Abstract
Periodic mesoporous organosilica (PMO) spherical particles with different organic contents were synthesized in one pot by reacting 1,2-bis(triethoxysilyl)ethane (BTSE) with tetraethylorthosilicate (TEOS) using a spray-drying technique. The scanning electron microscopy observation of spray-dried products clearly showed the formation of spherical particles. The 29Si magic angle spinning nuclear magnetic resonance data revealed that the organic contents due to ethane fragments embedded in the frameworks were controllable and consistent with the BTSE/TEOS molar ratios of precursor solutions. Transmission electron microscopy, small-angle x-ray scattering, and N2 adsorption data of PMO with controlled organic contents indicated that the ethane fragments were embedded in the frameworks with the formation of ordered mesostructures. PMO with a high organic content (BTSE/TEOS=0.50) only showed a hydrophobic property. According to the same procedure, benzene groups were also integrated to a similar degree in the frameworks by using 1,4-bis(triethoxysilyl)benzene.
Introduction
Since the first reports of mesoporous silica prepared using surfactants in the early 1990 s [Citation1–3], ordered mesoporous silica has attracted great attention because of its structural features, such as tunable mesopores, high surface area, large adsorption capacity and ordered pore arrangement [Citation4–7]. In general, mesoporous structures are constructed using supermolecular assemblies formed through the self-organization of amphiphilic molecules as structure-directing agents. It has been considered that ordered mesoporous materials are potentially applicable to biosensors, drug-delivery systems and adsorption systems, and in biomedicine on the basis of their structural characteristics [Citation4–7]. For further functionalization of ordered mesoporous silica, the organic modification of silicate surfaces is vital, and functional organic layers are grafted onto mesopore surfaces [Citation3, Citation8–17]. However, such organically functionalized mesoporous silica has unsatisfactory properties because organic moieties cannot be distributed homogeneously on silicate surfaces. In contrast, organic fragments are dispersed homogeneously in periodic mesoporous organosilica (PMO) [Citation17–22]. PMO with embedded organic groups is prepared through the hydrolysis and condensation of bridged silsesquioxanes, such as (R′O)3Si-R-Si(OR′)3 (R, R′; organic group). Problems in the reduction of mesostructural ordering have been addressed by further design of organic groups with large molecular sizes and/or with an increase in organic content [Citation21, Citation22].
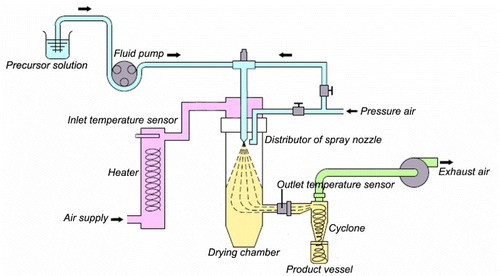
Several spherical particles of surfactant-templated mesoporous materials (e.g. silica [Citation23–27], organosilica [Citation28–30], maghemite-organosilica [Citation31], carbon [Citation32] and metal oxide [Citation33]) have been prepared by spray drying (i.e. aerosol-assisted process) [Citation34]. In the spray drying process, the liquid feed (i.e. clear precursor solution) is sprayed through a nozzle into a hot steam gas, in which the supplied liquid is vaporized and droplets are dried immediately. The system used previously is not suitable for the continuous production of solids, because dried samples are collected using membrane filters [Citation23–34]. Recently, we developed a laboratory-made apparatus suitable for rapid mass production and industrial uses [Citation35–39]. In our system, resultant solid products obtained by spray drying are collected with a cyclone separator owing to a strong centrifugal force (scheme ), which is used for many hours without any complex processing.
In this study, the use of our spray-drying system for large-scale production was extended to the preparation of microspheres of PMO with ethane and benzene fragments. We demonstrated the facile preparation of PMO with different organic contents in the frameworks by changing the BTSE/TEOS molar ratios of initial precursor solutions. To the best our knowledge, a systematical study focusing on the control of organic contents has not been reported thus far, though several spray-dried mesoporous materials with different compositions have been designed by adjusting the composition of precursor solutions [Citation23–34]. We demonstrated here that ethane and benzene fragments were successfully embedded in the frameworks (up to a BTSE/TEOS molar ratio of 0.50) with the formation of ordered mesoporous structures.
Experimental details
Materials
Bridged silsesquioxanes, such as 1,2-bis(triethoxysilyl)ethane ((C2H5O)3Si-C2H4-Si(OC2H5)3, BTSE) and 1,4-bis(triethoxysilyl)benzene (C2H5O)3Si-C6H4-Si(OC2H5)3, BTEB), were obtained from Aldrich. Tetraethylorthosilicate (TEOS, extrapure reagent) was purchased from Nacalai Tesque, Inc. All organosilane compounds were used as received. The triblock copolymer Pluronic F127 (PPO106-PPO70-PEO106) was obtained from Aldrich. Hydrochloric acid (pH 2) and ethanol (99.5%) were obtained from Nacalai Tesque, Inc. and Koso Chemical Co., Ltd, respectively.
Laboratory-made spray dryer
The spray dryer used in this study is laboratory-made equipment based on the Spray Dryer GB22 (Yamato Scientific Co.). The system diagram of the apparatus is illustrated in scheme . Droplets are generated from the two-flow spray nozzle (inner and outer diameters: 406 and 1270 μm, respectively). The nozzle tip pressure can be varied from 0.05 to 0.30 MPa. The inlet temperature of the drying chamber can be controlled from 50 to 280 °C. The temperature display is digital with an accuracy of ±1 °C. In the cyclone, the centrifugal force is created by a circular flow, which throws particles toward a wall. Spray-dried samples are collected with the cyclone collector. The drying chamber, the cyclone and the cyclone collector are all made of temperature-resistant, superhard glass.
Preparation of clear precursor solutions
Pluronic F127 (2.0 g) was dissolved completely in ethanol (2.5 g). The mixtures of BTSE and TEOS (see table ) in ethanol (2.5 g) were prepared separately, and then, an aqueous solution of HCl (3.1 ml, pH=2) was added to the mixtures dropwise. After vigorous stirring for about 30 min to obtain clear solutions, the mixtures were combined with the surfactant solution (resultant precursor solutions were also clear).
Table 1 Amounts of TEOS and BTSE in precursor solutions.
Spray drying of precursor solutions
The clear precursor solutions were spray-dried with our laboratory-made apparatus in order to obtain spherical PMO particles. The aspirator rate and the inlet temperature were set to 0.45 m3 h−1 and 250 °C, respectively. The nozzle pressure was kept constant at 0.20 MPa. The droplets of the precursor solutions were carried by an air flow, and the solvents were evaporated in the drying chamber. Subsequently, spherical particles were recovered by the cyclone collector. The particles were washed twice with an ethanolic HCl solution (2.8 g of conc. HCl in 150 ml of ethanol) to extract the surfactant.
Characterization
The morphology of samples was observed with a Hitachi S-4800 field emission scanning electron microscope (FE-SEM). Transmission electron microscopy (TEM) images of samples were acquired with a JEOL-3000F microscope. The accelerating voltage of the electron beam was 200 kV. For TEM observations, powder samples were sonicated in ethanol and mounted onto Cu microgrids. Small-angle x-ray scattering (SAXS) patterns were recorded with a NANO-Viewer (Rigaku). Nitrogen adsorption–desorption isotherms were measured with Quantachrome Autosorb-1 at 77 K. All samples were outgassed at 100 °C for 24 h before the measurements. Specific surface areas and pore size distributions were calculated using adsorption branches in the Brunauer–Emmett–Teller (BET) [Citation40] and Barrett–Joyner–Halenda (BJH) methods [Citation41], respectively. After purging with N2 for 1 h, thermogravimetry (TG) analysis was performed in an air flow with an SII EXTAR TG/DTA 6200 setup at a heating rate of 2 °C min−1. Solid-state 29Si magic angle spinning (MAS) nuclear magnetic resonance (NMR) measurements were performed with a Varian VNMR6.1C (300 MHz) spectrometer at a spinning rate of 5 kHz and a resonance frequency of 59.59 MHz with a 90° pulse length of 3.6 μs and a recycle time of 100 s. Solid-state 13C CP/MAS NMR measurements were performed on the same apparatus using a cross-polarization (CP) technique (spinning rate: 4 kHz; resonance frequency: 75.43 MHz; recycle time: 5 s). Samples were preheated at 110 °C for 6 h under vacuum, and then, the water adsorption–desorption isotherm was measured with a BELSORP 18 (Bel Japan Inc.) setup at 25 °C.
Results and discussion
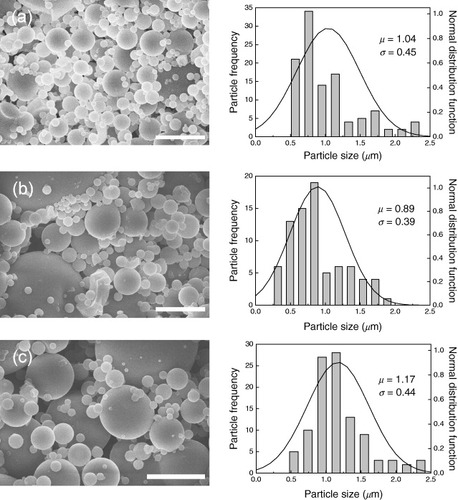
Using our laboratory-made spray dryer (scheme ), clear precursor solutions with different BTSE/TEOS ratios were spray-dried to obtain spherical particles of PMO with different organic contents. The FE-SEM images of the spray-dried PMO samples are shown in figure , clearly indicating the formation of excellent spherical particles. All the samples were morphologically controlled by spray drying. Particle size distributions were calculated by counting the spheres (well separated or slightly aggregated) with diameters less than 2.5 μm (figure ). The average (μ) and standard deviation (σ) of the particle sizes were also estimated, and then, particle size distributions were fitted with the normal distribution (equation (Equation1))
The average diameters of PMO particles were almost the same (between 0.8 and 1.2 μm), independent of the number of ethane fragments embedded in the frameworks. When the condensation degree of silica networks is low, the size distribution of spray-dried particles only depends on the concentration (amount) of soluble species. With the increase in the condensation degree of the soluble species, the diameter of spherical particles increases according to the high viscosity of the precursor solutions [Citation39]. Consequently, the viscosity of the precursor solutions should not change markedly in the present system because the preparation of the precursor solutions is conducted under acidic conditions in a short time.
Figure 1 SEM images with 5 μm scale bar of (a) Sample 1, (b) Sample 2 and (c) Sample 3, and corresponding particle size distributions. The line in the particle size distribution indicates the normal distribution function.
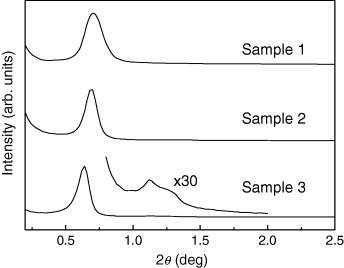
The SAXS patterns and TEM images of the samples are shown in figures and , respectively. The SAXS pattern of Sample 1 (pure mesoporous silica) showed only one broad peak (d=12.0 nm), indicating mesoscopic periodicity. The TEM image of Sample 1 (figure (a)) revealed that closely packed cage-type mesopores were formed in the spherical spray-dried particles. Similar spherical mesopores were also observed by tilting the sample during the TEM observation. The result well supports the formation of periodic cage-type mesopores in all the spherical particles. The pore-to-pore distance was estimated to be 11.7 nm, which agrees well with the d-spacing deduced from the SAXS pattern. All data for Sample 2 were similar to those for Sample 1. In contrast, Sample 3 was somewhat different from Samples 1 and 2. In addition to the main peak, higher order peaks were observed in the SAXS pattern. Although the peaks were very weak, they could be indexed to the (11) and (20) diffractions of a 2D hexagonal structure (space group: P6mm) when the main peak was assigned to the (10) peak. However, as shown in figure (c), the TEM image of Sample 3 was similar to those observed for Samples 1 and 2.
Figure 3 TEM images of (a) Sample 1, (b) Sample 2, (c) Sample 3 and (d) PMO with benzene fragments. Scale bars in the images are 50 nm.
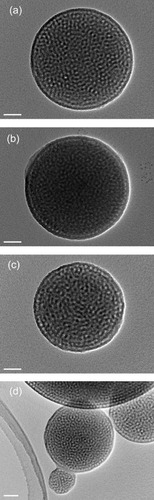
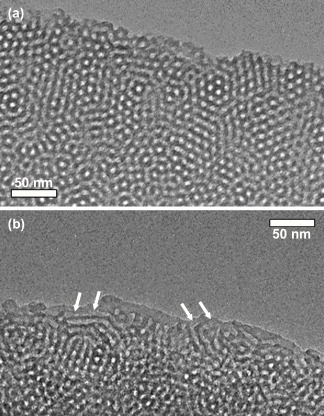
Detailed TEM observations of Samples 1 and 3 were carried out for further understanding of the formation of mesostructures. TEM images of sliced Samples 1 and 3 are shown in figure . The TEM image of Sample 3 clearly exhibited tubular mesochannels due to a 2D hexagonal structure in a very small region, as indicated by arrows in figure (b). The 2D hexagonal structure is partially formed on the surfaces of the spherical particles. Accordingly, it is rational to conclude that cage-type mesopores are mainly formed in the spherical particles. The pore-to-pore distances measured from the TEM images were 12.5 nm (Sample 2) and 13.8 nm (Sample 3). The SAXS results confirmed that the d-spacing (pore-to-pore distance) of the samples increased with the increase in the number of organic fragments embedded in the frameworks. In this study, the number of Si atoms is the same in all the precursor solutions for Samples 1, 2 and 3. Accordingly, the presence of organic groups in the frameworks leads to the increase in wall thickness because the volume of the ethane fragment (Si-C-C-Si in Samples 2 and 3) is larger than that of the pure silica framework (Si-O-Si in Sample 1). This would be related to the increase in pore-to-pore distance, as indicated in [Citation42, Citation43].
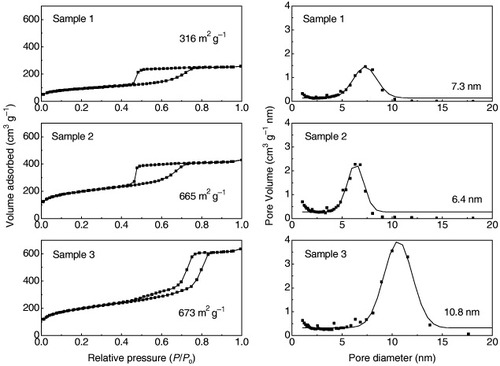
The N2 adsorption–desorption isotherms of the PMO spherical particles are shown in figure . The isotherms of Samples 1 and 2 showed type IV behaviors with clear H2 hysteresis loops, strongly indicating the presence of periodic cage-type mesopores connected through small windows [Citation44, Citation45]. In contrast, the isotherm of Sample 3 showed a type IV behavior with an H1 hysteresis loop, similar to that of 2D hexagonal SBA-15 [Citation46]. Most regions in the spherical particles are occupied by cage-type mesopores, but tubular mesopores are present on the surfaces of the spherical particles (explained above with the TEM and SAXS results of Sample 3). The adsorption–desorption behavior of nitrogen into a porous material would mainly be governed by the porous structures on the surfaces of the spherical particles. Accordingly, nitrogen adsorbs and desorbs into the spherical particles of Sample 3, as in the case of tubular mesoporous materials.
Figure 5 N2 adsorption–desorption isotherms and pore size distributions for (a) Sample 1, (b) Sample 2 and (c) Sample 3. BET surface areas and average pore diameters are noted in the figures.
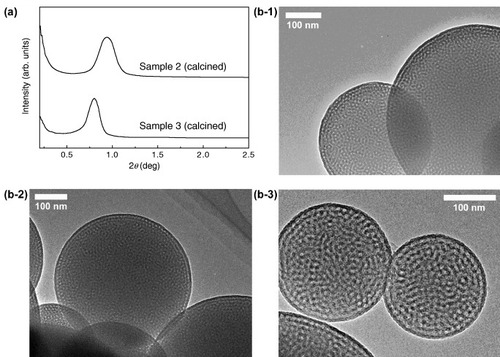
The 29Si MAS NMR spectra of Samples 1, 2 and 3 are shown in figure (a). For Sample 1, three peaks were observed at −109.7 (Q4, (SiO)4Si), −100.9 (Q3, (SiO)3SiOH) and −91.2 (Q2, (SiO)2Si(OH)2) ppm. For Samples 2 and 3, in addition to Qn peaks (n=2–4), peaks derived from BTSE appeared at −63.1 (T3, (SiO)3SiC) and −56.4 (T2, (SiO)2SiC(OH)) ppm for Sample 2, and −64.8 (T3), −56.4 (T2) and −47.4 (T1, (SiO)SiC(OH)2) ppm for Sample 3. For Samples 2 and 3, the integrated ratios of Qn (derived from TEOS) to Tn (derived from BTSE) were, respectively, estimated to be 1:4 and 1:1, which are in good agreement with the molar ratios of the precursor solutions (see table ). The results confirm that the initial compositions of the precursor solutions are strictly retained in the final products obtained by spray drying. The 13C CP/MAS NMR spectra of Samples 1, 2 and 3 are also shown in figure (b). The signals assignable to carbon atoms in ethane groups (Si-CH2-CH2-Si) in the PMO frameworks were observed at 4.35 ppm (Sample 2) and 4.71 ppm (Sample 3). Several peaks marked by filled circles and open triangles originate from the triblock copolymer and ethoxy groups, respectively. The ethoxy groups were formed during the treatment of the samples with an acidic ethanol to remove the triblock copolymer. The TG analysis of the spray-dried samples (Samples 2 and 3) showed large mass losses in the 400–700 °C range owing to the decomposition of ethane fragments [Citation17]. After calcination at 700 °C, organic fractions, such as ethane groups in the frameworks, were completely removed, and the frameworks were converted to pure silica. The SAXS patterns and TEM images of the calcined samples prove that mesostructural ordering is maintained even after the complete removal of the ethane fragments (figure ).
Figure 6 (a) 29Si MAS NMR and (b) 13C CP/MAS NMR spectra of Samples 1, 2 and 3. Peaks marked by filled circles and open triangles are due to the original surfactant and ethoxy groups, respectively.
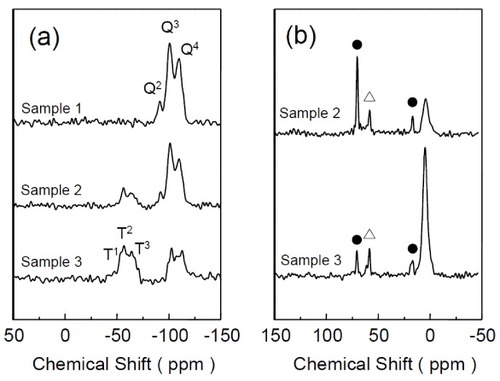
Figure 7 (a) SAXS patterns and TEM images of calcined (at 900 °C for 2 h) products of (b-1) Sample 1, (b-2) Sample 2 and (b-3) Sample 3.
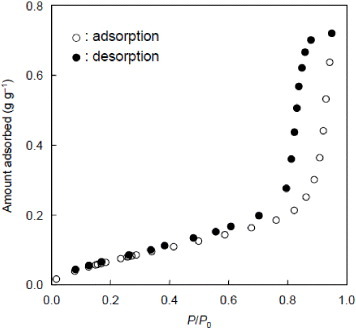
The water adsorption–desorption behavior observed on the surface of Sample 3 was investigated because the organic content of Sample 3 was the highest among the samples used in this study. In general, ordered mesoporous silica is hydrophobic (type V isotherm). It shows a large hysteresis loop and capillary condensation occurs depending on mesopore size [Citation12, Citation13, Citation47, Citation48]. It is considered that the water adsorption–desorption behavior observed over PMO is different from that observed for ordered mesoporous silica because of the presence of hydrophobic organic groups. The water adsorption–desorption isotherm of Sample 3 is shown in figure . Capillary condensation started at P/P0=0.8; the value was higher than that expected from mesopore size [Citation48] and was strongly related to the hydrophobicity owing to the presence of ethane groups. Interestingly, the hysteresis loop was markedly reduced, indicating that water molecules were easily removed from the PMO surface owing to a strong hydrophobicity. More hydrophobic phenyl-containing PMO spherical particles were also prepared using BTEB (instead of BTSE). The TEM image of the spray-dried sample prepared at a 0.25 BTEB/TEOS mole ratio revealed that the benzene fragments were successfully incorporated with the formation of ordered mesostructures (figure (d)).
Conclusions
Spherical particles of PMO with ethylene and phenylene groups were fabricated by spray drying. The spray-drying process is suitable for the rapid mass production of fine solid products and its use can be simply extended to the production of PMO with various organic groups. The organic contents of resultant solid products can be controlled according to the molar ratios of bridged silsesquioxanes to tetraalkoxysilanes in clear precursor solutions. This study provides a route for the mass production of PMO materials with unique properties and functions, because phenyl groups are extremely hydrophobic and reactive and therefore are suitable for further design of organic groups in the frameworks. This study will open a new stage of mass production of versatile periodic mesoporous silica with embedded and surface-attached organic groups.
Acknowledgments
This work was mainly supported by the World Premier International (WPI) Research Center Initiative on ‘Materials Nanoarchitectonics (MANA)’ by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. The study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) and the Murata Science Foundation. TS and MM acknowledge Grants-in-Aid for Scientific Research from JSPS and the Nanotechnology Support Project of MEXT, respectively. During this study, PG worked as a student intern at NIMS.
References
- YanagisawaTShimizuTKurodaKKatoC 1990 Bull. Chem. Soc. Japan 63 988 http://dx.doi.org/10.1246/bcsj.63.988
- KresgeC TLeonowiczM ERothW JVartuliJ CBeckJ S 1992 Nature 359 710 http://dx.doi.org/10.1038/359710a0
- BeckJ S et al 1992 J. Am. Chem. Soc. 114 10834 http://dx.doi.org/10.1021/ja00053a020
- SayariA 1996 Chem. Mater. 8 1840 http://dx.doi.org/10.1021/cm950585+
- CormaA 1997 Chem. Rev. 97 2373 http://dx.doi.org/10.1021/cr960406n
- WanYZhaoD Y 2007 Chem. Rev. 107 2821 http://dx.doi.org/10.1021/cr068020s
- ArigaKHillJ PLeeM VVinuACharvetRAcharyaS 2008 Sci. Technol. Adv. Mater. 9 014109 http://dx.doi.org/10.1088/1468-6996/9/1/014109
- YanagisawaTShimizuTKurodaKKatoC 1990 Bull. Chem. Soc. Japan 63 1535 http://dx.doi.org/10.1246/bcsj.63.1535
- ClarkJ HMacquarrieD J 1999 Chem. Commun. 853
- SayariAHamoudiS 2001 Chem. Mater. 13 3151 http://dx.doi.org/10.1021/cm011039l
- AnwanderR 2001 Chem. Mater. 13 4419 http://dx.doi.org/10.1021/cm0111534
- KimuraTKurodaKSugaharaYKurodaK 1998 J. Porous Mater. 5 127 http://dx.doi.org/10.1023/A:1009641304742
- KimuraTSaekiSSugaharaYKurodaK 1999 Langmuir 15 2794 http://dx.doi.org/10.1021/la9815042
- MurataSHataHKimuraTSugaharaYKurodaK 2000 Langmuir 16 7106 http://dx.doi.org/10.1021/la991393m
- KimuraTSuzukiMTomuraSOdaK 2003 Chem. Lett. 32 188 http://dx.doi.org/10.1246/cl.2003.188
- YoshitakeH 2005 New J. Chem. 29 1107 http://dx.doi.org/10.1039/b504957a
- InagakiSGuanSFukushimaYOhsunaTTerasakiO 1999 J. Am. Chem. Soc. 121 9611 http://dx.doi.org/10.1021/ja9916658
- MeldeB JHollandB TBlanfordC FSteinA 1999 Chem. Mater. 11 3302 http://dx.doi.org/10.1021/cm9903935
- Yoshina-IshiiCAsefaTCoombsNMacLachlanM JOzinG A 1999 Chem. Commun. 2539
- AsefaTMacLachlanM JCoombsNOzinG A 1999 Nature 402 867
- HoffmannFCorneliusMMorellJFröbaM 2006 Angew. Chem. Int. Ed. Engl. 45 3216 http://dx.doi.org/10.1002/anie.200503075
- KapoorM PInagakiS 2006 Bull. Chem. Soc. Japan 79 1463 http://dx.doi.org/10.1246/bcsj.79.1463
- LuYFanHStumpAWardT LRiekerTBrinkerC J 1999 Nature 398 223 http://dx.doi.org/10.1038/18410
- AnderssonNAlberiusP C APedersenJ SBergströmL 2004 Microporous Mesoporous Mater. 72 175 http://dx.doi.org/10.1016/j.micromeso.2004.04.019
- ArevaSBoissièreCGrossoDAsakawaTSanchezCLindénM 2004 Chem. Commun. 1630
- KimS HLiuB Y HZachariahM R 2004 Langmuir 20 2523 http://dx.doi.org/10.1021/la034864k
- SörensenM HCorkeryR WPedersenJ SRosenholmJAlberiusP C 2008 Microporous Mesoporous Mater. 113 1 http://dx.doi.org/10.1016/j.micromeso.2007.10.045
- LuYFanHDokeNLoyD AAssinkR ALaVanD ABrinkerC J 2000 J. Am. Chem. Soc. 122 5258 http://dx.doi.org/10.1021/ja9935862
- JiXHuQHampseyJ EQiuXGaoLHeJLuY 2006 Chem. Mater. 18 2265 http://dx.doi.org/10.1021/cm052764p
- MelnykI VZubY LVéronEMassiotDCacciaguerraTAlonsoB 2008 J. Mater. Chem. 18 1368 http://dx.doi.org/10.1039/b717133a
- LópezB JBoissièreCChanéacCGrossoDVasseurSMirauxSDuguetESanchezC 2007 J. Mater. Chem. 17 1563 http://dx.doi.org/10.1039/b705871c
- YanYZhangFMengYTuBZhaoD 2007 Chem. Commun. 601
- TsungC KFanJZhengNShiQFormanA JWangJStuckyG D 2008 Angew. Chem. Int. Ed. Engl. 47 1 http://dx.doi.org/10.1002/anie.200802487
- LuYMcCaugheyB FWangDHampseyJ EDokeNYangZBrinkerC J 2003 Adv. Mater. 15 1733 http://dx.doi.org/10.1002/adma.200305331
- YamauchiYGuptaPFukataNSatoK 2009 Chem. Lett. 38 78 http://dx.doi.org/10.1246/cl.2009.78
- SakuraiMShimojimaAYamauchiYKurodaK 2008 Langmuir 24 13121 http://dx.doi.org/10.1021/la801065a
- YamauchiYSuzukiNSatoKFukataNMurakamiMShimizuT 2009 Bull. Chem. Soc. Japan at press
- YamauchiYTakeuchiFTodorokiSSakkaYInoueS 2008 Chem. Lett. 37 72 http://dx.doi.org/10.1246/cl.2008.72
- YamauchiYGuptaPSatoKFukataNTodorokiSInoueSKishimotoS 2009 J. Ceram. Soc. Japan 117 198 http://dx.doi.org/10.2109/jcersj2.117.198
- SingK S WEverettD HHaulR A WMoscouLPierottiR ARouquerolJSiemieniewskaT 1985 Pure Appl. Chem. 57 603 http://dx.doi.org/10.1351/pac198557040603
- BarretE PJoynerL GHalendaP P 1951 J. Am. Chem. Soc. 73 373 http://dx.doi.org/10.1021/ja01145a126
- MizoshitaNGotoYTaniTInagakiS 2008 Adv. Funct. Mater. 18 3699 http://dx.doi.org/10.1002/adfm.200800694
- HattonB DLandskronKWhitnallWPerovicD DOzinG A 2005 Adv. Funct. Mater. 15 823 http://dx.doi.org/10.1002/adfm.200400221
- MorishigeKTateishiMHiroseFAramakiK 2006 Langmuir 22 9220 http://dx.doi.org/10.1021/la061360o
- KimT WRyooRKrukMGierszalK PJaroniecMKamiyaSTerasakiO 2004 J. Phys. Chem. B 108 11480 http://dx.doi.org/10.1021/jp048582k
- ZhaoD YFengJ LHuoQ SMeloshNFredricksonG HChmelkaB FStuckyG D 1998 Science 279 548 http://dx.doi.org/10.1126/science.279.5350.548
- InagakiSFukushimaY 1998 Microporous Mesoporous Mater. 21 667 http://dx.doi.org/10.1016/S1387-1811(98)00075-4
- KimuraTSuzukiMMaedaMTomuraS 2006 Microporous Mesoporous Mater. 95 213 http://dx.doi.org/10.1016/j.micromeso.2006.05.027