Abstract
A new copper(II) complex [Cu(C12H23N3)4Br2·2H2O] was synthesized and its structure was characterized by x-ray crystallography and elemental analysis. The copper atom had a distorted octahedron coordination involving two bromide anions and four nitrogen atoms from the 1-decyl-1H-[1,2,4]triazole ligands. Moreover, the electrochemical behavior and electrocatalysis of the carbon paste electrode (Cu-CPE) bulk-modified by the complex have been studied in detail. The Cu-CPE showed excellent electrocatalytic activities toward the reduction of hydrogen peroxide and nitrite, and the detection limit was much lower than that mentioned in earlier reports. This bulk-modified CPE has good reproducibility, long-term stability and surface renewability, which appear promising for constructing chemical sensors.
Introduction
The significant contemporary interest in organic–inorganic hybrid materials reflects both the fundamental chemistry of the rational design of complex materials and practical applications as diverse catalysis [Citation1], optical materials [Citation2, Citation3], membranes [Citation4–6] and sorption materials [Citation7, Citation8]. One synthetic strategy for the design of inorganic–organic hybrid materials is to select suitable inorganic materials and organic ligands with structure-directing properties [Citation9–11]. In these hybrid materials, metal complexes with diverse structural arrangements not only serve as charge-compensating units but also modify the magnetic and optical properties, electronic conductivities and electrocatalysis. Electrochemical reactions catalyzed by metal complexes had attracted considerable attention during the past several decades [Citation12, Citation13]. Recently, considerable efforts have been directed toward the study of Cu(II) complexes incorporating pyridine, thioether, imidazole and imine donors, which not only stem from their fascinating structures but also from their potential applications as new materials [Citation14–17]. Wang et al studied the electrocatalytic reduction of NO2− by copper complexes containing the chelating ligand dipyrido[3,2-d:2′,3′-f]quinoxaline [Citation18, Citation19]. The electrochemistry and electrocatalysis of copper complexes with various ligands have been investigated by several groups [Citation20–22].
Currently, triazole ligands and their derivatives are considered for constructing coordination complexes [Citation23]. Those complexes are known for their rich coordination modes and wide applications as multidentate bridging ligands in coordination chemistry. However, to the best of our knowledge, the electrochemistry and electrocatalysis of copper complexes containing triazole have been insufficiently studied. The significance of our work is to use a new copper(II) complex of 1-decyl-1H-[1,2,4]triazole as a bulk modifier to fabricate a renewable chemically modified carbon paste electrode by direct mixing. This modified electrode showed excellent electrocatalytic activities toward the reduction of hydrogen peroxide and nitrite, and the possible mechanisms were proposed.
Experiments
Chemicals and measurement
All the chemicals were of analytical reagent grade and used without further purification. Elemental composition was measured with a Perkin-Elmer 1400C analyzer (USA). Electrochemical measurements were performed using an Autolab PGSTAT-30 digital potentiostat/galvanostat (EcoChemie BV, Utrecht, Netherlands). A three-electrode cell was used in the experiments. The working electrode was modified CPE. The counter electrode was a platinum wire. The reference electrode was Ag|AgCl||KCl (1 M), and all the potentials reported in this work were measured relative to this electrode (236.3 mV at 25 °C). The supporting electrolytes used for electrochemical experiments were 0.1 M, pH 6.1, Britton–Robinson (B–R) buffer solutions. All the solutions were deaerated with pure nitrogen for 30 min and kept under nitrogen atmosphere during the experiments. All the measurements were performed at room temperature (25±2 °C).
Preparation and physical measurement of the title complex
Synthesis of [Cu(C12H23N3)4Br2·2H2O] was performed as follows: Copper bromide (224 mg, 1 mmol) and 1-decyl-1H-[1,2,4]triazole (836 mg, 4 mmol) were dissolved in 50 ml of ethanol. After stirring for 2 h, the resulting blue precipitate was obtained by filtration. Recrystallization from acetonitrile gave a yield of 78.2%. The C, H and N contents were determined by elemental analysis (calculated for C48H96Br2CuN12O2: C 52.57%, H 8.82%, N 15.33%; measured: C 52.55%, H 8.83%, N 15.29%).
X-ray diffraction was measured at 20 °C using MoKα radiation (λ=0.71073 Å) with a graphite monochromator. The absorption correction applied to the collected data was empirical, based on Ψ-scans. The structure of the title complex was solved by direct methods and refined by least squares on Fobs2 using the SHELXTL [Citation24] software package. All nonhydrogen atoms were refined anisotropically, and the hydrogen atom positions were determined on the map of difference Fourier synthesis and refined isotropically. The molecular graphics were plotted using SHELXTL. Atomic scattering factors and anomalous dispersion corrections were taken from the International Tables for x-ray Crystallography [Citation25].
Preparation of CPE bulk-modified by the title complex
The traditional carbon paste electrode (CPE) was prepared by hand-mixing of graphite powder with paraffin oil at a ratio of 70 : 30 (w/w) in an agate mortar: 0.3 g of graphite powder and 0.03 g of the title complex were mixed and ground together using agate mortar and pestle for about 30 min to achieve an even, dry mixture. Paraffin oil (0.1 ml) was added to the mixture and stirred with a glass rod. Then the homogenized mixture was used to fill glass tubes (3 mm inner diameter) to a depth of 0.8 cm. Electrical contact was established with a copper rod and the surface of Cu-CPE was polished on a piece of weighing paper to a mirror finish just before use.
The different quantity ratios of graphite powder and the title complex were investigated to determine the optimum preparation conditions. We investigated the graphite powder and the title complex at the ratios of 20 : 1, 14 : 1, 10 : 1, 8 : 1 and 5 : 1 (w/w), and found the 10 : 1 ratio optimal. When the amount of the title complex was low, the electrochemical signal was weak. Conversely, when the amount of the title complex was high, the conductivity of the modified carbon paste electrode was low.
Results and discussion
Crystal structure of the title complex
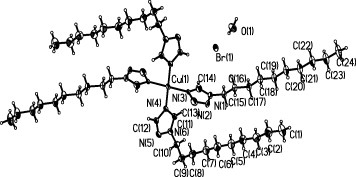
The molecular structure of [Cu(C12H23N3)4Br2·2H2O] with the atomic numbering scheme is shown in figure . Crystal data and structure refinement of the title complex are listed in table . The structure contains one Cu(C12H23N3)42+ cation, two Br− anions and two water molecules. The coordination sphere of the copper atom is completed with two long contacts to the two symmetry-equivalent bromide anions. These contacts are strongly elongated owing to a Jahn–Teller distortion. The coordination polyhedron around the copper atom can be described as a strongly distorted octahedron [Citation26]. The copper atom is coordinated with two bromide anions and four nitrogen atoms from the 1-decyl-1H-[1,2,4]triazole ligands. The Cu–N distances of Cu(1)–N(4)#1 2.018(2) Å, Cu(1)–N(4) 2.018(2) Å, Cu(1)–N(3) 2.031(3) Å and Cu(1)–N(3)#1 2.031(3) Å are comparable to the corresponding values in a similar complex [Citation26]. There is a potentially weak intramolecular hydrogen bond interaction. The donor–acceptor distance is 3.4293 Å for C(13)···Br(1). There are three intermolecular hydrogen bond interactions; the donor–acceptor distances were 3.3712 Å for O(1)···Br(1), 3.2209 Å for C(11)···O(1) and 3.0186 Å for C(12)···O(1). There is one type of π–π stacking interaction. The center-to-center distance is 3.951 Å, and the perpendicular interplane distance is 3.468 Å between the plane [N(4)#–N(5)#–N(6)#–C(11)#–C(12)#] and the plane [N(4)–N(5)–N(6)–C(11)–C(12)]. There is also one type of C–H···π supramolecular interaction. The distance between C(10)–H(10B) to the plane [N(1)–N(2)–N(3)–C(13)–C(14)] is 3.745 Å. In the solid state, all the above intermolecular interactions in this structure have stabilized the crystal structure.
Table 1 Crystal data and structure refinement for the title complex.
Electrochemical behavior of Cu-CPE
The electrochemical behavior of the Cu-CPE was investigated by cyclic voltammetry in the aqueous solution. We chose a 0.1 M KCl aqueous solution, 0.1 M, pH 7.0, Britton–Robinson (B–R) buffer solution and 0.1 M, pH 7.0, phosphate buffer solution as the supporting electrolyte solutions. It was found that the modified electrode in B–R buffer solution had strong current responses and symmetric peak shape. Then, we investigated the electrochemical behavior of modified CPE in the pH range from 4.0 to 8.0 B–R buffer solution and found that the electrochemical signal was relatively stable at pH 6.1. Therefore, we studied the electrochemical behavior and electrocatalytic properties in 0.1 M, pH 6.1, B–R buffer solution.
The cyclic voltammograms (CVs) of Cu-CPE were investigated. In the potential range from 0 to −1.3 V, there was no redox peak at the bare CPE. At the modified Cu-CPE in figure , when the scan rate was 0.05 V s−1, the curve a had two well-defined redox peaks at −0.724 and −0.582 V, with the formal potential (E0′) at −0.653 V, corresponding to the electrochemical process of Cu(II)/Cu(I). The separation of the cathodic and anodic peak potential, ΔE=0.142 V, ipc/ipa=1.06, indicated that the electrochemical behavior of the title complex on the electrode was quasi-reversible.
Figure 2 CVs of the Cu-CPE in 0.1 M, pH 6.1, B–R buffer solution. Curves (a–e) correspond to the scan rates 0.05, 0.06, 0.07, 0.08 and 0.10 V s−1, respectively. Inset: peak current versus the scan rate.
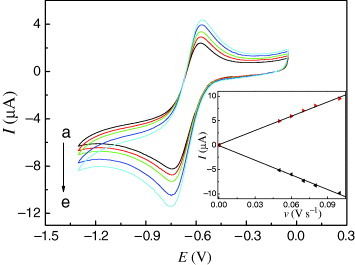
The effect of scan rate on the electrochemical behavior of the Cu-CPE can be seen from figure . When the scan rate was varied from 0.05 to 0.10 V s−1, the peak potentials changed gradually: the cathodic peak potentials shifted to the negative direction and the corresponding anodic peak potentials shifted to the positive direction with increasing scan rate. The plot of the peak current versus the scan rate is shown in the inset of figure . The anodic and cathodic currents are proportional to the scan rate, suggesting that the redox process is confined to the surface.
Electrocatalytic activity of the Cu-CPE
Electrocatalytic effect on reduction of hydrogen peroxide
Hydrogen peroxide is used in pollution control, bleaching of textile and paper products and in the processing of petrochemicals, minerals, food and various products. Moreover, hydrogen peroxide is the product of various oxidases in countless biological reactions, and this fact has been used in the development of first-generation biosensors [Citation27]. It is well known that the electroreduction of hydrogen peroxide requires a large overpotential, and no obvious response is observed on a bare CPE.
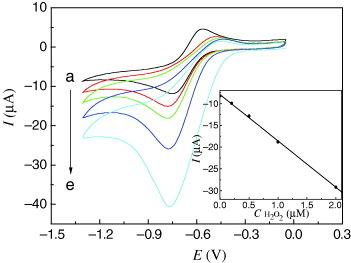
The electrocatalytic activity toward hydrogen peroxide of Cu-CPE is shown in figure . With the addition of hydrogen peroxide (curves a–e), the reduction peak currents increased markedly. Meanwhile, the corresponding oxidation peak currents decreased, which indicated that Cu-CPE had good electrocatalytic activity toward the reduction of hydrogen peroxide. The possible reaction processes could be described using the following equations [Citation19, Citation28]:
The inset of figure shows that the catalytic current is linear versus hydrogen peroxide concentration in the range of 0.2–2.0 μM. The linear regression equation was Ipc(μA)=–10.63C(μM)–7.939 with a correlation coefficient of 0.998. The detection limit was 0.16 μM based on the signal-to-noise ratio of 3. This value is much lower than that of an earlier report in which another modified electrode was used [Citation29].
Electrocatalytic effect on reduction of nitrite
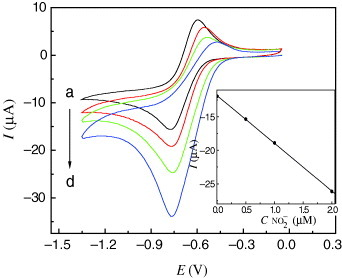
Nitrite is a common pollutant originating from both agricultural and industrial sources. Many substrates such as porphyrins and heteropolyanion systems have been used for the electrocatalytic reduction of nitrite in the past [Citation30]. The electroreduction of nitrite required a large overpotential, and no response was observed at a bare electrode. We found that the Cu-CPE also displayed excellent electrocatalytic activity toward the reduction of nitrite in 0.1 M, pH 6.1, B–R buffer solution. As indicated by curves a–d in figure , with the addition of nitrite, the reduction peak currents increased while the corresponding oxidation peak currents decreased. The possible reaction processes could be described as follows [Citation19]:
The inset of figure shows that the catalytic current varies linearly with nitrite concentration in the range of 0.5–2.0 μM. The linear regression equation is Ipc(μA)=–7.115C(μM)–11.85 with a correlation coefficient of 0.999. The detection limit was 0.35 μM based on the signal-to-noise ratio of 3. This value was much lower than that (2.3 μM) of an earlier report in which another modified electrode was used [Citation31].
Figure 4 CVs of Cu-CPE in 0.1 M, pH 6.1, B–R buffer solution (a–d) containing 0, 0.5, 1.0 and 2.0 μM nitrite with the scan rate of 0.08 V s−1. Inset: peak current versus nitrite concentration.
The electrode surface could be renewed by squeezing some carbon paste out of the glass tube, and a fresh surface was exposed whenever needed. This was particularly useful for electrocatalytic study since the catalytic activity is known to decrease when the electrode is degraded. Compared with other modified film electrodes, the electrode bulk-modified with the title complex showed high stability. The potential was stable over 200 cycles at a scan rate of 0.1 V s−1 and the current response remained almost unchanged. When the bulk-modified CPE was stored at room temperature for at least 2 months, the current response decreased by only 2.6%.
Conclusions
In summary, the structure of a new complex of [Cu(C12H23N3)4Br2·2H2O] was characterized by x-ray crystallography. The title complex was used to fabricate bulk-modified carbon paste electrodes because of its insolubility. The electrochemical behavior and electrocatalysis of the Cu-CPE have been investigated. This modified electrode showed excellent electrocatalytic activities toward the reduction of hydrogen peroxide and nitrite, and the results were reproducible with a low detection limit, which was suitable for the quantitative analysis of environmentally hazardous materials. The advantages of the bulk-modified Cu-CPE were its stability, excellent catalytic activity, low detection limit and simplicity of preparation in comparison with other methods. These preliminary results suggest potential applications in electrochemical sensors.
Supplementary material
Crystallographic data of the structural analysis have been deposited at the Cambridge Crystallographic Data Centre, CCDC reference number 710357. Copies of this information can be obtained free of charge from The Director, 12 Union Road, Cambridge, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: [email protected] or http://www.ccdc.cam.ac.uk).
Acknowledgment
This work was supported by the Natural Science Foundation of Shandong Province (No. Z2007B01), P. R. China.
References
- NgoH LHuA GLinW B 2004 J. Mol. Catal. A 215 177 http://dx.doi.org/10.1016/j.molcata.2004.01.022
- SanchezCLebeauBChaputFBoiletJ P 2003 Adv. Mater. 15 1969 http://dx.doi.org/10.1002/adma.200300389
- EvansO RLinW B 2001 Chem. Mater. 13 3009 http://dx.doi.org/10.1021/cm010427k
- JannaschP 2003 Curr. Opin. Colloid Interface Sci. 8 96 http://dx.doi.org/10.1016/S1359-0294(03)00006-2
- JavaidAHugheyM PVarutbangkulVFordD M 2001 J. Membr. Sci. 187 141 http://dx.doi.org/10.1016/S0376-7388(01)00341-6
- HonmaINomuraSNakajimaH 2001 J. Membr. Sci. 185 83 http://dx.doi.org/10.1016/S0376-7388(00)00636-0
- SudikA CMillwardA ROckwigN WCoteA PKimJYaghiO M 2005 J. Am. Chem. Soc. 127 7110 http://dx.doi.org/10.1021/ja042802q
- RowsellJ L CMillwardA RParkK SYaghiO M 2004 J. Am. Chem. Soc. 126 5666 http://dx.doi.org/10.1021/ja049408c
- LisdatFDronovRMöhwaldHSchellerF WKurthD G 2009 Chem. Commun. 274 http://dx.doi.org/10.1039/b813559b
- LiuSKurthD GVolkmerD 2002 Chem. Commun. 976 http://dx.doi.org/10.1039/b200942k
- TakátsZWisemanJ MGologanBCooksR G 2004 Anal. Chem. 76 4050 http://dx.doi.org/10.1021/ac049848m
- CheungK CWongW LMaD LLaiT SWongK Y 2007 Coord. Chem. Rev. 251 2367 http://dx.doi.org/10.1016/j.ccr.2007.04.004
- ChenS M 1998 J. Electroanal. Chem. 457 23 http://dx.doi.org/10.1016/S0022-0728(98)00143-0
- BalamuruganRPalaniandavarMHalcrowM A 2006 Polyhedron 25 1077 http://dx.doi.org/10.1016/j.poly.2005.07.047
- KimE HKimD ILeeH SByunJ CChoiJ HParkY C 2007 Polyhedron 26 85 http://dx.doi.org/10.1016/j.poly.2006.07.033
- GaoG GXuLWangW JAnW JQiuY FWangZ QWangE B 2005 J. Phys. Chem. B 109 8948 http://dx.doi.org/10.1021/jp044403n
- SalimiAAlizadehVHadadzadehH 2004 Electroanalysis 16 1984 http://dx.doi.org/10.1002/elan.200303035
- WangX LLinH YLiuG CZhaoH YChenB K 2008 J. Organomet. Chem. 693 2767 http://dx.doi.org/10.1016/j.jorganchem.2008.05.031
- WangX LZhaoH YLinH YLiuG CFangJ NChenB K 2008 Electroanalysis 20 1055 http://dx.doi.org/10.1002/elan.200704146
- WangX LKangZ HWangE BHuC W 2002 J. Electroanal. Chem. 523 142 http://dx.doi.org/10.1016/S0022-0728(02)00742-8
- LiCCaoRO'HalloranK PMaHWuL 2008 Electrochim. Acta 54 484 http://dx.doi.org/10.1016/j.electacta.2008.07.039
- ZhaoZZhouBSuZMaHLiC 2008 Inorg. Chem. Commun. 11 648 http://dx.doi.org/10.1016/j.inoche.2008.02.032
- LiuJ JHeXShaoMLiM X 2008 J. Mol. Struct. 891 50 http://dx.doi.org/10.1016/j.molstruc.2008.03.011
- SheldrickG M 2000 SHELXTL 6.10 Madison, WI Bruker Analytical Instrumentation
- WilsonA J 1992 International Table for X-ray Crystallography Dordrecht Kluwer
- NätherCWriedtMJeßI 2002 Acta Crystallogr. E 58 m39 http://dx.doi.org/10.1107/S1600536802000314
- GonzalezG L LKahlertHScholzF 2007 Electrochim. Acta 52 1968 http://dx.doi.org/10.1016/j.electacta.2006.08.006
- LiYLinXJiangC 2006 Electroanalysis 18 2085 http://dx.doi.org/10.1002/elan.200603618
- SunNGuanLShiZLiNGuZZhuZLiMShaoY 2006 Anal. Chem. 78 6050 http://dx.doi.org/10.1021/ac060396i
- OunathanJ N YWoodK SMeyerT J 1992 Inorg. Chem. 31 3280 http://dx.doi.org/10.1021/ic00041a022
- HuangXLiYChenYWangL 2008 Sensors Actuators B 134 780 http://dx.doi.org/10.1016/j.snb.2008.06.028