Abstract
Zeolite faujasite (FAU), Linde type A (LTA) and FAU/LTA composite have been synthesized using tetramethylammonium cation (TMA+) as template, by adjusting only the concentration of Na+ ions in the initial solution (1.00 Al2O3 4.36 SiO2 : 2.39 (TMA)2 O : β Na2 O : 249.00H2 O). Na+ ions alter the phase composition of the product more than TMA+ or OH− ions. When Na2 O concentration [Na2 O] increases from 0.024 to 0.168, the product gradually changes from pure FAU to pure LTA via the formation of FAU/LTA composite with increasing LTA fraction. Interestingly, the induction periods of FAU and LTA in the FAU/LTA composite zeolite ([Na2 O] is 0.072) are both 13 h, quite different from the induction periods of their individual pure phases—45 h for FAU and 4 h for LTA. During the crystallization, the LTA/(FAU + LTA) fraction in the composite zeolite decreases in a nearly linear fashion. Scanning electron microscopy, thermogravimetry and differential thermal analysis indicate some difference between the properties of the FAU/LTA composite zeolite and of the mechanical mixture.
Introduction
Zeolites have been widely used as catalysts, adsorbents and ion-exchangers [Citation1] in virtue of their complicated and unique porous structures and special acid–basic properties. Since the discovery of natural zeolites in 1756, many studies have been made on the formation, application as well as adsorption mechanisms [Citation2] of these microporous materials, although the mechanisms of zeolite synthesis have not been fully elucidated yet due to the complexity of their formation.
Zeolite faujasite (FAU) and Linde type A (LTA) are two of the most important zeolites because of their extensive commercial applications. From the perspective of framework structure, both FAU and LTA zeolites are stacked up by sodalite cages. For FAU zeolite, sodalite cages are linked together by double six-membered rings and form large cavities called supercages. Whereas for LTA zeolite, sodalite cages are linked together by double four-membered rings and form α cage. These two kinds of zeolites can be synthesized from Na2 O–Al2 O3–SiO2–H2 O systems using tetramethylammonium cation (TMA+) as structure-directing agent [Citation3–5]. Previous study has demonstrated that TMA+, almost the same size with sodalite cage, can induce the formation of some porous structures or secondary building units of the zeolite [Citation6, Citation7], and only one TMA+ cation could be located in one sodalite cage [Citation4, Citation8]. Some inorganic ions, such as small alkali metal ions (Na+ and Li+), are hydrated in the synthesis solution. They can hold water molecules as a network [Citation9] and also have a structure-directing effect. It has been known that the crystallization of FAU and LTA is sensitive to synthesis parameters, such as Na+ concentration, Si/Al ratio, and aging time. Usually, small variation of these parameters could change the composition of the products [Citation10].
Recently, composite zeolites have become important. Some composite zeolite, such as β /MCM-41 [Citation11], ZSM-5/Y [Citation12, Citation13] and MAZ/ZSM-5 [Citation14] have been reported exhibiting obvious synergic effect in catalysis. FAU/LTA was observed by Mintova and Valtchev [Citation10] in their early synthesis of FAU nanozeolite. Utilizing this composite zeolite, Okubo and co-workers [Citation4] roughly studied some synthesis parameters affecting the FAU/LTA yield, and particularly investigated the local environments of TMA + in as-synthesized FAU and LTA zeolites. Navrotsky and co-workers [Citation15] revealed the interplay of kinetic and thermodynamic factors, governing the competition in formation of FAU and LTA, via in situ calorimetry.
Obviously, Na + ions play an important role in the formation of FAU and LTA zeolites, however, there is a lack of research on this aspect. In the present contribution, we study the effect of inorganic and organic ions (Na +, OH − and TMA +) in the synthesis system of Na2 O–Al2 O3–SiO2–(TMA)2 O–H2 O on the formation of FAU, LTA and FAU/LTA zeolites under the same reaction conditions, Si/Al ratio, aging time and heating temperature. Based on these achievements, the crystallization of the FAU/LTA composite zeolite can be controlled to obtain either FAU or LTA or FAU/LTA composite with various LTA/(LTA + FAU) fractions by adjusting the Na + concentration in the initial solution. The products were characterized by scanning electron microscopy (SEM), x-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA) and differential thermal analysis (DTA).
Experimental details
Synthesis method for the FAU/LTA composite zeolite
Appropriate amounts of deionized water, 25 wt.% aqueous tetramethylammonium hydroxide solution (25%, Tianjin Kermel Chemical Reagent Co., Ltd.), sodium hydroxide (96.0 wt.% NaOH, Tianjin Kermel Chemical Reagent Co., Ltd.), and aluminum isopropoxide (24.7 wt.% Al2O3, Sinopharm Chemical Reagent Co., Ltd) were mixed under vigorous stirring until the solution became clear. Then fumed silica (99.8 wt.% SiO2, Shenyang Chemical Co. Ltd.) was added to this solution. The final molar composition of any given synthesis solution was 1.00 Al2 O3 : 4.36 SiO2 : α (TMA)2 O : β Na2 O : 249.00H2 O. After stirring for 30 min, the resulting mixture was transferred into a Teflon-lined 50 ml stainless steel autoclave, sealed and heated in a convection oven at 115 °C for some time after aging for 3 days at room temperature with vigorous stirring. All samples were recovered and washed with deionized water in a centrifuge at 5000 rpm for 30 min, until the pH value of the lotion became less than 9, and dried in an oven at 90 °C for 24 h.
Characterization
Powder XRD (PANalytical X’pert PRO diffractometer using CuKα radiation, 40 kV, 40 mA) was used to determine sample phase and crystallinity. LTA/(LTA + FAU) weight percents of the final product were determined by working curves made from XRD patterns of pure FAU and LTA mixtures via external standard method, for which the (622) peak of LTA and (620) peak of FAU were selected [Citation16]. SEM (FEI Quanta 200FEG) was used to observe the crystal morphology and size. FTIR spectra were recorded with Bruker Equinox 55 spectrometer at 4 cm- 1 resolution. The samples were prepared by mixing a small amount of zeolite (2 wt.%) with dried KBr and pressing into 13 mm diameter circular wafers (21 mg). TGA (PerkinElmer Pyris 1 TGA) and DTA (PerkinElmer DTA 7) were used to observe the decomposition of TMA + within the zeolite. The sample was heated at a rate of 10 °C min- 1 under air flow.
Results and discussion
Effect of Na+ concentration on phase composition of the products
In order to investigate the effect of Na + concentration on phase composition of the FAU/LTA zeolite, different amounts of Na + ions were introduced into the synthesis system of Na2 O–Al2 O3–SiO2–(TMA)2 O–H2 O. As we expected, not any zeolite product can be formed in the system of 1.00 Al2 O3 4.36 SiO2 : 2.39 (TMA)2 O : 249.00 H2 O without Na+, even though the reaction mixture is heated for 200 h at 115 °C. This demonstrates the important role of Na+ in promoting the formation of zeolite crystals. The effect of Na + on the composition of the products has been investigated for crystallization at temperature of 115 °C for 90 h using three groups of initial compositions listed table ; NaOH or NaBr provided Na + ions. Because the variation of Na + concentration is rather small, fumed silica without sodium is used as the silica source to control Na + concentration accurately. For these three groups of experiments, the Na + concentration in the initial solution is increased gradually, while the concentration of TMA + or OH− changes in a different way. For the group 1, TMA+ concentration is decreased appreciably to keep OH− concentration constant. For the group 2, the OH− concentration increases with Na + concentration to keep TMA+ concentration unchanged. For the group 3, both OH− and TMA + concentrations are kept constant by utilizing NaBr as the source of Na +.
Table 1 Synthesis batches with different Na +, OH− and TMA + contents used in the experiment.
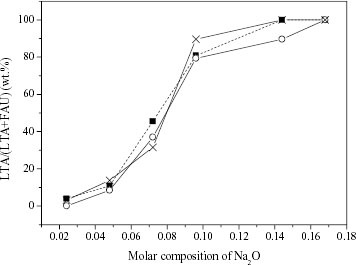
Figure shows the variety of phase compositions of the products as a function of Na+ concentration. Firstly, the effects of Na+ concentration on the phase composition of the products with these three groups of initial solutions are quite similar. When Na2 O concentration [Na2 O] in the synthesis solution increases from 0.024 to 0.168, more and more LTA in the FAU/LTA composite is formed. Accordingly the main component of the products changes from FAU to LTA gradually for all the three groups. Secondly, from the comparison of the results for group 2 and group 3, the LTA/(LTA+ FAU) percents increase with Na+ concentration, no matter whether Na+ is introduced by NaOH or NaBr. Thus the variety of OH− concentrations in the solution takes little effect on the formation of LTA, FAU or LTA/FAU zeolites. Also, results for group 1 and group 3 show that small variations of TMA+ concentration have little influence on the formation of LTA, FAU or FAU/LTA zeolites. These results demonstrate the important role of Na+ in the synthesis of FAU and LTA zeolites. It can be controlled to produce pure FAU or LTA zeolite or FAU/LTA composite with various LTA/(LTA + FAU) ratios by adjusting the Na+ concentration in the initial solution.
Figure 1 LTA/(LTA + FAU) percent of the solid products synthesized under the molar composition of initial solution from group 1 (▪), group 2 (∘) and group 3 (×).
During the formation of zeolite, both TMA+ and Na+ can draw the silicate and aluminate species together, but TMA+ and Na+ take different roles in the formation of FAU and LTA zeolites. Only Na+ allow them to react to form larger amorphous gel particles, while the large volume of the TMA+ prevent the species from getting sufficiently close to form new chemical bonds [Citation17]. Based on the claim of Melchior et al [Citation18], the immediate precursor to the FAU lattice is the hexagonal prism tertiary building unit, and the secondary building unit is the four-membered ring and six-membered ring. For LTA lattice, double four-membered ring is tertiary building unit, and four-membered ring and six-membered ring also are secondary building units. Our results suggest that Na+ not only has directing effect besides TMA+, but the directing effect varies with Na+ concentration, the synergistic effect of TMA + and Na+ induces the formation and phase selection for the FAU and LTA. When Na+ concentration is relatively low, double six-membered rings—the tertiary building unit for FAU phase—are formed during the aging and induction, by which the sodalite cages are bounded together to form FAU lattice in the subsequent crystallization. With the increase of Na+ concentration, double four-membered rings are formed beside double six-membered rings [Citation19–21]; as a result, FAU/LTA composite is synthesized. However, when the Na+ concentration is large enough, only double four-membered rings are formed, and thus pure LTA is produced.
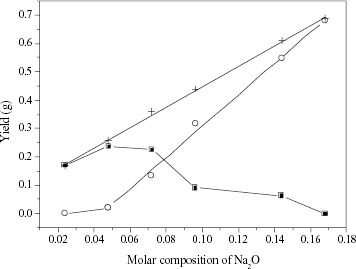
Figure shows the mass yields of the products obtained from initial solution of group 2 (from 40 g initial solution). The total solid yield of FAU/LTA composite increases linearly with the Na+ concentration. However, the mass of the LTA phase increases slowly when [Na2 O] is less than 0.048, and increases much faster when [Na2 O] is more than 0.048. Differently, the mass of the FAU phase tends to increase when [Na2 O] is less than 0.048, and then decreases slowly, or even disappears completely, at [Na2 O] of 0.168. These results further proved the important role of Na+ in the zeolite formation. The TMA+ ions are possibly responsible for the formation of sodalite cage, while Na+ is the key factor that determines by what manner (double four-membered ring or double six-membered ring) and how many sodalite cages are connected together.
Crystallization of FAU, LTA and FAU/LTA composite zeolite
To explore the effect of Na+ concentration on the formation process of the zeolite, the crystallization of FAU, LTA and FAU/LTA zeolites has been studied under the initial feed compositions of runs 7, 9 and 12 (see table ), respectively. The crystallization curves are presented in figure . For run 7, the pure crystalline phase of FAU emerges at 45 h, and its crystallinity rapidly increases to 100% at 76 h (curve A in figure ). For run 12, the pure crystalline phase of LTA begins to form as early as at 4 h, and its crystallinity increases to 100% at 21 h (curve B in figure ). For run 9, the product is FAU/LTA composite zeolite, in which both FAU (curve D) and LTA (curve E) phases appear simultaneously at about 13 h. The crystallinity of LTA increases to the maximum of 37% at 31 h, and it remains almost unchanged when the crystallization time is further prolonged from 31 h to 53 h. The crystallinity of FAU increases rapidly to 43% at 37 h, then increases slowly to ∼46 % at 53 h. In other words, the crystallinity of LTA reaches its equilibrium value earlier than FAU in the crystallization of FAU/LTA composite zeolites that influences the composition of the product. The total crystallinity of the FAU/LTA composite zeolites (curve C) is only 80% at 53 h, which is attributed to their smaller crystal size compared with pure FAU or pure LTA (as confirmed by SEM photos below).
Figure 3 Crystallizing curves of pure FAU (A), pure LTA (B), FAU/LTA composite (C), FAU in the FAU/LTA composite (D) and LTA in the FAU/LTA composite (E).
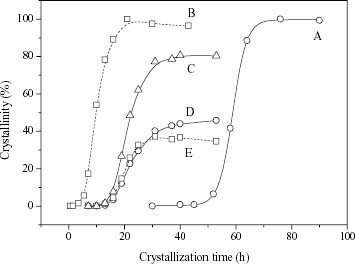
From the crystallization curves of FAU and LTA as well as FAU/LTA composite zeolites (figure ), it can be found that the induction period of FAU zeolite is much longer than that of LTA zeolite at the same crystallization temperature under the feasible conditions for the formation of their individual pure phases. This is possibly derived from the great host–guest stabilization effect of FAU that has a larger pore volume than LTA [Citation17, Citation22, Citation23]. However, the induction periods of FAU and LTA are almost the same in the FAU/LTA composite zeolites (figure ), which indicates that there are interactions between FAU and LTA phases in the formation of FAU/LTA composite zeolite. In the synthesis solution of FAU/LTA composite zeolite, the Na+ concentration exceeds the value which is suitable to obtain pure FAU, and additional Na+ would cause the formation of some double four-membered rings. However, this amount of double four-membered ring is much smaller than needed for growing pure LTA, so the crystallization of LTA is delayed. On the other hand, the formation of this small amount of double four-membered ring possibly creates an appropriate microenvironment to promote the formation of double six-membered ring, and the crystallization of FAU phase is thus accelerated. As a result, the induction periods of FAU and LTA in the FAU/LTA composite zeolite are the same (13 h).
The products, synthesized in run 9 for various durations, were studied by FTIR transmittance as shown in figure . The internal tetrahedral bending peak (∼470 cm- 1) exists for all the samples, and it shifts from 474 to 469 cm- 1 with the formation of crystalloid due to the incorporation of alumina tetrahedron into the zeolite framework [Citation24]. Before the crystal phase can be detected by XRD, the vibration peak of six-membered ring (418 cm- 1) appears firstly, and then the emergence of crystal phase is characterized by the double ring external linkage peak (573 cm- 1), internal linkage symmetrical stretching peak (690 cm- 1), external tetrahedral symmetrical stretching peak (780 cm- 1) and internal tetrahedral asymmetrical stretching peak (1033 cm- 1) [Citation25]. The peaks change suddenly from 14 to 16 h. During these 2 h, the internal tetrahedral asymmetrical stretching peak (1105 cm- 1) and the internal tetrahedral symmetrical stretching peak (808 cm- 1), both induced by fumed silica, weaken or disappear. However, the peaks at 573 and 1033 cm- 1 of zeolite crystal grow rapidly. It means that the formation of the zeolite crystal happens very quickly in the synthesis solution.
Figure 4 FTIR spectra of the products synthesized for different crystallizing time with initial feed composition of run 9.

Among the characteristic peaks described above, changes of the samples described by FTIR transmittance correspond well to the crystallization curve of FAU/LTA composite shown in figure . The six-membered rings, as secondary building unit of the zeolite, are formed during the induction period, while the double ring structure, tertiary building unit of the zeolite, is formed afterwards. Along with the formation of double ring structure, the zeolite crystalline phase is thus formed as detected by XRD, so the double ring structure may be the key to directly induce the formation of the zeolite crystalline phase.
Figure shows the solid weight of the products and the LTA/(FAU + LTA) percent of the FAU/LTA composite zeolite synthesized in run 9, for various durations, from 24.5 g of initial solution. Extending of the crystallization time on stream decreases the weight of the solid obtained from the synthesis solution from 0.81 g to nearly zero during the first 10 h. The solution color changes from white to transparent correspondingly. It means that the silica and alumina source dissolve gradually to form a transparent solution during the induction period, and XRD shows that these solid substances are amorphous. From 10 to 25 h, the solid weight increases from 0 to 0.17 g quickly, and the synthesis solution becomes white again. During this period, the amorphous phase decreases gradually, accompanying the appearance of the FAU/LTA composite zeolite with the increase of the crystallization time, and it does not disappear until 25 h. When the crystallization time is prolonged to 90 h, 0.21 g of the product is synthesized. From the above results, we can conclude that the formation of the FAU/LTA composite zeolite follows the liquid phase crystallizing mechanism [Citation26].
Figure 5 Solid yield (▪) and LTA/(FAU + LTA) weight percent (▴) of the products versus crystallization time under initial feed composition of run 9. The mass of initial solution is 24.5 g.
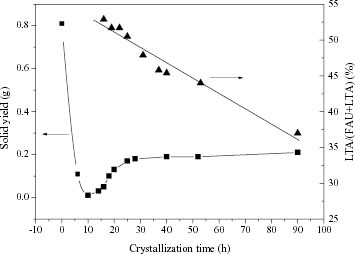
As for the phase composition of the product during the crystallization, figure shows that the LTA/(FAU + LTA) fraction declines linearly from 53% at 16 h to 37% at 90 h. In the later period (40–90 h) of the crystallization, the LTA/(FAU + LTA) fraction drops 8%, although the solid weight increases only by 0.02 g. This may be due to the different crystallization rates of FAU and LTA. As mentioned in the discussion of crystallization of FAU/LTA, much more FAU is formed than LTA during that period.
Characterization of the FAU, LTA and FAU/LTA composite zeolite
Figure shows the SEM images of the pure FAU, FAU/LTA composite and pure LTA, which are synthesized at 115 °C under the initial composition of runs 7, 9 and 12, respectively. All the samples have well-defined shape without intergranular amorphous substance. The pure FAU synthesized for 76 h (figure (a-1)) has typical octahedral shape with particle size of about 100 nm, and its morphology is unchanged as the crystallization extended to 90 h (figure (a-2)). However, the particle size of pure LTA with typical cubic shape grows from ∼300 nm at 21 h (figure (c-1)) to ∼600 nm at 90 h (figure (c-2)). With regards to the particle sizes, the FAU and LTA in the composite zeolite (figure (b-1)) are approximately twice smaller than each pure phase, and their morphology changes little when the crystallization extends to 90 h (figure (b-2)). Some interaction, restricting the zeolitic crystals from augmenting, may occur between FAU and LTA phase during the formation of FAU/LTA composite.
Figure 6 SEM micrographs of pure FAU synthesized in run 7 for76 h (a-1), 90 h (a-2), FAU/LTA composite from run 9 for 40 h (b-1), 90 h (b-2), and pure LTA from run 12 for 21 h (c-1), 90 h (c-2).

To investigate the amount of TMA+ ions enclosed in the zeolite, the results of TGA and DTA analyses for pure FAU, pure LTA, the FAU/LTA composite with 37% of LTA and the FAU-LTA mechanical mixture with 37% LTA are shown in figure . The exothermic peaks appearing at 341 and 480 °C for pure FAU (figure (a)) can be attributed to combustion of TMA + located in supercage and sodalite cage, respectively. Also the exothermic peaks located at 360 and 449 °C for LTA (figure (b)) belong to the oxidative decomposition of TMA + in α cage and sodalite cage [Citation4], respectively. For comparison, the DTA and TGA curves of the FAU/LTA composite zeolite and the mechanical mixture are presented together in figures (c) and (d), respectively. As expected, two exothermic peaks appear for both the FAU/LTA composite zeolite and the mechanical mixture in the DTA curves. The first peak at 354 °C for the composite is caused by combustion of TMA + located in supercage (FAU) and α cage (LTA), another peak at 465 °C results from the combustion of TMA+ located in sodalite cages of FAU and LTA. Whereas for the DTA curve of the mechanical mixture, two exothermic peaks were located at 358 and 480 °C, respectively, with the same attribution as in the FAU/LTA composite. The decomposition peaks of TMA + inside the FAU/LTA composite shift to lower temperatures compared with those of the mechanical mixture. Similar phenomena have been observed for MCM-41/β composite zeolite [Citation10]. From TGA results for the samples mentioned above (table ), the total weight loss due to the combustion of TMA+ for both the FAU/LTA composite and FAU-LTA mechanical mixture are calculated, and showed almost same results. In addition, both the LTA/(FAU + LTA) percent of the composite and the mechanical mixture, calculated from the total TGA weight loss of pure FAU and pure LTA, agree with XRD results.
Figure 7 TGA and DTA curves of the solid products of pure FAU (a), pure LTA (b), DTA (c) and TGA (d) curves of the FAU/LTA composite and FAU-LTA mixture.
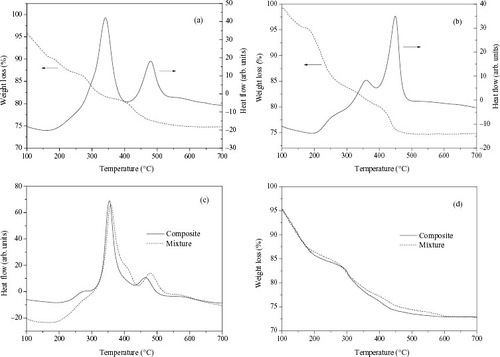
Table 2 Weight loss of FAU, LTA, FAU/LTA (37% LTA) composite zeolite and FAU-LTA mixture (37% LTA) from TGA measurements.
Conclusions
Pure FAU, LTA as well as FAU/LTA composite zeolite can be synthesized in the system of Al2 O3–SiO2–Na2 O–H2 O using TMA+ as structure-directing agent. The Na+ concentration in the initial solution is a crucial factor that determines the phase composition of the product. Based on the present achievements, pure FAU, pure LTA as well as FAU/LTA composite with various LTA/(LTA + FAU) weight percents can be selectively synthesized by adjusting the Na+ concentration in the initial solution. The crystallizing process of FAU/LTA composite zeolite is quite different from that of pure FAU or LTA.
Acknowledgment
This project was supported by the National Key Project of Fundamental Research (No. 2009CB623501).
References
- BarrerR M 1982 Hydrothermal Chemistry of Zeolites London Academic
- BonenfantDKharouneMNiquettePMimeaultMHauslerR 2008 Sci. Technol. Adv. Mater. 9 013007 http://dx.doi.org/10.1088/1468-6996/9/1/013007
- HolmbergB AWangH TNorbeckJ MYanY S 2003 Micropor. Mesopor. Mater. 59 13 http://dx.doi.org/10.1016/S1387-1811(03)00271-3
- FanWSatoshiSGaoF FOguraMOkuboT 2006 Micropor. Mesopor. Mater. 89 227 http://dx.doi.org/10.1016/j.micromeso.2005.11.001
- HolmbergB AWangH TYanY S 2004 Micropor. Mesopor. Mater. 74 189 http://dx.doi.org/10.1016/j.micromeso.2004.06.018
- de MoorP -P E ABeelenT P MKomanschekB UBeckL WWagnerPDavisM Evan SantenR A 1999 Chem. Eur. J. 5 2083 http://dx.doi.org/10.1002/(SICI)1521-3765(19990702)5:7<2083::AID-CHEM2083>3.0.CO;2-F
- BoyettR EStevensA PFordM GCoxP A 1996 Zeolites 17 508 http://dx.doi.org/10.1016/S0144-2449(96)00073-5
- HayashiSSuzukiKShinSHayamizuKYamamotoO 1985 Chem. Phys. Lett. 113 368 http://dx.doi.org/10.1016/0009-2614(85)80383-3
- GabelicaZBlomNDerouaneE G 1983 Appl. Catal. 5 227 http://dx.doi.org/10.1016/0166-9834(83)80135-3
- MintovaSValtchevV 1999 Studies in Surface Science and Catalysis vol 125 IKiricsi J BNagy H GKarge GPályi Amsterdam Elsevier p 141
- GuoW PHuangL MDengPXueZ YLiQ Z 2001 Micropor. Mesopor. Mater. 44–45 427 http://dx.doi.org/10.1016/S1387-1811(01)00217-7
- ChenH LShenB JPanH F 2003 Chem. Lett. 32 726 http://dx.doi.org/10.1246/cl.2003.726
- ShenB JChenH LGuoJ TPanH F 2005 Bull. Chem. Soc. Japan 78 2238 http://dx.doi.org/10.1246/bcsj.78.2238
- WangPShenB JGaoJ S 2007 Catal. Commun. 8 1161 http://dx.doi.org/10.1016/j.catcom.2006.10.021
- YangS YLiQ HWangM JNavrotskyA 2006 Micropor. Mesopor. Mater. 87 261 http://dx.doi.org/10.1016/j.micromeso.2005.08.016
- TreacyM M JHigginsJ B 2001 Collection of Simulated XRD Powder Patterns for Zeolites New York Elsevier
- CundyC SCoxP A 2003 Chem. Rev. 103 663 http://dx.doi.org/10.1021/cr020060i
- MelchiorM TVaughanD E WPictroskiC F 1995 J. Phys. Chem. 99 6128 http://dx.doi.org/10.1021/j100016a058
- OguraMKawazuYTakahashiHOkuboT 2003 Chem. Mater. 15 2661 http://dx.doi.org/10.1021/cm0218209
- DuttaP KShiehD CPuriM 1987 J. Phys. Chem. 91 2332 http://dx.doi.org/10.1021/j100293a025
- SugiyamaSYamamotoSMatsuokaONozoyeHYuJZhuGQiuSTerasakiO 1999 Micropor. Mesopor. Mater. 28 1 http://dx.doi.org/10.1016/S1387-1811(98)00271-6
- PetrovicINavrotskyA 1997 Micropor. Mater. 9 1 http://dx.doi.org/10.1016/S0927-6513(96)00060-0
- PiccioneP MLabertyCYangSCamblorM ANavrotskyADavisM E 2000 J. Phys. Chem. B 104 10001 http://dx.doi.org/10.1021/jp002148a
- XuR RPangW QTuK G 1987 Zeolite Molecular Sieves Structure and Synthesis Changchun Jilin University Press p 93
- FlanigenE MKhatamiHSzymanskiH A 1971 Molecular Sieve Zeolites. I (Advances in Chemistry Series vol 101) E MFlanigen L BSand Washington, DC American Chemical Society p 201
- ColinS CPaulA C 2003 Chem. Rev. 103 663 http://dx.doi.org/10.1021/cr020060i