UA also had a strong correlation with MDRD-GFR in patient group. But CrCl was found to have no significant correlation with MDRD-GFR in patients (r: 0.040, p: 0.779) and a weak correlation in control subjects (r: 0.387, p: 0.038).
ROC Analysis
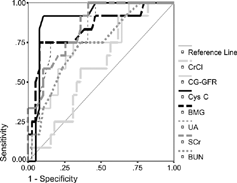
In order to determine the diagnostic accuracies of the investigated parameters for the reflection of the renal function and GFR, ROC plots were constructed. Nonparametric ROC curves were generated by plotting the sensitivity vs. 1-specificity; giving the ideal test a specificity equal to 1 and a specifity equal to 1 (corresponding to 1-specificity equal to zero). The GFR determined with MDRD formula was used as the reference method. The cut-off limit to define the borderline between normal and abnormal glomerular filtration was 80 mL/min/1.73 m2. On the basis of the MDRD-GFR values, patients with PH were divided into two groups: 12 patients with reduced GFR (≤ 80 mL/min/1.73 m2) and 39 with normal GFR (> 80 mL/min/1.73 m2). The area under the ROC curve (AUC) value for Cys C was found to be superior (AUC: 0.900) to the other markers.
The comparison of the AUCs indicated that the diagnostic accuracy of Cys C was significantly better than were those of CG-GFR, CrCl, and BUN (p: 0.043, p: 0.002 and p: 0.026; respectively). But the AUCs for the other parameters were not significantly different (p > 0.05) although there was a tendency toward the best area forCys C. summarizes the AUC and 95% Confidence Interval (CI) values, % sensitivities and specificities, and positive (PPV) and negative (NPV) predictive values of the all investigated parameters. A graphical comparison of the ROC plots is shown in .
Table 4. Diagnostic Accuracies of the Parameters.
DISCUSSION
In the present study; we compared the diagnostic accuracy of serum Cys C to that of the traditional markers of renal function such as SCr, BUN, UA, BMG, CrCl, and CG-GFR in patients with PH. Our aim was to evaluate the efficacy of Cys C as an early marker of renal involvement in this patient group. Since renal function decreases physiologically and in parallel to this, serum Cys C levels start to increase after the sixth decade; all of our subjects were between 30 and 60 years old. Any malignancies or medications that might affect Cys C levels were also not in question for any of the subjects. We used MDRD-GFR as a reference method for GFR estimation and we found Cys C levels increased as GFR decreased. We also found that the diagnostic accuracy of serum Cys C is considerably better than is that of SCr, BUN, UA, BMG, CrCl, and CG-GFR in discriminating between patients with PH having normal or slightly reduced GFR.
In the assessment of GFR, indirect markers such as SCr, BUN, UA, BMG, and some formulas like CrCl and CG-GFR are frequently used. These tests are rapidly and easily performed in the clinical laboratory. Unfortunately, it has been shown that they have several problems related to renal and nonrenal factors that need to be addressed to ensure the correct interpretation of the results. In our study, we also observed that these parameters are not sensitive enough to discriminate the subjects with and without renal involvement. Although there was a statistically significant difference between the two study groups for serum, urine, and GFR parameters; most of the patients were within reference values for the renal serum markers, except Cys C and BMG. This finding is in agreement with the previous studies indicating that the parameters such as SCr, CrCl, BUN, and UA are not predictive of a slight decrease in GFR.Citation[21-25] In our patient group; 21 had elevated serum Cys C levels and 12 had MDRD-GFR < 80 mL/min/1.73 m2 but SCr, BUN, and UA levels were in normal ranges for all patients. Even though UA had a strong correlation with MDRD-GFR in the patient group, it was not proved to be predictive of a slight decrement in GFR. The predictive value of albuminuria for renal morbidity and mortality is well established in diabetes. Although a comparable link between albuminuria and future renal impairment is highly likely, screening for microalbuminuria in HT is not yet a routine in clinical practice. Recent studies recommended that CrCl should no longer be measured because of the difficulty of collecting accurately timed urine samples. From this point of view, we estimated GFR by both CG and MDRD formulas. However, we used MDRD-GFR as a reference method because CG formula estimates GFR as a function of SCr, age, body weight, and gender. On the other hand, the MDRD formula includes both serum variables such as SCr, BUN, and albumin and demographic variables like age, gender, and ethnicity. The MDRD prediction equation could be easily implemented in clinical practice and it seems to be more accurate than do the other equations; it does not require collection of timed urine sample or measurement of weight and height. Therefore, we accepted MDRD-GFR values as the reference method and evaluated the correlations of all our variables with this parameter.
Dharnidharka et al.Citation[4] performed a meta-analysis of the recent articles about Cys C and found that Cys C has a greater correlation coefficient with GFR than does SCr. In accordance with this finding, we observed Cys C to be the serum parameter that had the best correlation with MDRD-GFR in the control group and SCr had the best correlation with MDRD-GFR in patient group (). There was a strong correlation between CG-GFR and MDRD-GFR in both of the study groups, but CrCl was found to have no significant correlation with MDRD-GFR in patients and a weak correlation in control subjects.
In some of the studies, the correlation between GFR and Cys C was found to be greater as the degree of renal impairment increased.Citation[4], Citation[21], Citation[26] In our study, 12 out of 51 patients had MDRD-GFR < 80 mL/min/1.73 m2 (23.5%) and the lowest GFR value was 59.36 mL/min/1.73 m2. Thus, 76.5% of the patients were considered to have no renal involvement and the rest had mild renal impairment. That might be the reason why we found weaker correlations for Cys C compared to SCr.
However, correlation coefficients may reflect only a linear association and not always translate into agreement or diagnostic accuracy. The best method for assessment of diagnostic accuracy of a test is ROC analysis. According to the AUC values, Cys C was found to have the best diagnostic accuracy between the investigated parameters with the greatest value of AUC (0,900). The difference between AUCs of Cys C and other parameters was statistically significant for only BUN, CG-GFR, and CrCl; although, there was a tendency toward the greatest area for Cys C.
Our results are in agreement with the previous studies suggesting Cys C to be superior to other markers as a predictor of GFR in primary hypertension.Citation[27&28] However,we have some limitations in this study. We probably could have found the differences between the parameters statistically more significant if we had had a larger patient population with a wider distribution range for the degree of renal dysfunction or if we had used a gold-standard method like inulin clearance for the determination of GFR.
We suggest serum Cys C measurement to be a better parameter in discriminating patients with GFR < 80 mL/min/1.73 m2 compared to the other traditional markers in primary hypertensive patients. One of the main purposes of GFR estimation in clinical practice is to detect and screen for patients with mild renal dysfunction, especially in clinical situations like diabetes or hypertension. In this regard, it is most important to detect early renal dysfunction, that is, patients with GFR below 80 mL/min/1.73 m2. In this report, we have indicated that Cys C might replace SCr or CG formula as a marker of early renal impairment in primary hypertensive patients. Measurement of Cys C may possibly be beneficial for the early detection and treatment of renal involvement in this group of patients. In conclusion, the role of Cys C as an early marker of renal damage in primary hypertensive patients merits further longitudinal studies.
REFERENCES
- Siewert-Delle, A.; Ljungman, S.; Andersson, O K.; Wilhelmsen, L. Does treated primary hypertension lead to end-stage renal disease? A 20-year follow-up of the primary prevention study in Göteborg, Sweden. Nephrol. Dial. Transplant. 1998, 13, 3084–3090. [PUBMED], [INFOTRIEVE], [CSA]
- Luft, F C. Hypertensive nephrosclerosis-a cause of end-stage renal disease? Nephrol. Dial. Transplant. 2000; 15: 1515–1517. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Perrone, R D.; Madias, N E.; Levey, A S. Serum creatinine as an index of renal function: new insights into old concepts. Clin. Chem. 1992, 38, 1933–1953. [PUBMED], [INFOTRIEVE]
- Dharnidharka, V R.; Kwon, C.; Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am. J. Kidney Dis. 2002, 40 (2), 221–226. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Newman, D J.; Cystatin, C. Ann. Clin. Biochem. 2002, 39, 89–104. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Price, C P.; Finney, H. Developments in the assessment of glomerular filtration rate. Clin. Chim. Acta 2000, 297, 55–66. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Laterza, O F.; Price, C P.; Scott, M G. Cystatin C: An improved estimator of glomerular filtration rate. Clin. Chem. 2002, 48 (5), 699–707. [PUBMED], [INFOTRIEVE], [CSA]
- Kyhse-Andersen, J.; Schmidt, C.; Nordin, G.; Andersson, B.; Nilsson-Ehle, P.; Lindstrom, V.; Grubb, A.;. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin. Chem. 1994, 40, 1921–1926. [PUBMED], [INFOTRIEVE], [CSA]
- Finney, H.; Newman, D J.; Gruber, W.; Merle, P.; Price, C P. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II). Clin. Chem. 1997, 43, 1016–1022. [PUBMED], [INFOTRIEVE], [CSA]
- Pergande, M.; Jung, K. Sandwich enzyme immunoassay of cystatin C in serum with commercially available antibodies. Clin. Chem. 1993, 39, 1885–1890. [PUBMED], [INFOTRIEVE]
- Harmoinen, A P.; Kouri, T T.; Wirta, O R.; Lehtimaki, T J.; Rantalaiho, V.; Turjanmaa, V M.; Pasternack, A I.. Evaluation of plasma cystatin C as a marker for glomerular filtration rate in patients with type 2 diabetes. Clin. Nephrol. 1999, 52 (6), 363–370. [PUBMED], [INFOTRIEVE], [CSA]
- Risch, L.; Blumberg, A.; Huber, A. Rapid and accurate assessment of glomerular filtration rate in patients with renal transplants using serum cystatin C. Nephrol. Dial. Transplant. 1999, 14, 1991–1996. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Le Bricon, T.; Thervet, E.; Froissart, M.; Benlakehal, M.; Bousquet, B.; Legendre, C.; Erlich, D. Plasma cystatin C is superior to 24-h creatinine clearance and plasma creatinine for estimation of glomerular filtration rate 3 months after kidney transplantation. Clin. Chem. 2000, 46, 1206–1207. [PUBMED], [INFOTRIEVE], [CSA]
- Orlando, R.; Mussap, M.; Plebani, M.; Piccoli, P.; De Martin, S.; Floreani, M.; Padrini, R.; Palatini, P. Diagnostic value of plasma cystatin C as a glomerular filtration marker in decompensated liver cirrhosis. Clin. Chem. 2002, 48, 850–858. [PUBMED], [INFOTRIEVE], [CSA]
- Mangge, H.; Liebmann, P.; Tanil, H.; Herrmann, J.; Wagner, C.; Gallistl, S.; Schauenstein, K.; Erwa, W. Cystatin, an early indicator for incipient renal disease in rheumatoid arthritis. Clin. Chim. Acta 2000, 300, 195–202. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Kazama, J J.; Kutsuwada, K.; Ataka, K.; Maruyama, H.; Gejyo, F. Serum cystatin C reliably detects renal dysfunction in patients with various renal diseases. Nephron 2002, 91, 13–20. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Levey, A S.; Bosch, J P.; Lewis, J B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Annu. Intern. Med. 1999, 130, 461–470. [CSA]
- Cockcroft, D W.; Gault, M H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41., [PUBMED], [INFOTRIEVE]
- Du Bois, D.; Du Bois, E F. A formula to estimate the approximate surface area if height and weight are known. Arch. Intern. Med. 1916, 17, 863–871.
- Zweig, M H.; Campbell, G. Receiver-operating characteristics (roc) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 1999, 39, 561–577.
- Mussap, M.; Vestra, M D.; Fioretto, P.; Saller, A.; Varagnolo, M.; Nosadini, R.; Plebani, M. Cystatin C is a more sensitive marker than creatinine for the estimation of GFR in type 2 diabetic patients. Kidney Int. 2002, 61 (4), 1453–1461. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Randers, E.; Erlandsen, E J.; Pedersen, O L.; Hasling, C.; Danielsen, H. Serum cystatin c as an endogenous parameter of the renal function in patients with normal to moderately impaired kidney function. Clin. Nephrol. 2000, 54(3), 203–209. [PUBMED], [INFOTRIEVE], [CSA]
- Coll, E.; Botey, A.; Alvarez, L.; Poch, E.; Quinto, L.; Saurina, A.; Vera, M.; Piera, C.; Darnell, A. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am. J. Kidney Dis. 2000; Jul, 36 (1), 29–34.
- Hoek, F J.; Kemperman, F A.W.; Krediet, R T. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol. Dial. Transplant. 2003, 18, 2024–2031. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Perlemoine, C.; Beauvieux, M C.; Rigalleau, V.; Baillet, L.;Barthes, N.; Derache, P.;Gin, H. Interest of cystatin C in screening diabetic patients for early impairment of renal function. Metabolism 2003, 52 (10), 1258–1264.
- Mussap, M.; Ruzzante, N.; Varagnolo, M.; Plebani, M. Quantitative automated particle-enhanced immunonephelometric assay for the routine measurement of human cystatin C. Clin. Chem. Lab. Med. 1998, 36 (11), 859–865. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Watanabe, S.; Okura, T.; Liu, J.; Miyoshi, K.; Fukuoka, T.; Hiwada, K.; Higaki, J. Serum cystatin C level is a marker of end-organ damage in patients with essential hypertension. Hypertens. Res. 2003; Nov, 26 (11), 895–899. [CROSSREF]
- Seco, M L.; Rus, A.; Sierra, M.; Caballero, M.; Borque, L. Determination of serum cystatin c in patients with essential hypertension. Nephron 1999, 81 (4), 446–447. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]